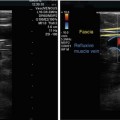
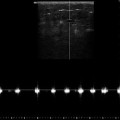
Fig. 12.1
Refluxive saphenofemoral junction tributaries. (a) Antegrade flow (blue) across the saphenofemoral junction during muscular systole. The epigastric vein (EV) is shown in red demonstrating blood which is refluxing into the saphenofemoral junction tributaries (see online material). (b) Spontaneous flow in the great saphenous vein (great saphenous vein) in the same patient standing. The slow, drawn-out, long-lasting reflux is typical of reflux originating in refluxive saphenofemoral junction tributaries. (c) Flow in the great saphenous vein after ligation of the saphenofemoral junction without ligation of the refluxive saphenofemoral junction tributaries. The reflux in the great saphenous vein persists unchanged (CFV = common femoral vein, VSM lig = ligated GSV) (Dr. Horst Gerlach, Mannheim; by kind permission)
Copyright: Dr. Horst Gerlach, Mannheim
3.
In the case of a competent terminal valve and incompetent preterminal valve, interruption of the saphenofemoral junction leaving the tributaries untouched does not have a significant effect on saphenous reflux. In this case the classic crossectomy (interruption of saphenofemoral junction and all tributaries) or the ablation with endoluminal devices distal to the confluence of the refluxive side branches is proposed.
In the presence of a shunt type III, the doctor must decide according to the ultrasound findings whether or not a CHIVA 2 strategy can be used. CHIVA 2 is ligation and transection of the tributaries of a saphenous trunk, with preservation of the saphenofemoral junction. With this strategy the refluxive great saphenous vein remains in continuity with the common femoral vein. Here there is a risk that a post-operative saphenous thrombosis might extend into the deep veins. The following preoperative duplex ultrasound criteria may help in the decision whether to use CHIVA 2 in order to lessen the chance of this complication.:
1.
Diameter of the great saphenous vein 15 cm from the groin greater than 10 mm: do not use CHIVA 2 strategy but operate directly on the saphenofemoral junction.
2.
Aneurysmal enlargement of the great saphenous vein in the region of the saphenofemoral junction: do not use CHIVA 2 strategy but operate directly on the saphenofemoral junction.
3.
Diameter of the great saphenous vein between 6 and 10 mm: administer low molecular weight heparin in prophylactic dose for 10 days with CHIVA 2 strategy.
4.
Diameter of great saphenous vein less than 6 mm: CHIVA 2 strategy may be followed without administration of low molecular weight heparin.
Perforating veins are only treated when they constitute the point of insufficiency. They are examined following the criteria mentioned in Chap. 9. A perforating vein should only be interrupted if it is found to be refluxive during calf diastole. This is very seldom the case in the first intervention.
12.3 Ultrasound in Endovenous Treatment of Varicose Veins with Thermal Procedures
Endovenous obliteration for the treatment of varicose veins has gained a firm place in varicose trunk vein therapy in the last 10 years. In particular, thermal procedures have reached clinical maturity in recent years. Their ablative effects are controllable in comparison to foam sclerotherapy which has also become widely practised. The two competing thermal procedures are endovenous radiofrequency ablation (Proebstle et al. 2011) and endovenous laser ablation (Schwarz et al. 2010; Prince et al. 2011). In both procedures the treatment can be controlled using ultrasound. The same is true for the newer procedures of mechanochemical ablation (Elias and Raines 2012) and endovenous cyanoacrylate glue embolization (Almeida et al. 2013).
The principal technical steps for all endoluminal procedures are:
1.
Setting up access to the vein
2.
Introduction of a catheter or laser fibre
3.
Peri-venous injection of a tumescent or local anaesthetic solution
4.
Release of the thermal energy during withdrawal of the fibre
All these steps are usually performed under continuous ultrasound control.
12.3.1 Prerequisites
An ultrasound machine which can be part of the standard operating room equipment is a prerequisite for continuous preoperative monitoring. Portable, space-saving, laptop-size machines are particularly suitable. The machine must have a data storage facility and documentation function. It should also be fitted with a linear probe which should ideally be able to work at frequencies between 8 and 15 MHz. The system should include functions which allow a duplex ultrasound image to be recalled as needed at any time during the operation. The first priority is an excellent quality B-scan, in which the anatomical structures and the catheters and fibres in use can be clearly recognised in all phases of the operation.
The operating doctor can ensure complete ultrasound sterility throughout the operation by using the following:
1.
Sterile latex or plastic cover for the transducer
2.
Sterile ultrasound gel
3.
Sterile plastic keyboard cover
12.3.2 Interventions for Trunk Vein Insufficiency
12.3.2.1 Choice of the Most Suitable Entry Point into the Vein
The first step to the endoluminal treatment is the introduction of the needle into the vein. With duplex the optimal point is to be found. One possibility is to use the most distal point of reflux as the point of entry into the vein to be treated. In the case of the great saphenous vein, this is usually found in the proximal lower leg or the distal thigh. In general, all the entry locations can be chosen on the inner side of the leg, so long as this is consistent with the pathological findings. In very obese patients, where the vein lies too deep, an alternative is to choose an entry point via a more superficial venous tributary provided it is below the distal end point of reflux. Alternatively, if the vein calibre permits, this can be accessed further distally. There are very few entry problems with the small saphenous vein on account of its relatively superficial position. Puncture of the vein at the ankle may be hampered by bony prominences like the lateral malleolus.
12.3.2.2 Venous Access in Practice
Endovenous obliteration procedures are usually performed via a percutaneous puncture. Entry into the vein through a cutdown or using a phlebectomy hook is a possibility; however, it detracts from the minimally invasive nature of the operation and experience with the procedure will generally render these techniques obsolete. Insertion of a peripheral venous catheter or needle followed by a guidewire is performed under ultrasound control. During puncture, the transducer head can be oriented transversally or longitudinally to the axis of the vein, according to the operating doctor’s personal preference (Fig. 12.2).
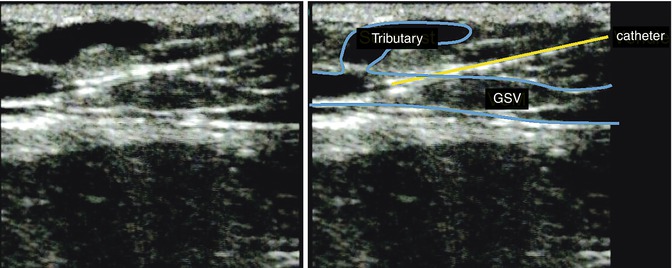

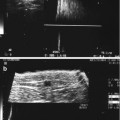
Fig. 12.2
Axial representation of the great saphenous vein (great saphenous vein) in the proximal lower leg region during introduction of an 18G, 40 mm long, peripheral venous catheter
Copyright: [Author]
The following measures facilitate ultrasound-guided percutaneous venous access:
1.
Achieving a vein diameter as large as possible at the puncture site by:
1.
Placing the patient in the reverse Trendelenburg position, with the legs angled downwards approximately 15°–30°
2.
Maintaining a warm operating room temperature to avoid cold-induced venospasm
3.
Occasionally by inflating a tourniquet proximal to the puncture point
2.
Technical tips on achieving successful cannulation:
1.
Stretching the skin at the intended puncture site. In patients with slack skin or obesity, manual stretching is recommended, if necessary with the help of an operating room assistant. This stabilises the vein and prevents it from yielding during attempted puncture.
2.
In patients with thick skin, an initial puncture with a pointed blade helps to maintain a sharp needle. It also reduces any friction between the skin and needle which helps to prevent cutaneous recoil and subsequent needle displacement out of the vein.
3.
Cannulation with the bevel of the needle downwards is preferred into small superficial veins when a shallow approach is used. With deeper punctures and a steeper angle of entry, an upward-directing bevel is more likely to lie across the lumen.
12.3.2.3 Introduction of the Laser Fibre or Radiofrequency Catheter
Whether the treatment method is radiofrequency, endoluminal laser ablation or one of the newer procedures, a suitable diameter tube or angiography catheter is then slid over the introduced guidewire. Once the guidewire is removed, this will allow the introduction of the laser fibre, radiofrequency catheter or other endovenous ablative device. With bare laser fibres, the use of a sheath of the same length as the vein to be treated is recommended. This reduces the risk of perforation since an unprotected bare laser fibre, when advanced, can lodge into the vein wall. The process of introducing the tube or catheter need not necessarily be controlled by ultrasound, so long as the wire and catheter advance easily and without resistance.
However, the final resting positioning of the end point of the endoluminal ablation device close to the deep vein system requires precise ultrasound control. This location must be documented in relation to the distance from the saphenofemoral or saphenopopliteal junction; knee flexion must be avoided as this can cause a change in the final position. If the tip of the endovenous ablation device is positioned too close to the deep vein system, this can lead to thrombus extension into deep veins. This is true for all endovenous procedures. For this reason the point of the catheter or laser fibre must not be positioned less than 1–2 cm from the saphenofemoral junction (Fig. 12.3a). If a cyanoacrylate glue deployment catheter is used, this distance should be 5 cm in order to avoid unintentional introduction of the cement applicator into the deep vein system (Fig. 12.3b). In treatment of the small saphenous vein, the catheter or fibre should be positioned up to the point where the vein lies directly under the crural fascia which is just as it turns down towards the popliteal vein.
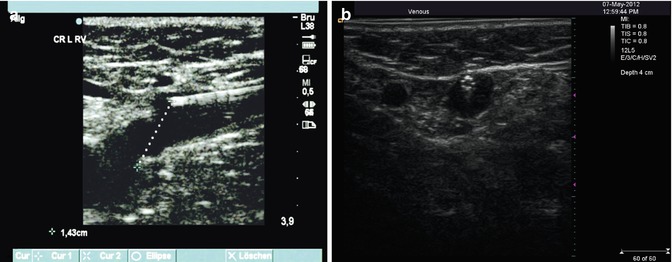

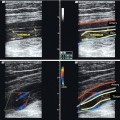
Fig. 12.3
(a) Longitudinal view through the groin demonstrating the termination a laser fibre before the saphenofemoral junction. (b) Transverse view of the patented 4 F Teflon catheter from the acrylic cement system. The eight microscopic air channels in the catheter wall at its termination make it very easy to identify on ultrasound
Copyright: [Author]
However, the use of ultrasound support may be advisable, and in some cases indispensible, during positioning of the guidewire, particularly if the wire cannot be advanced without resistance. Possible reasons for this might be a previously undiscovered segment of hypoplasia which can occur in up to 25 % of patients; Sect. 2.4.4). Other impediments include marked tortuosity which occurs in 1–2 % of patients or calibre variations in around 7 % of patients (Ricci and Caggiati 1999). With complete vein occlusion, a second puncture proximal to the occlusion is required in order to ablate the remaining segment. With very tortuous veins, some passages may be impossible to navigate. When it proves difficult to push the wire forward, the course of the vein is first analysed in three dimensions using ultrasound. The passage where the hindrance occurs is very often S-shaped. In such cases, it is sometimes possible to advance the wire by stretching the course of the vein using digital pressure on the skin at the affected region (Fig. 12.4 ) or by simply straightening the leg.
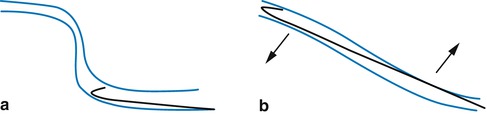

Fig. 12.4
Diagram of how to advance through a tortuous vein by manipulating the course of the vein. (a) Original course. (b) The vein is straightened using manual traction of the skin and subcutaneous tissue
Copyright: [Author]
12.3.2.4 Application of Local Anaesthetic (Tumescence)
The principal advantage of carrying out the operation under tumescent anaesthesia, as compared to general or regional anaesthesia, is that peri-venous structures can be protected from the effects of the heat generated by radiofrequency or laser. In particular, with tumescent anaesthesia, the patient can inform the operating doctor if there is any heat damage to the principal nerves. For example, if radiating pain occurs in the heel area due to irritation of the sural nerve, the energy source can quickly be turned off and the catheter repositioned a few millimetres along. Tumescent anaesthesia seems not to be required in mechanochemical ablation or acrylic cement deployment.
Tumescent anaesthetic is injected into the peri-venous space, preferably in a slight Trendelenburg position (Fig. 12.5). In this way the analgesic effect is obtained immediately, and the operation can be started directly after the injection. The use of an automated roller pump is easier than using direct injections by hand. Both injection of the tumescent solution and the Trendelenburg position lead to a significant reduction of the vein diameter. This means that a smaller volume of blood is present in the vein around the catheter. In endovenous laser ablation, a small amount of remaining blood results in better energy transfer to the vein wall because it facilitates the formation of steam bubbles and convection currents. This is in contrast to radiofrequency closure where the operator applies additional manual pressure over the catheter point to try to expel the remaining blood from the vein lumen. This is because radiofrequency works by conductive heat transfer making the presence of blood unnecessary or counterproductive. In contrast to peri-venous injection fluid constrained within the saphenous compartment, tumescent solution around a superficial vein disperses more easily. Therefore, care must be taken to ensure an adequate volume of tumescence remains between the skin and vein. This will avoid thermal damage to the dermis and consequent hyperpigmentation.
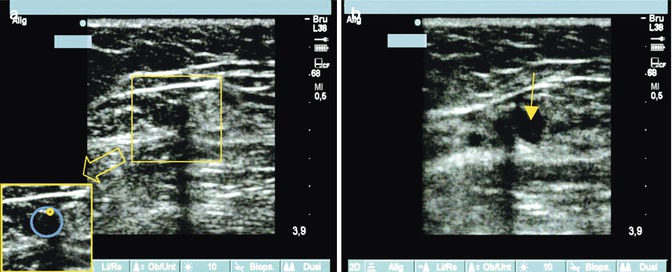

Fig. 12.5
(a) Transverse view of a great saphenous vein in the thigh with the laser fibre in the vein demonstrating an acoustic shadow caused by the laser fibre (see detail). (b) Immediate peri-venous injection of the tumescent solution (echo-poor, arrowed), which only partially compresses the great saphenous vein. The laser fibre can be visualised to the side with its characteristic acoustic shadow
Copyright: [Author]
12.3.2.5 Energy Transfer During Treatment
During radiofrequency ablation, ultrasound monitoring is superfluous, since the principal focus of the operator is on maintaining the target temperature at the catheter point. This temperature is easier to maintain if the operator applies pressure over the catheter point. This is considerably easier using manual compression applied directly with the hand than with the ultrasound probe.
In contrast to radiofrequency ablation, manual pressure over the laser fiber tip should be avoided. This is especially true when a bare fibre is used, because this action may facilitate vein wall perforation. Furthermore, residual blood is needed to obtain a better, more regular transfer of laser energy to the vein wall. This takes place through convection currents or, more accurately, via a turbulent flow of steam bubbles and blood (Fig. 12.6). Continuous ultrasound monitoring of the endovenous laser process in case of bare fibres allows the formation and spreading of steam bubbles to be observed and in particular allows significant perforations of the vein wall to be recognised in time. This is identified when steam and gas bubbles spread into the surrounding tissues during an extraluminal release of laser energy. In this event lasering is stopped and the fibre is withdrawn a few millimetres, after which normal endovenous ablation can be resumed.
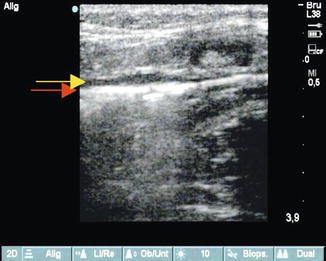

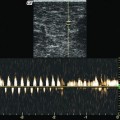
Fig. 12.6
Longitudinal view of the proximal great saphenous vein. Release of laser energy from a 600 μm diameter fibre (30 W, 940 nm) demonstrating steam bubbles. The strong echo reflection in the vein (red arrow) stands out from the surrounding echo-poor tumescent anaesthetic (yellow arrow)
Copyright: [Author]
12.3.2.6 Ultrasound Surveillance
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree