Ultrasound clinical application
1976
1986
1991
Ophthalmic
17
17
50
Fetal, neonatal, pediatric imaging
46
94
720
Cardiac (adult)
430
430
720
Peripheral vascular
720
720
720
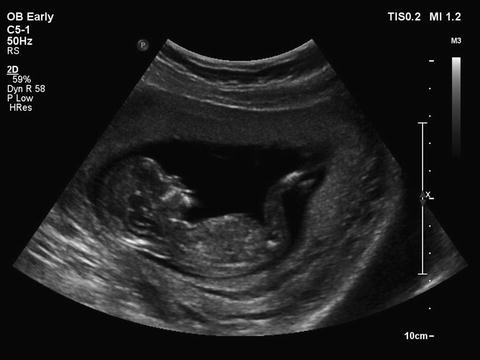
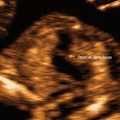
Fig. 1.1
In early pregnancy the entire fetus is within the ultrasound beam. Gestational age of 12 weeks
Non-thermal Effects
Ultrasound bioeffects may also occur through non-thermal or mechanical processes [38, 39]. These include acoustic cavitation, if gas bubbles are present, as well as radiation torque and force and acoustic streaming secondary to propagation of the ultrasound waves. Included in this category are physical (shock wave) or chemical (release of free radicals) effects. Bubble cavitation seems to be the major factor in mechanical effects [40, 41], as it has been demonstrated to occur in living tissues when insonated [42, 43]. Non-thermal mechanisms have been implicated in biological effects of ultrasound in animals, such as local intestinal [44], renal [45], and pulmonary hemorrhages [46], although cavitation could not always be incriminated. Furthermore, since there does not seem to be gas bubbles in the ovarian vasculature or parenchyma, nor the fetal lungs or bowels (where effects have been described in neonates or adult animals), the risk to the ovum and fetus from mechanical effect appears to be minimal [47]. However, the use of contrast agents (including agitated saline) to image the fallopian tubes or the endometrial cavity for instance, introduces these potential cavitation foci [48]. Details on the cavitation phenomenon can be found in various publications [38, 40, 43, 49, 50]. Another described result of mechanical energy is hemolysis [51]. Again, it is evident, however, that the presence of some cavitation nuclei is necessary for hemolysis to occur. In the presence of such contrast agents, fetal red blood cells are more susceptible to lysis from ultrasound exposure in vitro [52]. In addition to the above, fetal stimulation caused by ultrasound (Doppler) insonation has been described, with no apparent relation to cavitation [53]. This effect may be secondary to radiation forces associated with ultrasound exposures. These forces were suspected at the earliest stages of ultrasound research [54] and are known to possibly stimulate auditory [55] and other sensory tissues [56]. The main effects of non-thermal damage have been demonstrated in mammalian tissues containing gas where capillary bleeding has been observed [39, 42, 57]. This potentially pertains to the neonatal lung, intestine and also, as noted above, in the presence of ultrasound microbubble contrast agents. Several non-thermal mechanisms, not related to cavitation have also been described: radiation force, acoustic streaming, modification of electrical potentials, effect on cardiac performance and stimulation of bone repair [12]. None of these have been demonstrated in humans and no harmful effects of diagnostic ultrasound, secondary to non-thermal mechanisms have been reported in human fetuses.
The Output Display Standard (OSD)
Until 1992 acoustic outputs of clinical ultrasound machines had specific limits. For instance, the upper limit of the spatial peak temporal average intensity or I SPTA (the most clinically useful intensity used to determine acoustic power of the ultrasound beam) for adult use was 720 mW/cm2 and for fetal use, 94 mW/cm2, which in fact, already had been increased from a previous maximum value of 46 mW/cm2. It was assumed that higher outputs would generate better images and, thus, improve diagnostic accuracy. Hence, end-users required ultrasound manufacturers to increase their machines output. Some worry, however, was expressed regarding the actual amount of energy absorbed by a human fetus during an ultrasound examination. This amount cannot be measured precisely. Not only the lack of an internal recording device is a major issue but, in addition, elements such as variations in maternal body habitus, fetal position changes, and gestational age progression render such a task impossible. To allow clinical users of ultrasound to use their instruments at higher powers than originally intended and to reflect the two major potential biological consequences of ultrasound (thermal and mechanical), the American Institute of Ultrasound in Medicine (AIUM), the National Electrical Manufacturers’ Association (NEMA) and the US food and Drug Administration (FDA), with representatives from the Canadian Health Protection Branch, the National Council on Radiation Protection and Measurements (NCRP) and 14 other medical organizations developed a standard related to the potential for ultrasound bioeffects [12]. The Standard for Real-Time Display of Thermal and Mechanical Indices on Diagnostic Ultrasound Equipment, generally known as the Output Display Standard or ODS, was an attempt to provide quantitative safety-related information. This information was to appear on-screen during an exam, so that the end-users would be able to see how manipulation of the instrument controls during an examination causes alterations in the output and, thus, on the exposure, providing, from a clinical standpoint, a rough estimate to compare various modes of examination. As a consequence, the acoustic output for fetal use, as expressed by the I SPTA went from a previous value of 94–720 mW/cm2 (see Table 1.1). It is interesting to observe from the table that, for fetal imaging, the I SPTA was allowed to increase by a factor of almost 16 from 1976 to the most recent values in 1992; yet virtually all epidemiological information available regarding fetal effects predates 1992. A further remarkable fact is that intensity for ophthalmic examination was increased from the original 17–50 mW/cm2, a value approximately 12 times lower than the present allowed maximal value for fetal scanning. Furthermore, the clinical categories included in the analysis consisted of ophthalmic, fetal (without specification of gestational age), cardiac, and peripheral vascular examinations. Pelvic imaging (abdominal or transvaginal), including, naturally, examination of ovaries in ovulation induction, is not mentioned. The indices to appear on-screen (Fig. 1.2) were the thermal index (TI), to provide some indication of potential temperature increase and the mechanical index (MI), to provide indication of potential for non-thermal (i.e., mechanical) effects [58, 59]. The TI calculation is based on the formula:

 where W is the acoustic power while scanning and W deg is the acoustic power required to achieve an increase in temperature of 1 °C under similar conditions [60]. The TI has three variants [35]: TI for soft tissue (TIS), to be used mostly in early pregnancy when ossification is low (as well as in ART for ovulation studies), for bones (TIB), to be used when the ultrasound beam impinges on bone, at or near the beam focus, such as late second and third trimesters of pregnancy and for transcranial studies (TIC) when the transducer is essentially against bone, mostly for examinations in adult patients. These indices were required to be displayed if equal to or over 0.4. It needs to be made very clear that TI does not represent an actual or an assumed temperature increase. It bears some correlation with temperature rise in degrees Celsius, but in no way allowing an estimate or a guess as to what that temperature change actually is in the tissue. The TI represents reasonable “worst-case” estimate of the temperature rise resulting from the exposure. It can, thus, be used to assess the potential for harm via a thermal mechanism, the higher the TI, the higher this potential. Calculations are also on the ultimate temperature reached after prolonged exposure. This time will be short (less than 5 min) with a narrow beam and good tissue perfusion, as is the case in late first-trimester scanning. When bone is present, this time is very short, approximately 30 s. An important point to remember is that experimental data has clearly demonstrated that this worst-case elevation of temperature may be a gross underestimation, by as much as a factor of 2 or even 6, and, more rarely, an overestimation [12]. Furthermore, exposure time is not part of the equation, nor is it in the second index, the MI, which represents the potential for cavitation in tissues, but is not based on actual in situ measurements. The MI is defined as:
where W is the acoustic power while scanning and W deg is the acoustic power required to achieve an increase in temperature of 1 °C under similar conditions [60]. The TI has three variants [35]: TI for soft tissue (TIS), to be used mostly in early pregnancy when ossification is low (as well as in ART for ovulation studies), for bones (TIB), to be used when the ultrasound beam impinges on bone, at or near the beam focus, such as late second and third trimesters of pregnancy and for transcranial studies (TIC) when the transducer is essentially against bone, mostly for examinations in adult patients. These indices were required to be displayed if equal to or over 0.4. It needs to be made very clear that TI does not represent an actual or an assumed temperature increase. It bears some correlation with temperature rise in degrees Celsius, but in no way allowing an estimate or a guess as to what that temperature change actually is in the tissue. The TI represents reasonable “worst-case” estimate of the temperature rise resulting from the exposure. It can, thus, be used to assess the potential for harm via a thermal mechanism, the higher the TI, the higher this potential. Calculations are also on the ultimate temperature reached after prolonged exposure. This time will be short (less than 5 min) with a narrow beam and good tissue perfusion, as is the case in late first-trimester scanning. When bone is present, this time is very short, approximately 30 s. An important point to remember is that experimental data has clearly demonstrated that this worst-case elevation of temperature may be a gross underestimation, by as much as a factor of 2 or even 6, and, more rarely, an overestimation [12]. Furthermore, exposure time is not part of the equation, nor is it in the second index, the MI, which represents the potential for cavitation in tissues, but is not based on actual in situ measurements. The MI is defined as:
 It is a theoretical formulation of the ratio of the peak rarefaction pressure to the square root of the ultrasound frequency (hence, the higher the frequency, the lesser risk of mechanical effect, which is an advantage in endovaginal scanning). As for the TI, exposure time is not part of the calculation. Both the TI and MI can and should be followed as an indication of change in output during the clinical examination. A major component of the implementation of the ODs was supposed to be education of the end-user. Unfortunately, this aspect of the ODS does not seem to have succeeded as end-users’ knowledge of bioeffects, safety, and output indices is lacking. Both in Europe [61] and the USA [62], approximately 70 % of clinicians (physicians and sonographers, including nurses who perform ultrasound) show very poor, or no knowledge of bioeffects and safety issues, do not know what TI and MI represent and do not even know that these appear on-screen during clinical ultrasound examinations. This is true in several other countries [63–65] as well as among residents/fellows [66] and sonographers, regardless of their seniority [67]. Furthermore, several assumptions were made when formulating the indices, which bring questions on their clinical value [68]. Details can be found in the NCRP report 140 [12]. These indices, however, are the best mean we have, nowadays, to estimate, in real time, changes occurring in acoustic output of the instrument, although various modifications have been offered, in particular in regard to exposure time [69, 70]. There is, in fact, little information on energy output and exposure in clinical obstetrical ultrasound. Only relatively recently has it been shown that, if one considers TI and MI to be some indication of acoustic output, then the levels are low in the first [71, 72], second and third trimesters [73], and even Doppler studies [74]—although higher levels of TI can be reached in this modality—as well as 3D/4D examinations [75]. It should also be noted that in some countries, the number of prenatal ultrasound examinations has reached 10 per pregnancy and it is presently unknown whether there is a cumulative dose effect to exposure [76].
It is a theoretical formulation of the ratio of the peak rarefaction pressure to the square root of the ultrasound frequency (hence, the higher the frequency, the lesser risk of mechanical effect, which is an advantage in endovaginal scanning). As for the TI, exposure time is not part of the calculation. Both the TI and MI can and should be followed as an indication of change in output during the clinical examination. A major component of the implementation of the ODs was supposed to be education of the end-user. Unfortunately, this aspect of the ODS does not seem to have succeeded as end-users’ knowledge of bioeffects, safety, and output indices is lacking. Both in Europe [61] and the USA [62], approximately 70 % of clinicians (physicians and sonographers, including nurses who perform ultrasound) show very poor, or no knowledge of bioeffects and safety issues, do not know what TI and MI represent and do not even know that these appear on-screen during clinical ultrasound examinations. This is true in several other countries [63–65] as well as among residents/fellows [66] and sonographers, regardless of their seniority [67]. Furthermore, several assumptions were made when formulating the indices, which bring questions on their clinical value [68]. Details can be found in the NCRP report 140 [12]. These indices, however, are the best mean we have, nowadays, to estimate, in real time, changes occurring in acoustic output of the instrument, although various modifications have been offered, in particular in regard to exposure time [69, 70]. There is, in fact, little information on energy output and exposure in clinical obstetrical ultrasound. Only relatively recently has it been shown that, if one considers TI and MI to be some indication of acoustic output, then the levels are low in the first [71, 72], second and third trimesters [73], and even Doppler studies [74]—although higher levels of TI can be reached in this modality—as well as 3D/4D examinations [75]. It should also be noted that in some countries, the number of prenatal ultrasound examinations has reached 10 per pregnancy and it is presently unknown whether there is a cumulative dose effect to exposure [76].

Fig. 1.2
The TI and MI acoustic indices as demonstrated on the monitor screen during routine ultrasound examination. In this picture, the MI is 0.9 and the TIS, 0.1


Ultrasound and the Ovum
Ultrasound has permeated the field of infertility and reproductive endocrinology, from diagnosing uterine anomalies [77], following development of the follicle [78], evaluating tubal patency [79], to its use in embryo transfer [80]. A study from 1982 demonstrated premature ovulation in women who underwent ultrasound examination of the ovaries (B-mode) in the late follicular phase [81]. The authors compared patients in induced ovulation cycles, followed by ultrasound (study group) or only by hormone levels (control group). They investigated timing of follicle rupture after the onset of LH surge or administration of hCG. Rupture never occurred before the 37th hour in control patients (no ultrasound in the follicular phase). However, (premature) ovulation was observed at 26–36 h in about 50 % cases in the study group (ultrasound during the previous 3 days or in the 36 h immediately following the ovulatory stimulus). This study was very concerning but has never been reproduced. Ultrasound-guided oocyte aspiration for in vitro fertilization and embryo transfer was reported in the early 1980s [82, 83]. It has now become routine [84]. There are only a few, relatively dated, studies aimed at determining the interaction between ultrasound exposure and successful fertilization. Most are, in fact, concerned with success or lack thereof of the procedure in terms of pregnancy rates and not possible bioeffects. Some researchers have reported deleterious effects of ultrasound on the menstrual cycle, particularly decrease in ovulation rates in mice [85] and premature ovulation [81], as well as reduced cumulative pregnancy rates in mice [86] and in humans [87]. Others have demonstrated no effects on the ovulation process or egg quality, including DNA and RNA synthesis [88], or on fertilization rate and embryonic development following in vitro fertilization and embryo transfer [89]. In general, the clinically available data on ultrasound exposure of oocytes during meiosis are confusing. Some researchers reported a deleterious effect on the fertility of patients undergoing artificial insemination with a reduction in the cumulative rate of pregnancy [87]. A study of ultrasound exposure of meiotically active, preovulatory oocytes showed no differences between rats exposed to ultrasound after the LH surge and controls in terms of pregnancy rate, number of corpora lutea, implantations, pups, and mean pup and placental weights at autopsy on day 22 of pregnancy [90]. Others have claimed an increase in the success rate, allowing ultrasound monitoring of follicular growth [91], although, evidently, this is not a direct effect of ultrasound but of improved intervention timing. An attempt to clarify this was described by Mahadevan and colleagues [89]. They wanted to determine how oocytes obtained under ultrasound guidance affected the pregnancy rate. The results obtained with 3.5-MHz probes suggest that exposure of human oocytes to ultrasonic waves during the different phases of meiosis does not significantly influence the developmental potential of the in vitro fertilized embryos. Unfortunately, no researcher describes any of the relevant exposure parameters discussed earlier, except for ultrasound frequency.
Ultrasound in Early Gestation
There are many valid medical indications to perform ultrasound in early gestation [2, 92]. These are described in various parts of this book and include, among others, pregnancy location, accurate gestation dating, confirmation of viability, verification of number of fetuses, and early anatomy survey. All of these examinations are, generally, performed with B-mode, a mode with relatively low acoustic output. However, more recently, screening for genetic abnormalities, such as NT and early assessment of structural abnormalities are described in the literature in early (11–15 weeks) pregnancy [5, 93, 94]. While most of these are also performed with B-mode, often Doppler is used to detect blood vessels and/or to visualize and analyze cardiac valves [95], exposing the fetus to much higher energy levels (see below]. One needs to keep in mind that, even with B-mode, dwell time is important since prolonged examination can result in higher exposure levels [96]. Interesting, somewhat worrying and unexplained data have been published on an increased incidence of fetal anomalies in fetuses resulting from ART [97, 98]. Some concerning effects on the chorionic villi in women who had transvaginal ultrasound during the first trimester were reported [99]. There was a time–effect relation with activation of an enzyme pathway responsible for apoptosis through a mitochondrial pathway with exposures of 20 and 30 min but not 0 (control group) or 10 min.
Fetal Susceptibility to External Insults
The growing fetus is very sensitive to external influences. This is especially true in the first 10–12 weeks of gestation [100, 101]. Known teratological agents include, for instance, certain medications or drug of abuse taken by the pregnant woman, exposure to X-rays and elevated temperature, secondary to infectious diseases [102]. Gestational age is thus a vital factor when considering possible bioeffects: milder exposure during the preimplantation period or more severe exposures during embryonic and fetal development can have similar results and can result in embryonic/fetal death and abortion or a wide range of structural and functional defects. Most at risk is the central nervous system (CNS), due to a lack of compensatory growth of undamaged neuroblasts. In experimental animals the most common defects are of the neural tube as well as microphthalmia, cataract, and microencephaly, with associated functional and behavioral problems [14]. Other prominent defects are seen in craniofacial development, such as facial clefts [103], the skeleton [104], the body wall, teeth, and heart [105]. Hyperthermia in utero [due to maternal influenza for instance] was long known to potentially induce structural anomalies in the fetus [106–108] but it has been described also as an environmental risk factor for psychological/behavioral disturbances [109] and, more particularly, schizophrenia [107]. It is stressed that these are not ultrasound-induced hyperthermia effects. Yet ultrasound has been shown to induce temperature increase in vivo [11, 21, 24, 29, 110–112], albeit not in humans. Subtle effects are possible, such as abnormal neuronal migration with unclear potential results [113]. One specific single specific effect has been described in various publications: a mild increase in the prevalence of non-right handedness among male children exposed to prenatal ultrasound with no other neurological, intellectual, behavioral, or physical anomalies [114–116]. Ultrasound has been implicated, in chicks who were insonated in ovo, in learning and memory disturbances [117]. A study failed to demonstrate a relation between ultrasound insonation in utero and decreased intellectual performance [118]. In mice, the etiology of symptoms similar to those seen in autism was attributed to ultrasound [119]. Extrapolation to humans is not automatic, despite the argument, by some, that the increased incidence of this condition in children over the last 20 years or so is secondary to the similar increase in the use of ultrasound in obstetrics [120]. One study reported on approximately 750 children, half with autism spectrum disorders and half without [121]. The conclusion was that ultrasound in any of the three trimesters of pregnancy could not be correlated with an increased risk of ASD. In fact, there is a serious lack of data examining the role of ultrasound in the etiology of autism while rigorously excluding other confounding factors [122]. If one, however, considers together the facts that hyperthermia is potentially harmful to the fetus and that ultrasound may, under certain circumstances elevate tissue temperature, then precaution has to be recommended, particularly in early gestation and especially with modes known to emit higher acoustic energy levels (such as pulsed Doppler).
Is Doppler Different and Can It Have Detrimental Effects on the Fetus in the First Trimester?
Ultrasound modalities can result in either scanned or unscanned exposure. Scanned conditions are associated with grey-scale B-mode images (the most commonly used real-time application), and Doppler images of tissue cross sections. Unscanned conditions are used for M-mode and pulsed-Doppler studies of tissue movement (such as cardiac valves) or blood velocity waveforms. This is clinically very important because for unscanned beams the power is limited to the area of the beam cross section, often very narrow (1 mm2) in the focal region. For scanned beams the acoustic power is not limited to a narrow area, but may cover large areas in the lateral direction, hence less risk of high exposure at a specific point. Furthermore, a variety of movements intervene during B-mode imaging, such as fetal body motion, observer’s hand movements, and maternal breathing. During a Doppler examination, however, it is necessary to have the transducer as steady as possible. This is because, in general, blood vessels or heart valves are small in comparison to the general organ or body size being scanned and even small movements will have more undesired effects on the resulting image. As described below, the most commonly used intensity (spatial peak temporal average intensity, I SPTA) associated with Doppler ultrasound is the highest of all the general-use categories, 1180 mW/cm2 for pulsed Doppler, as opposed to 34 mW/cm2 for B-mode, a 35-fold difference. Dwell time (duration of exposure) is also of major importance: Ziskin [123] reported that among 15,973 Doppler ultrasound examinations, the average duration was 27 min (and the longest 4 h!). A study in chicken seemed to clearly implicate Doppler [117]. Chicken eggs were insonated on day 19- of a 21-day incubation period. Exposure was to B-mode for 5 or 10 min or to pulsed Doppler for 1–5 min. Eggs were allowed to hatch and learning and memory tests were performed in the chicks on day 2. Impairment in ability to learn or in short, medium and long-term memory was not observed after B-mode exposure but was clearly demonstrated for those exposed to Doppler, with a dose–effect relationship. Furthermore, the chicks were still unable to learn with a second training session 5 min after completion of the initial testing. Hearing the fetal heart beat is certainly a very satisfying experience for the expecting parents. Often this is accomplished by using pulsed Doppler. This is so engrained in the minds of the public that each time ultrasound is mentioned in a television series or a movie, one can hear the heart beat in the background although the image on-screen is only of a B-mode exam. In fact, using Doppler to “listen” to the fetal heart is not new [124, 125]. This should be discouraged and replaced by M-mode assessment. If Doppler is used, it is sufficient to “hear” 3–4 heart beats and thus limit the exposure [126, 127]. One of the major uses of ultrasound is the prenatal detection of fetal abnormalities. The organ most commonly affected by major genetic disorders is the heart, and hence, extensive research is conducted in imaging and functional assessment of the heart. While B-mode is used to assess structure, Doppler (pulsed [spectral] and color) are the ideal techniques to examine heart function. A vast amount of literature has been published on the value of ultrasound examination of the fetal heart, using various techniques, including Doppler analysis of flow across the cardiac valves and Doppler velocimetry of various fetal vessels [128–130]. The vast majority of published reports was, until recently, on B-mode examinations around 18–20 weeks. However, several authors have demonstrated the feasibility of examining the heart much sooner in pregnancy, beginning around 10 or 11 weeks [131–134]. Doppler analysis has long been a tool to study cardiac function, although mostly in the placenta, umbilical or uterine arteries [135]. Studies have been published of Doppler study of flow through cardiac valves, beginning at 6 weeks [136, 137]. It should be noted that it is technically extremely difficult to obtain these tracings and, thus, very prolonged dwell times may be necessary. Some have described performing a measurement of the heart diameter, heart rate and inflow and outflow waveforms “after 5 weeks” [138]. No details are available on exposure levels. It should also be remembered that, at these early stages of pregnancy, fetuses measure 1–2 cm in length and are completely included in the beam, therefore generating “total body scanning” in B-mode, which is necessary to position the Doppler gate. Analysis of ductus venosus flow as well as characteristics of flow across the tricuspid valve have been shown to be helpful in screening for chromosomal anomalies in the first trimester of pregnancy, as an adjunct to measurement of the NT. Waveform analysis of the ductus venosus reduces the false-positive rate of the screening test [139, 140]. For example, in fetuses with increased NT but with normal karyotype, from 11 to 13 6/7 weeks, absent or reversed A-wave (atrial contraction) in the ductus venosus is associated with a threefold increase in the likelihood of a major cardiac defect, whereas normal ductal flow is associated with a 50 % reduction in the risk for such defects [141–143]. It is clear that Doppler is an important tool to study fetal health in early (and late) pregnancy [130] but appropriate precautions need to be taken to limit exposure in terms of clear indication, time and acoustic output [144].
Acoustic Output
Based on various sources, it appears that acoustic output (as expressed by various intensities) is much higher in Doppler than in B-mode: for instance, 34 mW/cm2 for the I SPTA in B-mode versus 1080 mW/cm2 for spectral Doppler [35, 145]. Furthermore, as demonstrated in Table 1.2, the output has increased in all modes over the years [35]. If one compares outputs (as expressed by TI and MI, a clinically easy-to-use but somewhat remote expression of output) between first, second and third trimesters, differences are not major [72] but higher TI values are obtained when switching to Doppler mode [74]. The increase in TI is, generally, small but with some new machines, TI’s of up to 5–6 are displayed in Doppler mode (Fig. 1.3). Research has shown that excellent, diagnostic images can be obtained at low outputs, as defined by the TI values of 0.5 or even 0.1 [146]. This is illustrated in Fig. 1.4. Therefore, the switch-on default should be set up such that a low acoustic output power is initiated for each new patient, when starting an examination. Only if images are not satisfactory from a diagnostic standpoint, should the output be increased. Under pressure from Safety Committees of various societies, several ultrasound manufacturers have implemented this recommendation. Concerns about the fact that outputs are much higher in Doppler applications were expressed in three editorials [9, 147, 148]. In one of these, the authors raised the question whether research involving Doppler in the first trimester should even be considered for publication [147]. Based on these considerations, some recommend extreme caution when employing Doppler in the first trimester [149]. Furthermore, acoustic outputs, as published by the various ultrasound instruments manufacturers may not always be adequate [150] and an additional cause for concern is the increase in instruments outputs over the years [151]. Despite this, as detailed above, in recent years there has been a major recrudescence in the usage of Doppler in very early pregnancy. Unfortunately, one of the reason for this is the ignorance of many end-users of potential bioeffects, based on the “nothing has been shown” principle. Therefore, the risk is that this will become a routine standard, secondary to the push to utilize this modality by certain individuals, not necessarily knowledgeable of potential safety issues and that inexperienced end-users, wishing to imitate and adulate these “experts” will attempt to perform these exams for extremely extended period of time at pregnancy stages which are very susceptible to external insults (Christoph Brezinka, pers. comm.). Indeed, as mentioned earlier, a major issue is the lack of knowledge of ultrasound clinical users on output, bioeffects and safety, both in the USA [62] and abroad [61, 63, 64].


Table 1.2
Changes over the years in I SPTA (in mW/cm2), mean (and range) in various ultrasound modalitiesa
Ultrasound modality | 1991 | 1995 | 1998 |
|---|---|---|---|
B-mode | 17 (0.3–177) | 34 (0.3–991) | 94 (4.2–600) |
Pulsed Doppler | 1140 (110–4520) | 1659 (173–9080) | 1420 (214–7500) |
Color Doppler | 148 (25–511) | 344 (21–2050) | 470 (27–2030) |

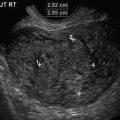
Fig. 1.3
Very high TI (5.7) may be obtained in Doppler mode (not an actual clinical examination). Note that this is a general obstetrics setting

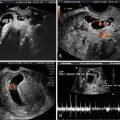
Fig. 1.4
Doppler velocimetry in the umbilical artery. (a) TIB is 2.4. (b) The TIB is 0.4 and the image is equally diagnostic
3D/4D Ultrasound
Three-dimensional (3D) as well as four-dimensional (4D) ultrasound are gaining recognition in obstetrics and gynecology. In prenatal diagnosis it adds to the detection of a wide range of anomalies, such as those involving the face, skeleton and extremities. The usefulness in early gestation is less obvious [152]. Characteristics are short acquisition time and post processing analysis, hence decreased exposure. As determined by TI and MI, acoustic output during 3D/4D exams does not seem excessive [75]. Figure 1.5 demonstrates low TI and MI during a 3D acquisition. The resulting reconstructed image is, obviously, a post-processing process. Sheiner et al. have shown that mean TIs during the 3D (0.27 ± 0.1) and 4D examinations (0.24 ± 0.1) were comparable to the TI during the B-mode scanning (0.28 ± 0.1; P = 0.343) [75]. The 3D volume acquisitions added 2.0 ± 1.8 min of actual ultrasound scanning time (i.e., including neither data processing and manipulation, nor 3D displays, which are all post-processing steps). The 4D ultrasound added 2.2 ± 1.2 min to the examination time. Amount of additional scanning time needed to choose an adequate scanning plane and to acquire a diagnostic 3D volume was not noted. Attractive views of the face, for instance, have led to its popularity among pregnant women who ask for non-medically indicated ultrasound (“keepsake ultrasound”). This is often performed in non-medical facilities, not for diagnostic purposes, in order to provide images for the family photo album. The issue was addressed long ago [153] but the practice has been opposed by various authors and professional, scientific organizations [154–164], although not by all [165] and with difference of opinions on whether practitioners involved in this activity should be sanctioned [166].
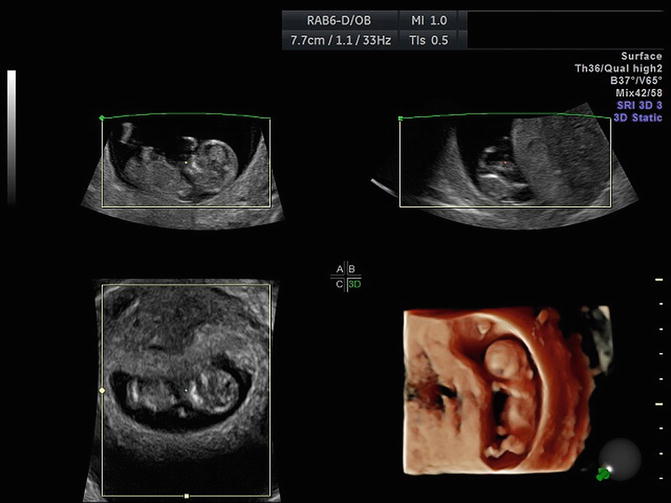

Fig. 1.5
Three-D acquisition with three orthogonal planes and reconstructed volume. The output power is determined by the acquisition plane (in general plane A), since the two other planes (B, C) and the reconstructed volume are computer-generated. In this acquisition, TIS was 0.5
How to Limit Fetal Exposure and Safety Statements
The answer is simple: perform ultrasound only with a clear indication, keep exposure to a minimum power and time, compatible with an adequate diagnosis (application of the ALARA principle), watch the TI (and, to a lesser degree) the MI on-screen and do not perform examinations with new techniques “simply because you can,” if they have not been scientifically shown to afford diagnostic advantages [96, 127, 144, 167]. In general, begin your exam with a low power output and increase only if necessary [167, 168]. Some scientists have clearly stated that Doppler should be avoided in the first trimester. Several ultrasound organizations, however, have publishing statements and/or guidelines specific for first-trimester ultrasound, with a particular emphasis on the use of Doppler in early pregnancy. The following statement which summarizes the various guidelines is copied from the AIUM’s website and is available to the public.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree