Robert F. James • Lacey B. Martin • John R. Gaughen, Jr • William J. Mack
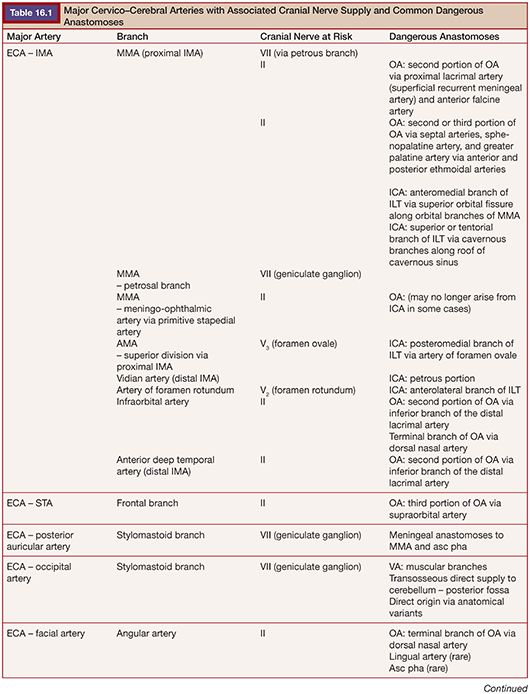
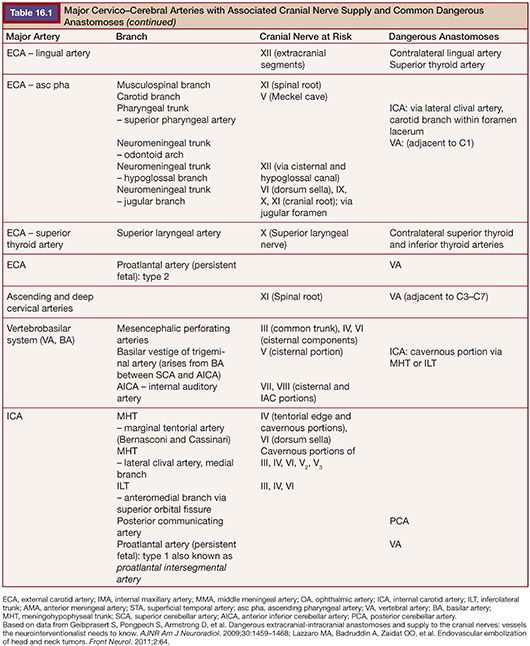
Highly vascular tumors of the head and neck often pose significant surgical challenges. Bleeding can result in decreased operative visibility and inadvertent injury to adjacent vital structures. Significant blood loss and volume depletion can lead to major morbidities. Embolization of vascular head and neck tumors before surgical resection may help to minimize blood loss, reduce operative time, and facilitate surgical resection. However, the additional endovascular procedure is not without risk to the patient. Multiple anastomotic connections exist between the arteries of the head and neck. Aberrant embolization through these channels can lead to neurologic injuries such as visual loss, paralysis, or ischemic injury to the cranial nerves. It is imperative that the interventionalist and the operating surgeon discuss the goals of embolization and the overall treatment plan before the endovascular procedure. The most common vascular tumors of the head and neck region suitable for preoperative embolization include meningiomas, paragangliomas, juvenile nasopharyngeal angiofibromas, hemangiopericytomas, and hemangioblastomas. Here we will discuss the relevant procedure-related devices and materials, techniques, clinical applications, and potential complications. Most discussion focuses on meningiomas as they are the most frequently encountered of the vascular head and neck tumors. Subsections are devoted to specific concerns for the other tumor types. Table 16.1 details important dangerous anastomoses with the cranial nerve blood supply.
DEVICE/MATERIAL DESCRIPTION
Catheters
A coaxial system of an outer guide catheter and an inner microcatheter is usually employed for transarterial endovascular embolization. We typically use guide catheters with a 0.053-in or 0.070-in inner diameter. The 6-Fr 070 Neuron guide catheter (0.070-in inner diameter; Penumbra, Inc., Alameda, California) can be used for the internal carotid artery (ICA) access, the 053 Neuron guide catheter for the external carotid artery (ECA) (0.053-in inner diameter), and either the 053 or 070 Neuron guide catheter for the vertebral artery (VA), depending on vessel diameter.
There are various-sized microcatheters with multiple features that are beyond the scope of this chapter. The Echelon-10 and Marathon (Covidien, Irvine, California) microcatheters can be used for routine embolizations. The Scepter C balloon catheter (MicroVention, Inc., Tustin, California) enables a new balloon-augmented embolization technique through a single microcatheter.1,2 Typically, 0.014-in diameter guidewires are used to help navigate the microcatheter because of their superior steering and trackability. However, smaller guidewires such as the 0.008-in Mirage (Covidien, Irvine, California) may be safer in small, distal cerebral vasculature (Note: the 0.008-in guidewires are essentially unsteerable and navigating bifurcations is often an exercise in persistence of the trial and error technique and/or uncanny tip reshaping proficiency). The size and angioarchitecture of the feeding vessel may require altering or modifying the microcatheter, often necessitating very small and “floppy” catheters for difficult-to-reach anatomy. In these situations, the Marathon flow-directed microcatheter could be advantageous. Unfortunately, guidewires 0.012 in or smaller are necessary when using the Marathon microcatheter due to its 0.013-in distal lumen diameter. However, the catheter is surprisingly compatible with the 0.014-in Traxcess guidewire (MicroVention, Inc., Tustin, California), as both the catheter and wire taper in a similar fashion. Preference is given to use the 0.014-in Traxcess guidewire when the Marathon microcatheter is required, as the Traxcess wire has superior steerability and provides a more rigid tracking platform compared to the other compatible guidewires.
Particles
The most common agent used for embolization of meningiomas and other head and neck tumors is a suspension of particles mixed with a contrast agent. Particles are small substrates that aggregate to obstruct the vessel. The choices of particle material and size impact results and potential complications associated with embolization. As differences in the granulometric distribution of particles exist, they may not match exactly with their advertised size range. Compressibility, elastic recovery, aggregation, and visualization of particles can all affect performance.
Polyvinyl alcohol (PVA) particles are nonspherical and are available in preparations of varying size ranges. Limitations with PVA embolization include difficulty with aggregation and microcatheter obstruction, leading to premature termination of the embolization or microcatheter exchange. Injection under increased pressure to clear the catheter should never be performed. This maneuver increases the risk of forcing particles into undesired territories once they are released.
Smaller particles allow deeper penetration into the tumor bed for more complete devascularization and increased tumor necrosis; however, they have a higher likelihood of reaching potentially dangerous or disabling arteries.3 Larger particles are safer but may not fully penetrate and devascularize the tumor bed and, therefore, may be less efficient at reducing surgical blood loss. Maintaining particle size greater than 150 µm is thought to reduce the risk of damaging the vasa nervorum of the cranial nerves.4,5 A large study of 201 embolized meningiomas found small particle size (45 to 150 µm) to be the sole risk factor for complications, hemorrhagic or otherwise.5 In high-flow situations, increasing particle size to greater than 500 µm may be beneficial, or an alternate class of embolic agent can be considered. Size selection of the PVA particles is a balance between desired tumor penetration and unwanted target embolization. In most situations, selecting particles with a diameter between 150 and 350 µm will provide optimal results.6
Trisacryl Microspheres
Calibrated spherical particles made of trisacryl and cellulose porous beads were developed to address the disadvantages of PVA particles, specifically their irregular size and nonspherical nature. Trisacryl microspheres are partially compressible and, as a result, allow for easier transit through the delivery catheter.7,8 Microspheres are thought to redistribute after initial clumping and delayed control angiography is therefore warranted. A study of 60 patients comparing trisacryl particles to nonspherical PVA particles found lower surgical blood loss with trisacryl embolization.9
Gelfoam
Gelfoam should be delivered via the transarterial embolization approach. For head and neck tumors, powder and sponge forms can be used.10 Gelfoam persists for 3 to 6 weeks before recanalization begins.
Liquid Embolics
The two most common liquid embolic agents are N-butyl cyanoacrylate (NBCA: Trufill; Codman & Shurtleff, Inc., Raynham, Massachusetts) and ethylene vinyl alcohol (Onyx; Covidien, Irvine, California). The choice of liquid embolic is a matter of preference, with each having advantages and disadvantages. Onyx offers a decreased theoretical risk of catheter retention, whereas NBCA has more versatility by altering the rate of polymerization with dilution strategies.
Advantages of liquid embolic agents include decreased peritumoral edema and the prevention of delayed recanalization (sometimes seen after particle embolization). They can be used with transarterial or direct puncture embolization techniques. Potential disadvantages include an inability to select the size of vessels that the liquid embolic will enter (compared to particle embolization). There is risk that the liquid embolic agent will occlude vessels prematurely without deep penetration into the tumor.11 Further, liquid embolics are more expensive than particles.
NBCA is a liquid embolic that is injected as a mixture with Ethiodol. The safety of tumor embolization with NBCA has been studied. Kim et al.12 examined 35 consecutive tumor patients embolized with NBCA (17% meningiomas). The authors suggest that NBCA had better fluoroscopic visibility than PVA particles, enabling precise identification of embolized vessels and tumor mass. Disadvantages include quicker polymerization and risk of catheter retention. Proper embolization with NBCA requires greater technical skill than PVA administration. NBCA is also considered more permanent than PVA particles. When dangerous anastomoses are encountered, coils can be used to obstruct their origins and prevent distal embolization of the liquid embolic agent into unwanted territories.12
Onyx is an effective embolic agent that can penetrate into tumor capillaries. A small case series reported no increased postembolization tumor edema and no hemorrhagic complications following Onyx embolization.11 The Onyx mixture is radiopaque and highly visible during angiography.13 Care must be taken to create a meniscus between the dimethyl sulfoxide (DMSO) and the Onyx. Mixing of Onyx and DMSO in the catheter hub can dilute the Onyx, rendering it less radiopaque and more difficult to visualize. This can increase the risk of embolization to unwanted vascular territories. Onyx can be injected over a longer time frame than NBCA, which makes it more controllable and therefore may result in more uniform lesion penetration.14
Coils
Coils are shaped (curled) pieces of metal, usually platinum, that are released into a vessel for occlusion. Detachable and pushable coils can serve as an alternative to particle and liquid embolization methods. Some debate exists regarding the appropriateness and use of coils in the setting of head and neck tumors. Coils may be suitable for masses with large feeding vessels, greater than 1.5 mm in diameter.15 However, some argue that proximal coil occlusion may not only create collaterals but may also prevent repeat access in case of tumor recurrences.16 Coils can be an excellent adjunct to liquid embolization, preventing penetration of the liquid embolic into unwanted territories without causing ischemia.
Other Embolic Agents
Additional agents that have been used in embolization of vascular head and neck tumors include fibrin glue, ethyl alcohol (ETOH), hydroxyapatite ceramics, phenytoin, hyperosmolar mannitol, and Lipiodol.17–22
TECHNIQUE
Preembolization Workup and Considerations
A complete medical history and physical examination is required before any embolization procedure. A review of noninvasive imaging is critical; findings on computed tomography (CT) and magnetic resonance imaging (MRI) studies can help guide the treatment. A complete angiographic evaluation of the tumor is recommended. For intracranial meningiomas, a comprehensive study includes a six-vessel intracranial and extracranial angiogram (bilateral ECA, ICA, and VA). Large vessel sacrifice may be warranted when paragangliomas encase the carotid artery. In these cases, angiography can be combined with balloon test occlusion to assess the feasibility of vessel sacrifice.23
Subsequently, superselective angiography of individual arterial pedicles, with specific attention to dangerous anastomoses and the at-risk blood supply of cranial nerves, is necessary to plan the embolization procedure (Table 16.1).24 Blood supply to most meningiomas is derived from the dural arterial vasculature (middle meningeal artery, posterior meningeal artery, tentorial artery of Bernasconi and Cassinari, etc.), arising from the ECA, VA, or ICA. A typical finding on superselective angiography is an intense vascular tumor blush from the arterial phase through the late venous phase, often with a “sunburst” type pattern.25 Tumors located in specific anatomical locations such as the orbital, parasellar, petroclival, and cervicomedullary regions will have associated, and predictable, patterns of these dangerous anastomoses or at-risk cranial nerve blood supply relative to their location.24 Careful study of the superselective angiographic images with a strong understanding of the usual dangerous anatomy is imperative for the avoidance of complications.
Procedural Considerations
Timing and Adjunctive Medications
Preoperative embolization is typically performed within several days of surgical resection. Delaying surgical resection at least 24 hours after embolization may be beneficial.26 One study suggests the optimal latency for meningioma resection following embolization may be 7 to 9 days, allowing for maximal tumor softening, decreased operative times, and lower Simpson grades.27 However, in tumors that have been embolized with excellent tumor bed penetration (especially with smaller particle size such as 60 to 150 µm), delay of surgical resection may lead to significant increases in peritumoral edema and mass effect.6 Most interventionalists advocate high-dose intravenous steroids during and after meningioma embolization procedures where the tumor is large or there is already a significant amount of edema present.5,6 Provocative testing with injection of Amytal and/or lidocaine with simultaneous neuromonitoring or immediate neurologic examination can help predict the risk of embolization to dangerous territories.28 ECA vasospasm may occur during the embolization procedure. Prophylactic application of a transdermal nitroglycerin patch or sublingual calcium channel antagonists may reduce the occurrence of vasospasm. Administration of papaverine (30 to 60 mg), nitroglycerin (100 to 300 µg), or verapamil (5 to 20 mg) through the catheter system may reduce vasospasm and allow more predictable results.
Endovascular Technique
Embolization should be performed by a well-trained neurointerventionalist in a biplane digital subtraction fluoroscopy suite. Intravenous sedation, monitored anesthesia care (MAC), or general endotracheal anesthesia should be provided according to the specific goals and challenges of the procedure. Arterial endovascular access is obtained most often by puncture of the common femoral artery and insertion of a 5-Fr or 6-Fr short sheath using the modified Seldinger technique. Alternatively, embolization may be performed using a direct puncture technique (DPT).
The patient should be given a bolus of intravenous heparin with a goal of 2 to 2.5 times the baseline activated clotting time (ACT) to help prevent thromboembolic complications. If not performed previously, a detailed angiographic study of the tumor blood supply is then performed via superselective angiography using microcatheters.
After the decision to proceed with embolization is made, a suitable guide catheter is selected to allow for a working platform within the major artery supplying the tumor. The ICA, ECA, or the distal V4 segment of the VA may be accessed by a 90-cm guide catheter in most patients. However, vessel tortuosity or patient height may dictate the use of a longer guide catheter. The outer diameter of the guide catheter is usually 5-Fr or 6-Fr, and the inner diameter should easily accommodate the desired microcatheter(s).
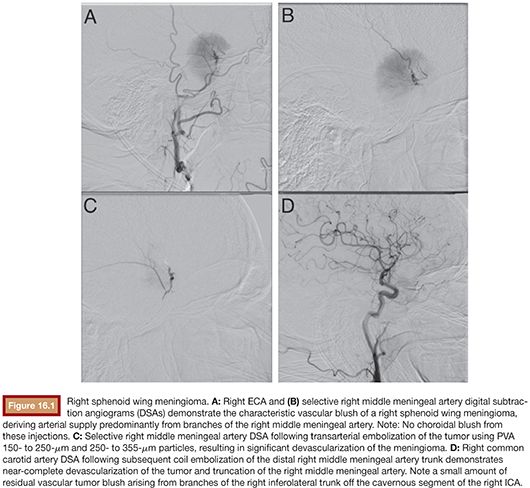
After the microcatheter is in position within the target arterial pedicle and the interventionalist is confident that safe embolization can be performed, the embolic material is selected and prepared. The embolic material is injected slowly with simultaneous biplane fluoroscopy to identify flow into the feeding artery and tumor bed. Reduction of tumor blush by 80% or more following embolization should be the goal, as reaching this threshold may be necessary to impart a beneficial effect.23 Injection should stop when the embolic agent no longer reaches the tumor or, in the case of particle embolization, when there is contrast stagnation of the feeding artery (Fig. 16.1). If there is any identification of embolic material tracking into undesired territories, or reflux along the microcatheter, the injection should cease immediately. Deep penetration of embolic agent into the tumor bed may result in complete devascularization of the tumor, often without embolization of all of the feeding arteries. In other circumstances, selection of additional pedicles may be necessary to obtain adequate embolization results. This typically requires obtaining a fresh microcatheter. After completion of the embolization, a thorough neurologic examination is performed to assess for ischemic or hemorrhagic complications. Close clinical surveillance is indicated over the following hours as delayed complications such as intratumoral hemorrhage, increased edema, and mass effect may develop. These complications can usually be quickly and reliably treated if recognized in a timely manner.4,5
Route of Embolization
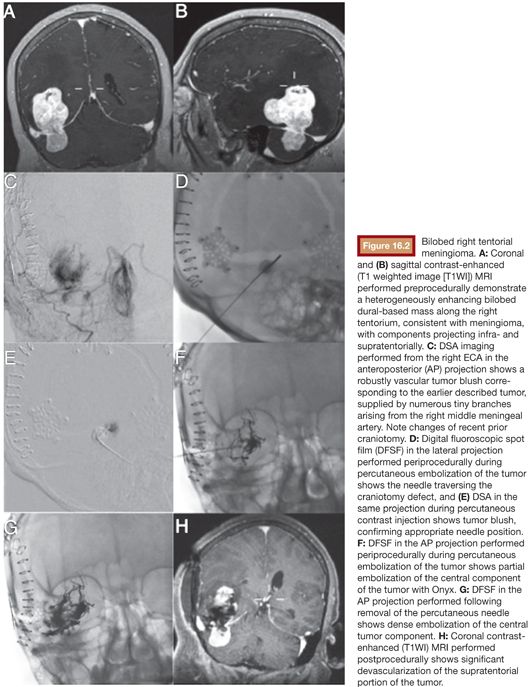
The approach to head and neck tumor embolization varies based on different factors including, but not limited to, the material chosen, the location of the tumor, and the comfort level of the operator. The traditional transarterial route has been used in most meningioma embolization procedures. Rarely, vascular anatomy prevents appropriate endovascular access to the tumor and, therefore, prevents intra-arterial treatment. In these situations, a percutaneous (or transmucosal) DPT may be the only available route for embolization.23 Occasionally, a previous craniotomy can allow DPT access for the embolization of intracranial meningiomas (Fig. 16.2). Typically, liquid agents with 18- to 20-gauge needles are employed for DPT alone or in combination with the transarterial approach. Although DPT may yield increased tumor penetration,14 systematic comparisons of transarterial and DPT embolizations have not been performed. DPT does eliminate the issue of catheter obstruction. DPT is not without risk of major complications.29 Care must be taken during DPT as the embolic material is driven by pressure and it may be pushed through physiologic collaterals into undesired territories because of the pressure gradient that is created.30 Pain from tumor necrosis may occur during the DPT procedure. The patient should be aware of this possibility and may require general anesthesia.31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree