Name
Manufacturer
Retrievable (R)/permanent (P)
Access (delivery size, French ID)
Material
Maximal IVC filter (mm)
MRI compatibility+
Minimum IVC diameter (mm)
Retrieval set
Pediatric use
Celect
Cook
R/P
Femoral, jugular (8.5 Fr)
Conichrome
30
Y (1.5, 3 T)*
15
Jugular (11 Fr)
Y
Gunther tulip
Cook
R/P
Femoral, jugular (8.5 Fr)
Conichrome
30
Y (1.5, 3 T)*
N/A
Jugular (11 Fr)
Y
Gianturco-Roehm Bird’s Nest
Cook
P
Femoral, jugular (12 Fr)
Stainless steel
40
Y (6 weeks delay)*
N/A
N/A
Tempofilter II
B Brawn
P
Jugular (6 Fr)
Phynox
28
N/A
N/A
N/A
Trapease
Cordis
P
Femoral, jugular, brachial (6 Fr)
Nitinol
30
Y (1.5 T)
N/A
N/A
Optease
Cordis
R/P
Femoral, jugular (6 Fr)
Nitinol
30
Y (3 T)*
N/A
N/A
Vena Tech LP
B Brawn
P
Femoral, jugular (7 Fr)
Phynox
35
Y
N/A
N/A
G2
Bard
R/P
Femoral (7 Fr), jugular (10 Fr)
Nitinol
28
Y (1.5 T)
N/A
Recovery Cone system (10 Fr)
Y
G2X
Bard
R/P
Femoral (7 Fr), jugular (10 Fr)
Nitinol
28
Y (3 T)*
N/A
Snare (7 Fr), Recovery Cone system (10 Fr)
Option
Rex Medical
R/P
Femoral, jugular (5 Fr)
Nitinol
30
Y (3 T)*
N/A
Snare and sheath combination
Safeflow
Rafael Medical
P
Femoral, jugular (6 Fr)
Nitinol
27
N/A
N/A
ALN
ALN
R/P
Femoral, jugular, brachial (7 Fr)
316 L amagnetic stainless steel
32
Y
N/A
Vena Tech LGM
B Brawn
P
Femoral, jugular (10 Fr)
Phynox
28
Y
N/A
N/A
Eclipse
Bard
R/P
Femoral (7 Fr), jugular (10 Fr)
Nitinol Electropolished G2X
28
Y (3 T)*
N/A
Snare (7 Fr), Recovery Cone system (10 Fr)
Simon Nitinol
Bard
P
Femoral, jugular, brachial (7 Fr)
Nitinol
28
Y
N/A
N/A
Meridian
Bard
R/P
Femoral (8 Fr), jugular, subclavian (10 Fr)
Nitinol-titanium alloy
28
Y (1.5, 3 T)*
N/A
Snare and 10 Fr sheath
N
Vena cava filters are classified as temporary, optional/retrievable/recoverable, or permanent:
Temporary filters: these are filters that must be removed or repositioned after a certain time. They are usually tethered devices with a segment (catheter/wire) exiting through the skin at the insertion site.
Optional or retrievable/recoverable filters: these are permanent filters with the option to be removed if caval filtration is no longer needed up to a predetermined time limit. They are excellent devices for prophylaxis (duration of PE risk is short) or as a bridge to anticoagulation. They are the preferred filters in children as there is an option to remove the filter and avoid a permanent device in a child with long life expectancy. They can also be left as a permanent device if the clinical status of the patient requires or if large thrombus burden is trapped in the filter.
Permanent filters: these devices should not be removed or repositioned.
Pre-procedure Assessment
d-dimer levels help to detect recent thrombus formation or prothrombotic states. Patients with an elevated d-dimer [d-dimer normal levels <0.51 μg/mL FEU (fibrinogen equivalent unit)] and clinical signs/symptoms suggestive of DVT have a high probability of venous thromboembolic events. These patients should be further investigated with imaging.
Ultrasound is the best imaging modality to assess the venous system and to determine the extent of thrombosis. Compressibility on ultrasound of deep veins on the lower limbs (common femoral veins, superficial femoral veins, or popliteal veins), upper limbs, and neck (jugular veins) is considered the gold standard exam to detect DVT. Other sonographic signs include lack of augmentation in the vessel lumen, lack of respiratory variability, or absent flow on pulsed and color Doppler.
The anatomy and patency of the IVC are confirmed with US, CT, or MRI. Vena cava anatomy and size is further evaluated during the procedure with a venogram.
Computed tomography angiography (CTA) of the chest/pulmonary arteries is required in cases of PE suspicion.
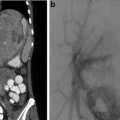
If a decision is made to insert a vena cava filter, the rationale to insert a vena cava filter in a child is slightly different from an adult (Fig. 11.1). Children have a long life expectancy and the indication for filter insertion generally is not permanent. Therefore, retrievable/recoverable or optional filters are preferred.
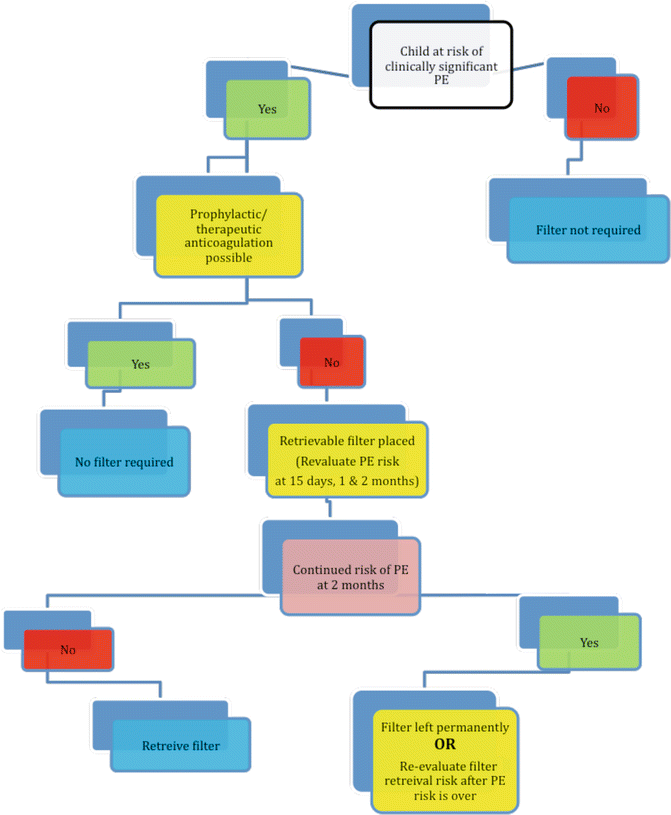

Fig. 11.1
Rationale algorithm to insert a vena cava filter in a child
Insertion Technique
Percutaneous approach is preferred as new lower profile delivery systems are available.
Get Clinical Tree app for offline access

1.
2.
Local anesthesia/sedation or general anesthesia according to the patient age.
3.
Sterile technique.
4.
Venous access: the vast majority of filters have a jugular or femoral delivery system. The most commonly used access sites are the right femoral vein and the right jugular vein. Alternative accesses such as the basilic vein, subclavian vein, and external jugular vein may be used in some patients. Access is obtained under real-time US guidance to reduce procedure length and potential complications such as arterial puncture [11] or arteriovenous fistula creation.
5.
Place a ruler on the angiographic table parallel to the IVC to determine the IVC diameter and to help mark the location of the renal veins. Alternatively, modern angiography equipment may isocenter the patient and estimate the size of objects quite accurately. Measurements from pre-procedure noninvasive imaging methods (US, CT, or MRI) may also be used. Measuring the IVC is critical in children.
6.
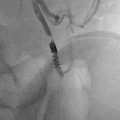
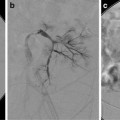
Perform A cavogram using a catheter (such as a pigtail catheter) positioned at the confluence of the common iliac veins (jugular or femoral approach) or through an angiocatheter placed in the non-involved femoral vein. Contrast volume injected depends on patient age/size. In general, 10–20 mL of contrast is needed to opacify the IVC. The maximum volume of contrast should not exceed 6–8 mL/kg [15]. Diluted nonionic iodinate contrast (50 %) can be used to minimize contrast exposure. Digital subtraction angiography (DSA) with suspended respiration, filming at 2–6 frames/s, is ideal (Fig. 11.2a).
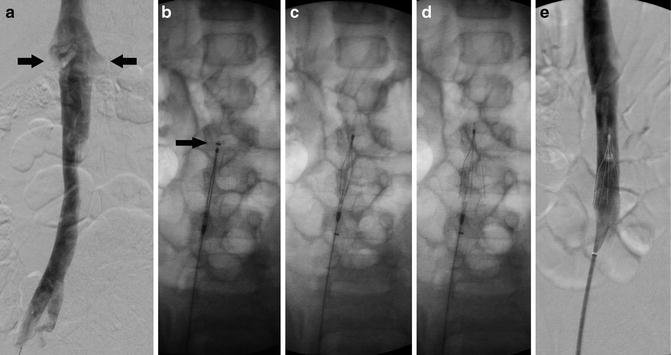

Fig. 11.2
Vena cava filter insertion technique (femoral approach): (a) Cavogram through the right common femoral vein delineating the inferior vena cava (IVC), its contours and diameter, the position of the renal veins (arrows), and the absence of clot in the lumen. (b) Introducer sheath with marker (arrow) positioned at the level that the hook of the filter (seen inside the sheath) will be deployed. (c) Introducer sheath pulled back exposing filter. (d) Vena cava filter released and deployed in the IVC. (e) Venogram to confirm position of the filter, clearance from the renal veins’ ostium, and patency of IVC
(a)
The cavogram defines the vena cava anatomy, its patency and its size. Absent IVC with azygos continuation occurs in 0.15 % of patients [16]. Drainage occurs through the azygos venous system precluding IVC filter placement. Megacava (IVC with a diameter >28 mm) is found in 1 % of patients and may be a contraindication for some filters due to the potential risks of filter migration.
(b)
The cavogram also determines the position of the renal veins, seen as a faint non-opacified inflow of blood in the pool of contrast in the IVC or by reflux of contrast into their orifices. Alternatively, the renal veins may be selectively catheterized with a guidewire to determine their locations.
(c)
The cavogram detects possible thrombus in the IVC, assisting on the location to place the IVC filter (always above the thrombus).
7.
Insert a stiff guidewire in the IVC, and advance a guiding/delivery sheath over the wire to the position where the filter will be deployed. The ruler and anatomical landmarks help determine the proper position. Serial dilation of the venous entry point may be necessary to allow insertion of large guiding/delivery sheaths.
8.
Remove the guidewire (unless you are using an over the wire filter), check the correct orientation of the filter, and advance the filter to the end of the delivery sheath (Fig. 11.2b).
9.
Recheck filter position.




(a)
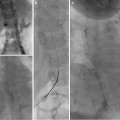
The majority of filters are placed in an infrarenal position (1 cm below the renal veins) to reduce the risk of renal vein thrombosis and filter migration (higher in suprarenal filters—27.5 % vs. 3 %) [17]. These may be challenging in small children where the infrarenal portion of the IVC is shorter than the filter length and in patients with scoliosis due to acute angulation of IVC (Fig. 11.3). Filter arms or legs should not be placed at the level of the renal veins due to the risks of filter dislodgement, filter tilting, filter fracture, and renal vein thrombosis.
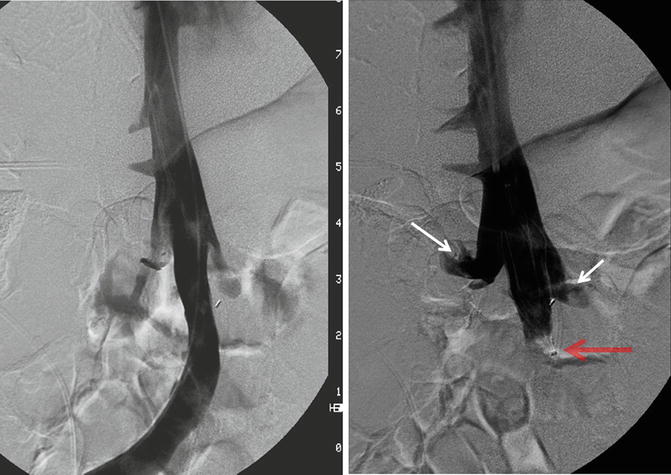

Fig. 11.3
Digital subtraction venography shows tortuous course of IVC due to severe scoliosis requiring partial placement of Trapease filter (red arrow) at the level of renal veins (white arrows)
(b)
Suprarenal filters are reserved for situations where the thrombus originates from the renal veins, the thrombus extends above an indwelling vena cava filter, or the infrarenal IVC is too short (some children) and cannot accommodate the vena cava filter.
(c)
The most common anatomical variant is a circumaortic renal vein present in 4–5.5 % of patients [18]. The filter should be placed below the circumaortic left renal vein to prevent inadvertent clot bypassing the filter through the circumaortic left renal vein and main renal vein.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree