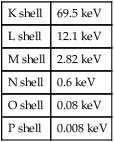
• Explain the process of characteristic x-ray photon production. • Explain the process of bremsstrahlung x-ray photon production. • Determine characteristic and bremsstrahlung photon energy. • Describe beam quantity and how milliamperage, kilovoltage peak, filtration, and distance affect it. • Describe beam quality and how kilovoltage peak and filtration affect it. • Interpret the discrete, continuous, and x-ray emission spectrums. • Explain the effects of milliamperage, kilovoltage peak, filtration, generator type, and target material on the x-ray emission spectrum. Figure 6-1 illustrates the inside of the x-ray tube. Exposure factors have been selected; electricity has traveled to the anode, cathode, and filament; and electrons have been boiled off of the filament and are streaming across to the anode at tremendous speeds. The filament electrons penetrate the face of the target to a depth of approximately 0.5 mm, interacting with the tungsten target atoms in their path. These filament electrons interact with target atoms to produce x-rays in two ways: characteristic interactions and bremsstrahlung (brems) interactions. It should be noted that most of the interactions (approximately 99%) do not result in x-rays but produce only heat. Keep in mind also that there are thousands of interactions taking place inside the target at once, but for the sake of explanation we focus on a single event in each case. Refer to the first part of Chapter 2 for a refresher on the general structure of the atom. Characteristic interactions involve the filament electron and an orbital electron of a target atom. In general, a filament electron enters a target atom, strikes an orbital electron, and if its energy is greater than the binding energy of the orbital electron it is removed from orbit. Recall from Chapter 2 that orbital shells fill from the shell nearest the nucleus outward and a vacancy in a shell makes the atom unstable. To correct this situation, outer-shell electrons drop to fill inner-shell vacancies. To do so the outer-shell electron must expend some of its potential energy. This energy is given off as a characteristic x-ray photon (Figure 6-2). Note that when the first orbital electron drops to fill the vacancy, it in turn leaves another. This second vacancy is also filled by an outer-shell electron that again must give up some of its energy, producing another characteristic photon. This process of outer-shell electrons filling inner-shell vacancies continues down the line, creating a cascading effect called a characteristic cascade. Each time an orbital electron moves to a lower orbit, a characteristic photon is produced. This process is not necessarily orderly. If a filament electron removes a K-shell electron from an atom, the most likely electron to fill the vacancy is an L shell because of proximity. But any outer-shell electron can fill the K-shell vacancy; it is just not as likely to do so. Notice that the removal of the orbital electron established the environment for x-ray production and it is the expending of energy during the cascade that produces characteristic x-rays. Characteristic photons are so called because their energy is “characteristic” or dependent on the difference in binding energy between the shells involved. The electron shells of each element have specific binding energies. Table 6-1 presents binding energies that can be assumed for tungsten. A tungsten atom has 74 electrons orbiting its nucleus in six different shells. The filament electron may interact with any of them, but medical imaging generally focuses on K-shell (innermost shell) interactions because they are the highest energy and the most useful for imaging purposes. Recall from Chapter 2 that each orbital electron is held in orbit by a binding energy and the closer the orbit, the stronger the bond. K-shell electrons in tungsten have the strongest binding energy at 69.5 kiloelectron volt (keV). For a filament electron to remove this orbital electron, it must possess energy equal to or greater than 69.5 keV. For all practical purposes using a general purpose x-ray machine, if a radiographer selects a kVp less than 70 on the control panel, there will be no photons produced from K-shell interactions. TABLE 6-1 part of the current through the tube. If, however, that same filament electron had 100 keV of energy, it would easily remove the K-shell orbital electron and be deflected in a new direction. It would still have 30 keV of energy left and with it interact with another atom. The second type of interaction in the target that produces x-rays is a brems interaction. Bremsstrahlung is a German word roughly meaning braking or slowing down radiation, which is exactly what this interaction involves where the filament electron is concerned. In this interaction the filament electron misses all of the orbital electrons and interacts with the nucleus of the target atom. Recall that the electron is negatively charged and the nucleus (containing all the protons) is positively charged, and there will be a force of attraction between the two. The strength of this attraction depends on how close the filament electron passes to the nucleus. The attraction causes the filament electron to slow down and change direction and in doing so it will lose kinetic energy. This energy is released as a brems photon (Figure 6-3
X-ray Production
Photons (Target Interactions)

A general-purpose x-ray tube.
Characteristic Interactions
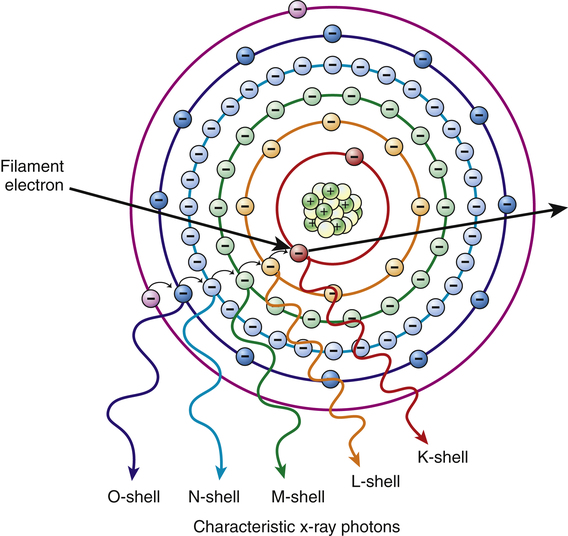
A characteristic interaction event. Note that as outer-shell electrons fill inner-shell vacancies, their excess energy is released as characteristic x-ray photons.
K shell
69.5 keV
L shell
12.1 keV
M shell
2.82 keV
N shell
0.6 keV
O shell
0.08 keV
P shell
0.008 keV
Bremsstrahlung Interactions
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


X-ray Production


