Situs solitus
Totalis (levocardia)
Mesocardia
Dextrocardia
Situs inversus
Totalis (dextrocardia)
Mesocardia
Levocardia
Heterotaxy
Polysplenia syndrome
Asplenia syndrome
Thoracoabdominal discordance
The next step in the segmental approach is the determination of the bulboventricular loop, which can be D-loop {–,D,–}, L-loop {–,L,–}, or X-loop {–,X,–}. With D-looping {–,D,–}, the bulboventricular tube bends and shifts to the right, placing the bulbus cordis, the precursor of the right ventricle, to the right of the primitive ventricle which is the precursor of the left ventricle. Therefore, in situs solitus with D-looping {S,D,–}, there is atrioventricular concordance with the right atrium connected to the right ventricle and the left atrium connected to the left ventricle (Fig. 12.1a). In early fetal development, the ventricular portion of the heart, the ventricular mass, is in the right hemithorax. Subsequently, the bulboventricular loop shifts to the left hemithorax, resulting in levoversion. In the case of partial shift of the bulboventricular loop to the midline or failure to shift to the left, the result is situs solitus with mesoversion and situs solitus with dextroversion, respectively.
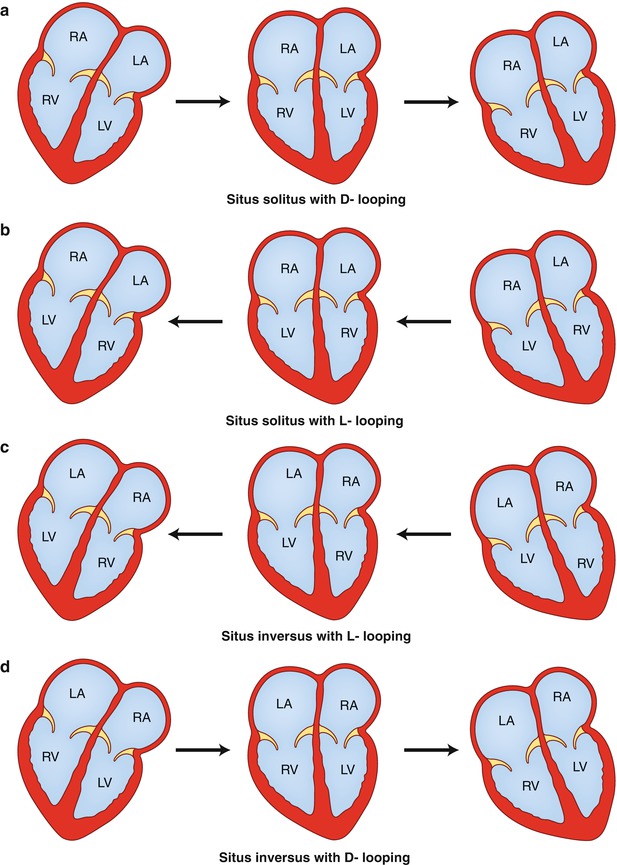

Fig. 12.1
Drawings demonstrate variations in looping and horizontal shift of the cardiac apex. (a) Situs solitus with D–looping resulting in atrioventricular concordance. The cardiac mass normally shifts from right to left, transitioning from dextroversion to mesoversion and finally to levoversion. (b) Situs solitus with L–looping resulting in atrioventricular discordance. The cardiac mass usually shifts from left to right resulting in dextroversion. (c) Situs inversus with L–looping results in atrioventricular concordance. The cardiac mass shifts from left to right, leading to dextroversion. (d) Situs inversus with D–looping results in atrioventricular discordance. The cardiac mass shifts from right to left, leading to levoversion. RA right atrium, LA left atrium, RV right ventricle, LV left ventricle
In L-looping {–,L,–}, the heart tube folds and rotates to the left, which places the bulbus cordis to the left of the primitive ventricle. That means that the ventricular mass is in the left hemithorax, and the bulboventricular loop tends to rotate to the right hemithorax. Therefore, situs solitus with L-looping {S,L,–} results in atrioventricular discordance, with the right atrium connected to the left ventricle and the left atrium connected to the right ventricle (Fig. 12.1b) [10]. As above, the degree of shift of the cardiac mass determines the orientation (version) of the cardiac apex. Complete shift of the apex toward the right results in situs solitus with atrioventricular discordance and dextroversion; partial shift to the midline results in mesoversion and absence of shift results in levoversion. In a similar manner, formation of an L-loop (concordant loop) in cases of situs inversus results in atrioventricular concordance {I,L,–} (Fig. 12.1c), whereas a D-loop (discordant loop) results in atrioventricular discordance {I,D,–} (Fig. 12.1d). Therefore, a complete, partial, or a failure of shift of the cardiac apex to the right further classifies these forms into situs inversus with dextroversion, situs inversus with mesoversion, and situs inversus with levoversion [1, 7, 10].
In X-loop {–,X,–}, a single ventricle is present, and ventricular looping is undefined. Single ventricle or univentricular heart has three major presentations according to the trabecular pattern. If the pattern is similar to either the right or the left ventricle, then it is called single right or single left ventricle, respectively. If the trabecular pattern is indeterminate, then it is called single “undifferentiated” ventricle. The atrial connection is through a common atrioventricular valve (common inlet; AV canal) or through two atrioventricular valves (double inlet; right and left AV valves). Both atrioventricular valves may be patent (double inlet) or one may be often atretic (single inlet). Anatomically, the two valves are different from the tricuspid and mitral valves, and therefore, they should be referred to as right and left atrioventricular valve. The exit of flow of the main ventricular cavity into the great vessels may be direct or through a rudimentary outlet chamber. In single right ventricle, the exit is usually direct, whereas in single left ventricle, the exit is through a rudimentary chamber (outlet chamber). The outlet chamber can be anterior, superior and to the right (normally related), or anterior, superior, and to the left (inverted) of the base of the main cavity. It is uncommon for the outlet chamber to be in front of the main cavity. A bulboventricular foramen (ventricular septal defect; outlet foramen) connects the two chambers. The aorta and the pulmonary artery are almost always transposed (D- or L-malposition), and both great vessels may arise from the rudimentary chamber or from the main cavity. Subvalvular or valvular pulmonary stenosis may be present, and restriction of the bulboventricular foramen may be found. The outlet foramen may straddle the right or left atrioventricular valve depending on its location.
After determining the cardiac situs and the position of the bulboventricular loop, the next step in the segmental approach is to identify the ventriculoarterial relationship [4, 6, 11]. Normally, the heart tube folds to the right {–,D,–}, and the developing ascending aorta is positioned to the right of the developing pulmonary artery in the truncus arteriosus with both structures at the same level. Growth in the subpulmonic free wall of the truncus elevates the pulmonic valve and advances it anteriorly with a rightward rotation; while resorption in the subaortic free wall results in the aortic-mitral fibrous continuity [12]. Therefore, normally the pulmonic valve is positioned anteriorly, superiorly, and to the left of the aortic valve (solitus) {–,–,S}. The mirror-image configuration results in an anteriorly placed pulmonic valve to the right of the aortic valve (inversus) {–,–,I}.
Defining the atrial situs (cardiac situs), the ventricular topology, and the position of the great vessels does not predict the atrioventricular and ventriculoarterial connections, which must be separately evaluated [4, 11]. Similarly, the orientation of the cardiac apex (ventricular mass) is independent of the position of the cardiac atria and depends on the degree of the horizontal shift of the cardiac mass. Defining the position of the cardiac apex after identification of the atrial situs and the cardiac loop determines the “version.” The terms dextroversion, mesoversion, and levoversion indicate the position of the cardiac apex, when this structure is not in the usual location for the corresponding visceroatrial situs. The terms dextrocardia, mesocardia, and levocardia refer to the position of the heart within the thoracic cavity and, in common parlance, are used interchangeably with the version terminology. The cardiac situs is usually concordant with the thoracic situs (tracheobronchial tree) and the position of the ventricular mass (cardiac apex) and must be determined to predict the probability and type of congenital heart disease. There are two forms of determinate situs, situs solitus with levoversion or levocardia, the normal configuration {S,D,S}, and situs inversus with dextroversion or dextrocardia {I,L,I}, the mirror-image configuration.
Interestingly, the degree of shift of the cardiac apex in the horizontal plane (the version) predicts the occurrence and complexity of congenital heart disease. Severe congenital heart disease is present in more than 90 % of people who have situs solitus with dextroversion and situs inversus with levoversion [1, 13]. The severity and occurrence rate is much lower in both situs solitus and situs inversus with mesoversion (Table 12.2).
Table 12.2
Congenital heart disease incidence for various configurations of visceroatrial situs
Situs configuration | Incidence (%) |
|---|---|
Situs solitus totalis | 0.8 |
Situs solitus mesocardia | 0.8 |
Situs solitus dextrocardia | 90 |
Situs inversus totalis | 5 |
Situs inversus mesocardia | 0.8 |
Situs inversus levocardia | 95–100 |
Polysplenia | 75 |
Asplenia | 99–100 |
The thoracic and abdominal organs are usually lateralized on either side of the midline. Situs solitus totalis {S,D,S} refers to the expected configuration of thoracic and abdominal organs with a trilobed right lung and a right-sided liver and inferior vena cava. The cardiac apex is toward the left and the spleen, the stomach and the aorta are also positioned on the left. In the case of normal thoracoabdominal concordance, the rate of congenital heart disease is 0.8 % [13]. The mirror-image configuration of thoracic and abdominal organs is referred to as situs inversus totalis {I,L,I} and has a 5 % incidence of congenital heart disease [13]. Approximately 20 % of the cases of situs inversus totalis are associated with Kartagener syndrome [14] comprised of the classic triad of situs inversus totalis, chronic sinusitis, and bronchiectasis [15]. Conversely, only half of the patients with primary ciliary dyskinesia have situs inversus [12, 13].
In the rare occasion of thoracoabdominal discordance (thoracic situs solitus with abdominal situs inversus or vice versa), there is high incidence of congenital heart disease [1, 16].
Heterotaxy Syndromes [1, 2, 17–19]
In situs ambiguus or heterotaxy, there is duplication of right- or left-sided structures and abnormal arrangement of organs and vessels, not in the expected configuration of situs solitus {S,D,S} or situs inversus{I,L,I}. Splenic abnormalities are a hallmark of heterotaxy, and there is a high association with complex anomalies of the cardiovascular system. Rather than a distinct set, there is a spectrum of anomalies. The two major forms of heterotaxy are polysplenia and asplenia or Ivemark syndrome [1].
Polysplenia is associated with multiple spleens and bilateral left-sided structures (left isomerism) (Fig. 12.2a) [1, 2, 20–22]. In contrast to asplenia, polysplenia is more common in females and has a milder course, often presenting in childhood with a left to right shunt. However, some present in adulthood with an atrial septal defect and approximately 25 % of patients have no significant cardiac anomalies. Polysplenia is characterized by bilateral bilobed lungs and two hyparterial bronchi. A common atrium is usually present with morphologic characteristics of bilateral left atria. Congenital cardiac defects include endocardial cushion defects, double-outlet right ventricle, and left-sided obstructive lesions such as coarctation of the aorta, subaortic stenosis, or less commonly hypoplastic left heart syndrome. In contrast to asplenia, right ventricular obstruction and transposition of the great vessels are unusual, although minor forms of pulmonic stenosis have been described. Anomalous systemic and pulmonary venous drainage are seen, including partial anomalous pulmonary venous return, duplicated superior vena cava and azygos, or hemiazygos continuation of the inferior vena cava.
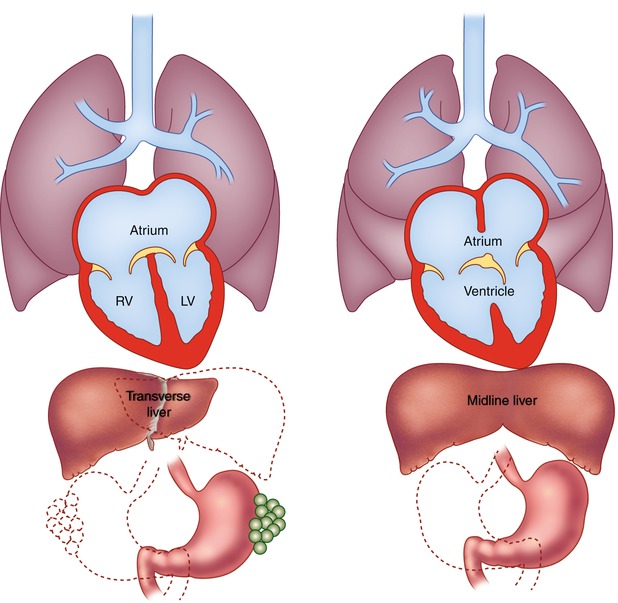

Fig. 12.2
Schematic diagrams of polysplenia and asplenia. (a) Polysplenia is characterized by bilateral bilobed lungs and bilateral hyparterial bronchi. Positions of multiple small rounded spleens and stomach are variable. Congenital heart disease occurs in 75 % of these patients and is usually mild. (b) Asplenia is characterized by bilateral trilobed lungs and bilateral eparterial bronchi. Liver is often midline, spleen is absent, and position of stomach is variable. Congenital heart disease is nearly universal. Associated cardiac defects are generally severe and include common atrium and single ventricle with pulmonary stenosis or atresia
The gastric fundus can be right or left, and intestinal malrotation is common [23]. Multiple spleens are characteristic and can be seen on either side of the abdomen, resulting in the absence of Heinz and Howell-Jolly bodies on peripheral blood smear. The liver is usually midline or symmetric, and biliary and pancreatic abnormalities may be present (Table 12.3) [24].
Table 12.3
Polysplenia
Cardiovascular anomalies |
Anomalous systemic venous connections (bilateral superior venae cavae) and interruption of the IVC with azygos continuation |
Anomalous pulmonary venous return (partial or total of the cardiac variety) |
Common atrium |
Primum or secundum atrial septal defect |
Ventricular septal defect often of the endocardial cushion type |
Double-outlet right ventricle |
Left-sided obstructive lesions (coarctation of the aorta, subaortic stenosis, hypoplastic left heart syndrome) |
Cardiac malposition |
Noncardiac anomalies |
Bilateral hyparterial bronchi and bilobed lungs |
Two or more spleens |
Transverse or symmetric liver |
Gallbladder anomalies including biliary atresia |
Intestinal malrotation |
Genitourinary abnormalities |
Asplenia (Fig. 12.2b) is characterized by an absent spleen and duplication of right-sided structures (dextro isomerism, bilateral right sidedness) [1, 2, 25–28]. It is twice as common in males and has a high association with congenital heart defects (99–100 %) that present early in life and carry a grave prognosis. Unrecognized asplenia is only rarely encountered in adulthood. Congenital absence of the spleen is associated with immune deficiency, infections, and Heinz and Howell-Jolly bodies on peripheral blood smear.
Asplenia is associated with bilateral trilobed lungs and two eparterial bronchi. Anomalous systemic and pulmonary venous drainage are common, including bilateral superior venae cavae, total anomalous pulmonary venous return, and D- or L-malposition of the great arteries. There is usually a common atrium with anatomic characteristics suggestive of bilateral right atria or a large atrial septal defect. An atrioventricular canal (atrioventricular communis) is often present, as is a single ventricle or a large ventricular septal defect (bulboventricular foramen). If two atrioventricular valves are present, both may be patent or either one may be atretic. Right ventricular outflow tract obstruction is common, usually pulmonic stenosis or atresia, resulting in pulmonary undercirculation and cyanosis. Aortic stenosis or atresia is unusual in asplenia.
In the abdomen, the spleen is characteristically absent and the liver is usually midline. There is commonly ipsilateral course of the inferior vena cava and the aorta, resulting in their juxtaposition below the diaphragm [29]. The gastric bubble can be on either side of the midline, and there is a high occurrence of intestinal malrotation [23]. Other associations include agenesis of the gallbladder, annular pancreas, imperforate anus, horseshoe kidney, and urethral valves (Table 12.4).
Table 12.4
Asplenia
Cardiovascular anomalies |
Bilateral superior venae cavae |
Common atrium or a large atrial septal defect |
Single ventricle or a large ventricular septal defect |
AV communis (atrioventricular canal) |
Transposition of the great arteries |
Anomalous pulmonary venous return (either supracardiac or infracardiac) |
Cardiac malposition |
Midline crossing of the IVC just below the diaphragm to enter the systemic venous atrium |
Common course of the IVC and the abdominal aorta |
Severe pulmonary stenosis or atresia |
Noncardiac anomalies |
Bilateral eparterial bronchi and trilobed lungs |
Absent spleen associated with Howell-Jolly bodies on peripheral blood smear and susceptibility to infections |
Transverse or symmetric liver |
Intestinal malrotation |
Gallbladder agenesis |
Annular pancreas |
Imperforate anus |
Horseshoe kidney |
Ureteral and urethral valves |
The Role of Imaging
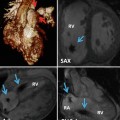
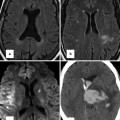
The presentation of situs abnormalities in the adult depends largely on the underlying congenital heart disease and falls into two main groups. The first group includes patients who have situs abnormalities without congenital heart disease that can escape early diagnosis. This includes the majority of adults with situs solitus with mesocardia and situs inversus with mesocardia or dextrocardia (Fig. 12.3) and a minority of patients with polysplenia. Situs abnormalities associated with mild congenital heart disease, such as simple shunt lesions or complex congenital heart disease that is physiologically mild, such as corrected transposition of the great arteries, can also remain undiagnosed during childhood. They may present and become clinically apparent later in life, when long-standing disease and comorbidities unmask the underlying defect, or they may be incidentally detected on imaging [30]. Severe forms of congenital heart disease that are usually diagnosed prenatally or at birth and undergo treatment early in life comprise the second group. These patients present in adulthood for follow-up imaging or for imaging to help diagnose and treat symptoms related to their long-standing disease or its treatment.
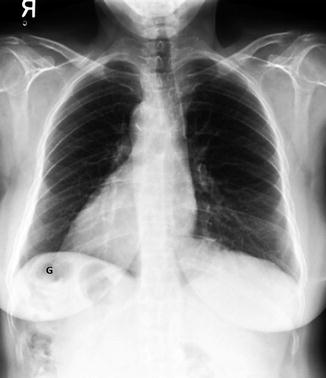

Fig. 12.3
A 60-year-old woman with situs inversus totalis and no associated congenital heart disease. There is tracheobronchial situs inversus with a right-sided hyparterial bronchus and a left-sided eparterial bronchus. Dextrocardia and right-sided aortic arch are present. The stomach (G) is in the right upper quadrant and the liver shadow is seen in the left upper quadrant
In describing a patient’s visceroatrial situs, the chest radiograph is useful to assess not only the location of the cardiac apex (ventricular mass) and gastric bubble but also the tracheobronchial situs, which is usually concordant with the atrial situs [1]. These structures are also well demonstrated on CT and MRI, which allow simultaneous evaluation of the abdominal viscera. Dextrocardia in adults can be associated with any form of situs and is present in up to half of patients with heterotaxy [9, 31]. When an anomaly of visceroatrial situs is identified, knowledge of the associated cardiac anomalies is key to making the appropriate findings and ultimately the correct diagnosis, which serves as a guide to patient management.
When CT or MRI is performed for a cardiac indication, it is typically to answer specific questions, since echocardiography remains the first-line cardiac imaging modality [32]. Tailoring the examination appropriately requires every effort to obtain relevant anatomic and surgical history and review of prior imaging. CT and MRI outperform echocardiography in demonstrating extracardiac vascular anatomy and systemic and pulmonary venous connections [33]. MRI is considered a reference standard for estimating ventricular volumes and function. Phase-contrast sequences for flow quantification are useful in documenting the presence or absence of shunts by calculating the pulmonary to systemic blood flow ratio (Qp:Qs) and regurgitant volumes and estimating the degree of stenosis [34]. There is increasing evidence that delayed enhancement and viability sequences have prognostic implications in these adults.
Situs solitus and situs inversus with mesocardia have the same rate of congenital heart disease as patients with normal situs or situs solitus totalis (0.8 %). However, when congenital heart disease is present, the diagnosis of corrected transposition of the great arteries should be considered [35




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree