Fig. 13.1
Abdominal radiograph demonstrates bilateral renal stones and bladder stones which are radiopaque (arrows)
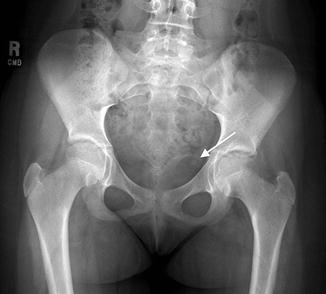
Fig. 13.2
Abdominal radiograph demonstrates a round calcification in the left pelvis (arrow). This calcification is more radiolucent in the center as compared to the periphery, consistent with a phlebolith. A ureteral stone would be most dense/radiopaque at its center
In the case of a known stone former with radiopaque stones, radiography can be used effectively to follow stone size progression or change in stone location. Abdominal radiographs can also be used preoperatively in preparation for SWL to ensure good visualization of the stone on fluoroscopy during the procedure and postoperatively to determine resolution or stone passage (Fig. 13.3). The sensitivity and specificity of radiography are improved when used in conjunction with ultrasound.
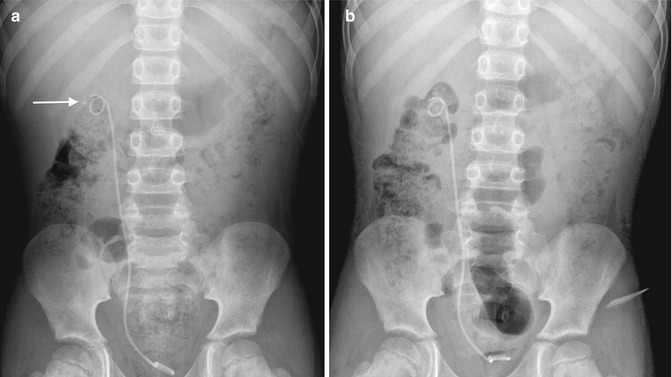

Fig. 13.3
A 7-year-old boy presenting with right flank pain was found to have an obstructing right ureteropelvic junction stone. (a) Abdominal radiograph after preoperative stent placement demonstrates a radiopaque stone in the right kidney (arrow). (b) Abdominal radiograph 2 weeks after SWL demonstrates eradication of the stone
Ultrasound
Given its lack of ionizing radiation, ultrasound (US) is often the initial modality of choice in evaluating a pediatric patient with suspected urolithiasis and nonemergent abdominal or flank pain [20, 21]. It is also lower in cost than CT, is noninvasive, does not require sedation, and can be performed portably, allowing bedside evaluation of sick patients.
The greatest strength of US in the setting of stone disease is in evaluating for obstruction, or hydronephrosis, easily depicted as fluid distension of the renal pelvis and calices [15] (Fig. 13.4). It can also provide extensive anatomical detail in patients with congenital duplications and other anomalies contributing to stone formation. US can also directly demonstrate stones. Typically, a renal stone appears as a very bright, or echogenic, rounded structure. Due to its high density, the stone will block penetration of the US beam, resulting in a black shadow behind it. This is referred to as posterior acoustic shadowing [2, 16] (Figs. 13.5 and 13.6). The exceptions are drug stones or matrix/protein stones which are of variable density and may not be distinguishable from surrounding soft tissue on ultrasound [16].
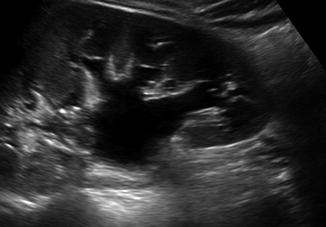
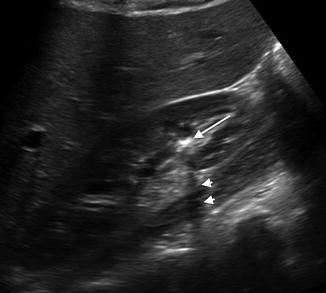
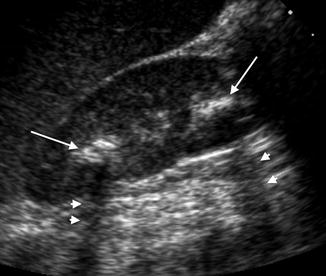

Fig. 13.4
A 15-year-old female with hydronephrosis due to an obstructing distal stone. This US shows a dilated renal pelvis communicating with dilated calyces. This patient required a ureteral stent for her obstruction

Fig. 13.5
A 15-year-old female with a nonobstructing lower pole renal stone. The stone is echogenic (long arrow) and causes posterior acoustic shadowing (arrow heads)

Fig. 13.6
An 8-year-old female with renal stones in the upper and lower poles. Both stones are echogenic (long arrows) with posterior acoustic shadowing (arrow heads)
Stones are most easily identified in the kidney and in the distended urinary bladder (Fig. 13.7). In general, US is poor at localizing ureteral stones, especially in the absence of ureteral dilatation. Palmer et al. found that the detection rate for ureteral calculi was only 38 % as compared to 90 % for renal stones [22]. Hydronephrosis may suggest a ureteral stone, but even in the presence of hydroureter, it is often difficult to see the ureteral stone if there is overlying bowel gas or surrounding soft tissue which obscures the ureter [2–15] (Figs. 13.8 and 13.9). If the dilated ureter at the level of obstruction can be seen, then the stone may be visible (Fig. 13.10).
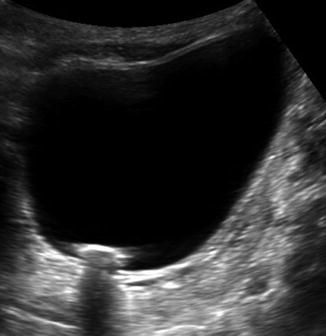
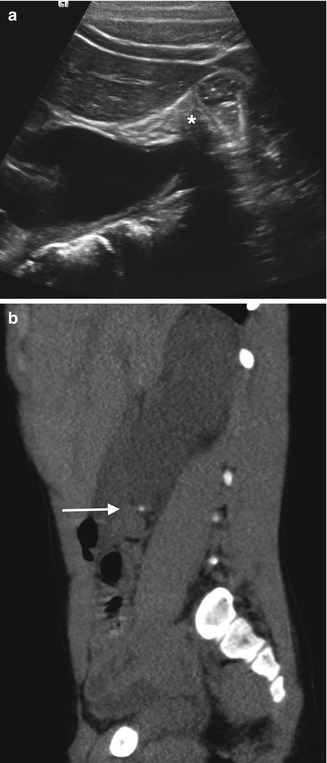
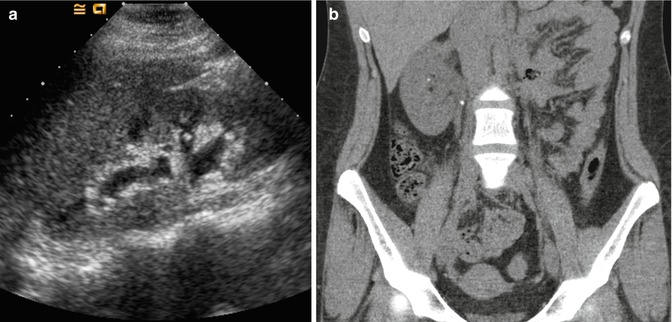
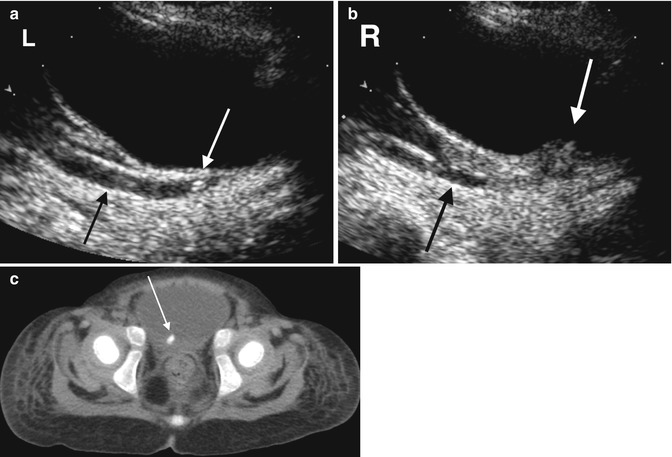

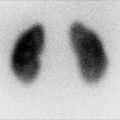
Fig. 13.7
Bladder stone on US. Similar to the renal stones, the bladder stone is echogenic and demonstrates clear posterior acoustic shadowing

Fig. 13.8
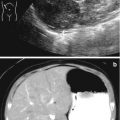
A 5-year-old boy with right-sided abdominal pain and vomiting. (a) An ultrasound shows obvious obstruction with hydronephrosis. However, when attempting to look at the proximal ureter, it is obscured by bowel gas (asterisk). (b) A CT scan was performed that same day for further work-up due to a strong suspicion of stone disease. A sagittal reformation of the CT shows a dense proximal ureteral stone (arrow) with proximal hydroureteronephrosis

Fig. 13.9
A 16-year-old female with a history of stones presents with right-sided flank pain and nausea. (a) US demonstrates mild right-sided hydronephrosis without identification of a stone. Given her history and clinical picture, there was high suspicion for a stone. (b) Subsequent noncontrast CT that same day finds a small stone in the proximal right ureter causing obstruction, as well as a small nonobstructing stone in the right kidney

Fig. 13.10
A 28-month-old female with abdominal pain. (a) An initial ultrasound demonstrates a left distal ureteral stone (white arrow) with proximal hydroureter (black arrow). (b) There is also thickening of the right side of the bladder at the ureterovesical junction (white arrow) with proximal hydroureter (black arrow) but no definite echogenic stone or posterior acoustic shadowing. (c) A CT one day later demonstrates a right-sided ureterovesical junction stone (arrow). The left distal ureteral stone seen on US is not present on the CT, but there is residual dilation of the left ureter (not shown), suggesting the stone had passed
A special feature of US is the artifact created by color Doppler imaging of a rough reflective surface, known as “twinkling.” Twinkling refers to the multicolor signal behind the stone simulating turbulence (Fig. 13.11). This can be used as supportive evidence when diagnosing stones by US. One study found that twinkling artifact had a 78 % positive predictive value for nephrolithiasis at subsequent CT. The true-positive rate of twinkling artifact for confirmed calculi at CT, however, was only 49 % with a 51 % false-positive rate [23]. This study used 5 mm CT sections to correlate with twinkle artifact, however, which may have in fact missed smaller stones that were accurately identified by twinkle artifact.
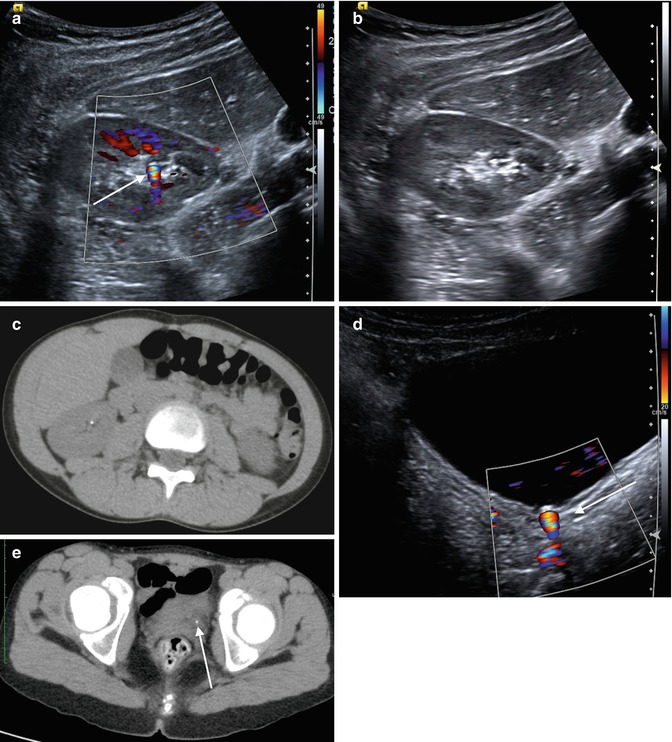

Fig. 13.11
An 11-year-old female with flank pain. (a) “Twinkle” is demonstrated on a color Doppler ultrasound image of the right kidney. Notice the focal multicolor signal emanating from the renal sinus (arrow). (b) The stone without the use of color Doppler is more difficult to see, particularly when surrounded by echogenic renal sinus fat. (c) The small renal stone is confirmed on CT imaging. (d) This patient also had a distal ureteral stone that demonstrates twinkle (arrow). (e) The stone is again confirmed on CT imaging (arrow)
Many studies have found US to lack the sensitivity and accuracy of CT in both identifying and characterizing stones. One study by Passerotti et al. found that US had an overall sensitivity of 76 % and a specificity of 100 % in comparison to CT for urolithiasis in the kidney, ureter, or bladder [24]. Ray et al. performed a literature review and only found a 45 % pooled sensitivity for US [15]. US has been found to both miss stones averaging 2.3 mm [24] and to overestimate stones <5 mm in size by 2 mm [15]. Palmer et al. compared US and CT in symptomatic patients and found US nondiagnostic in 41 % of cases versus 5 % of cases with CT [22]. As a result, a negative US in a symptomatic patient may still require further work-up with CT imaging. Confirmatory CT is also sometimes advocated after an equivocal US and even after a positive US to evaluate stone location and burden for appropriate planning of an intervention [22]. Given the likelihood of requiring subsequent CT, some would advocate beginning with a low-dose CT to decrease the overall number of imaging exams and the burden of cost. For example, Palmer et al. advocated beginning with a CT when evaluating children >11 years old with a positive family history of stone disease [22]. This issue remains controversial, however, and the aforementioned study by Passerotti concluded that although CT is more sensitive than US for the detection of renal stones, the difference between these exams may not be clinically significant; these authors therefore recommend US as the initial imaging exam for children with suspected stones due to the potential adverse effects of ionizing radiation from CT.
As previously mentioned, the sensitivity of US can be increased if used in conjunction with abdominal radiography. Mitterberger et al. found that the combined use of radiography and native tissue harmonic imaging ultrasonography had a sensitivity and accuracy for detecting ureteric calculi of 96 % and 95 %, respectively [25]. Both modalities may be effective in following a patient with known stone disease.
While readily accessible, US is operator dependent and potentially limited by a patient’s body habitus. Skeletal abnormalities such as extreme kyphosis/scoliosis may be prohibitive in visualizing the retroperitoneum with US. These factors must also be considered when choosing between US and CT.
Computed Tomography
Noncontrast computed tomography (CT) has surpassed many other imaging modalities in diagnosing and confirming stone disease; it is overall considered the gold standard imaging technique for urolithiasis. CT provides accurate information regarding the location and size of the stone and relevant surrounding anatomy [15] (Figs. 13.12, 13.13, 13.14, and 13.15). It can also be used to measure stone density to provide preliminary information regarding stone composition which may help direct therapy [18]. CT imaging has a high sensitivity and specificity independent of stone location (96–99 % sensitivity and 96–97 % specificity) [26, 27]. Using thin slice collimation of <3 mm, the sensitivity and specificity are virtually 100 % [15]. CT is typically used in the emergency setting due to its speed and high sensitivity/specificity as well as its ability to rule out other pathology. CT for the evaluation of renal stones does not require IV contrast, and in fact, contrast enhancement of the renal parenchyma and excretion of contrast into the collecting system may obscure small stones. Stones which are radiolucent or too small to be seen on abdominal radiographs can generally be seen on CT.
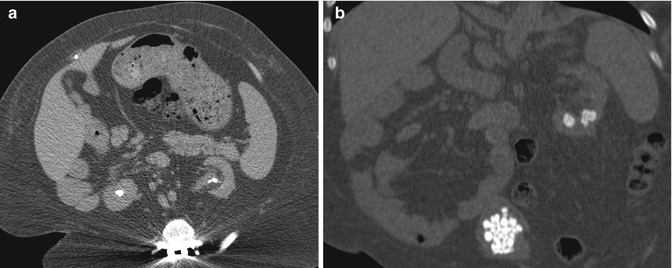
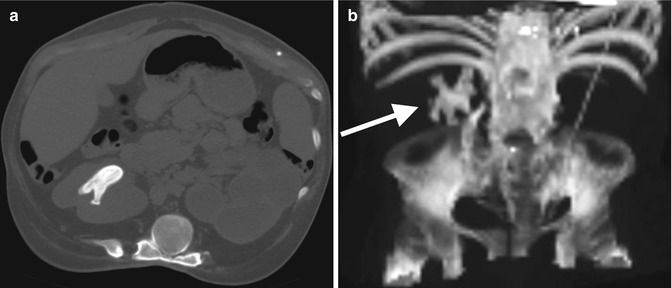
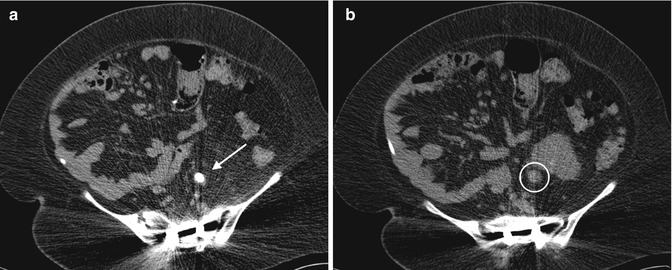
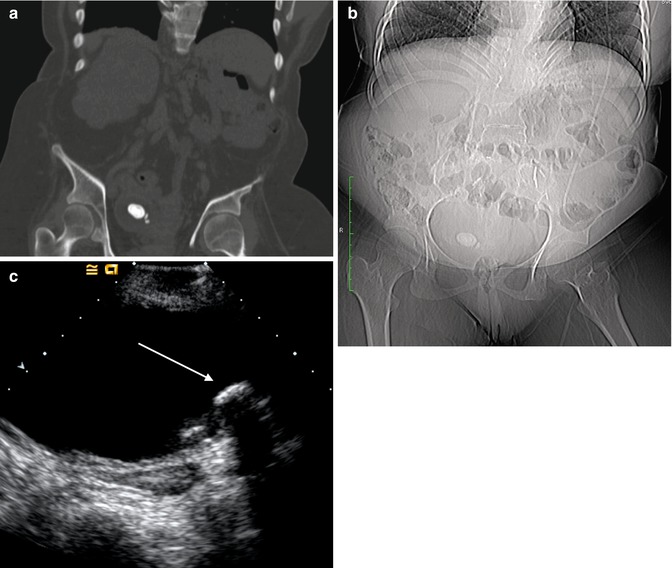

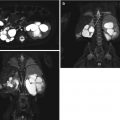
Fig. 13.12
CT imaging of stone disease. (a) This patient has multiple bilateral renal stones. (b) Coronal reformation of the same patient demonstrates bladder stones as well

Fig. 13.13
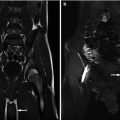
An 18-year-old female with spina bifida, neurogenic bladder, and a history of kidney stones was found on regular follow-up to have a right-sided staghorn calculus. (a) This stone is demonstrated in the axial plane on noncontrast CT. Notice how the stone fills at least 2 calyces on these images. She also has a nonfunctioning left kidney. (b) 3D volume rendering can be used to demonstrate the full size of the staghorn calculus (arrow)

Fig. 13.14
A 14-year-old boy with spina bifida and a neurogenic bladder status post bladder augmentation, Mitrofanoff catheterizable stoma, and bladder neck closure who has a history of kidney and bladder stones. (a) A CT demonstrates an obstructing left ureteral stone (arrow). Notice the soft tissue density around the stone representing the edematous ureter. (b) Proximal left ureteral dilation is seen (circle)

Fig. 13.15
This is a series of the same bladder stones as seen on various imaging modalities. (a) The stones as they appear on CT bone window. (b) The largest stone was big enough to be seen on the scout radiograph for the CT. (c) A view of the stones on US (arrow)
Secondary signs of ureteral stones can also be seen on CT, such as hydronephrosis, proximal ureteral dilatation, unilateral renal enlargement, decreased renal attenuation due to edema, and perinephric or periureteral edema (Figs. 13.16, 13.17, 13.18, and 13.19). In the event that no stone is seen on the CT scan, these secondary signs can suggest recent passage of a stone. A method to distinguish a ureteral stone from a phlebolith is to look for the “rim sign” – circumferential soft tissue density representing the ureter encircling the stone, which is not found with a phlebolith (Fig. 13.20). If the obstructing stone is associated with infection, pyelonephritis and ureteritis can also be demonstrated using contrast-enhanced CT (Fig. 13.21).
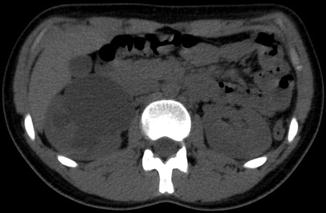
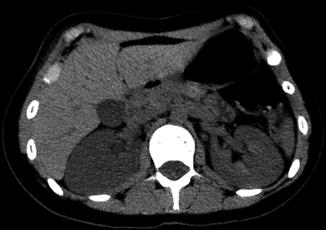
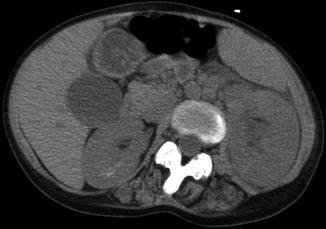
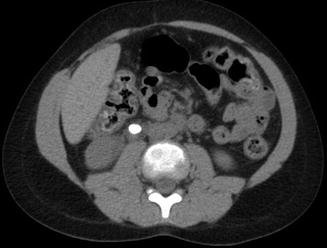
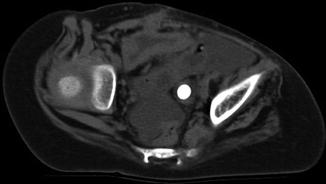
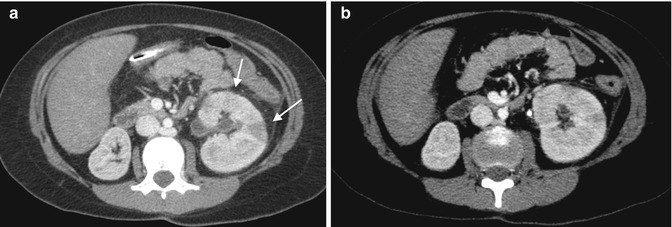

Fig. 13.16
Noncontrast CT demonstrates severe hydronephrosis of the right kidney

Fig. 13.17
Noncontrast CT demonstrates decreased attenuation of the right kidney as compared with the left kidney, consistent with edema. The right kidney also demonstrates mild hydronephrosis

Fig. 13.18
Noncontrast CT demonstrates left-sided perinephric stranding with renal edema and enlargement

Fig. 13.19
Right-sided periureteral edema around a ureteral stone

Fig. 13.20
The “rim sign” can be seen around this ureteral stone. It can be used to distinguish a stone from a phlebolith

Fig. 13.21
This is a patient presenting with fever and abdominal pain. (a) Contrast-enhanced CT shows wedge-shaped areas of hypoenhancement suggesting infection or pyelonephritis (arrows). There is also hyperenhancement of the urothelium signifying infection. (b) The obstructing stone can be seen in the proximal ureter on a more inferior slice
Despite all the advantages of CT imaging, a major concern is its use of ionizing radiation, particularly as it pertains to the pediatric patient. CT utilizes significantly more ionizing radiation than the other imaging modalities: the effective dose of an abdominal/pelvic CT is 4.3–8.5 mSv as compared to an abdominal radiograph which is 0.2–0.7 mSv and MRI or US which is 0 mSv [18]. To put it further in perspective, the effective dose of a single noncontrast CT (stone protocol) for a child is about 1–2 years worth of background radiation (3 mSv) [12]. As a result, it is not necessarily the automatic first choice of imaging in the pediatric population and should be thoughtfully used in these patients who may require multiple imaging procedures in the future.
As a result, there has been keen interest in low-dose CT imaging techniques to try to reduce the amount of radiation to which the patient is exposed without affecting its diagnostic capability. Newer CT protocols have been shown to decrease the radiation-measured entrance dose by 60–90 % [28], with published effective doses for a low-dose CT of 0.98–1.5 mSv as compared to 4.3–8.5 mSv for a standard abdominal/pelvic CT [18]. Techniques such as tube current modulation, low voltage protocols, and other adjustments to alter image acquisition and reconstruction can significantly reduce patient radiation exposure [18, 29]. In a model looking at effective dose and lifetime risk of cancer, low-dose imaging (40 mA, 10 ms, 125 kVp) as compared to standard dose (80 mA, 25 ms, 125 kVp) decreased the lifetime attributable risk of cancer from 23–144 cases per 100,000 to 5–31 cases per 100,000 [30]. Diagnostic accuracy has been shown to be maintained with low-dose protocols. Karmazyn et al. found that reducing the dose of radiation did not significantly reduce the detection of ureteral or kidney stones in children weighing <50 kg [31].
It is important to remember that even with lower-dose imaging techniques, there is no threshold under which radiation is safe. A study from the Netherlands found a linear-no-threshold model for the induction of chromosomal damage induced by ionizing radiation, seeing an induction of micronuclei in human fibroblasts in doses as low as 20 mGy. The damage was increased if cells were exposed during the S-phase of the cell cycle [32]. The only way to most effectively decrease the amount of radiation to which a patient is exposed is to choose nonionizing studies when possible (i.e., US). If CT has to be used, use it sparingly and limit the scan to only the area of interest [13].
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a very good modality for imaging the anatomy and function of the genitourinary tract using MR urography protocols and has the added benefit of lacking ionizing radiation. Its use in patients with urolithiasis, however, is limited [33]. MRI is unable to directly visualize stones; stones present instead as signal voids. It is also expensive, requires sedation for most young children, and is limited in its availability, especially in the emergent setting and at night [18].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree