Fig. 26.1
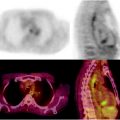
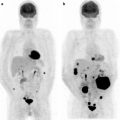
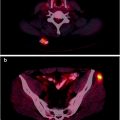
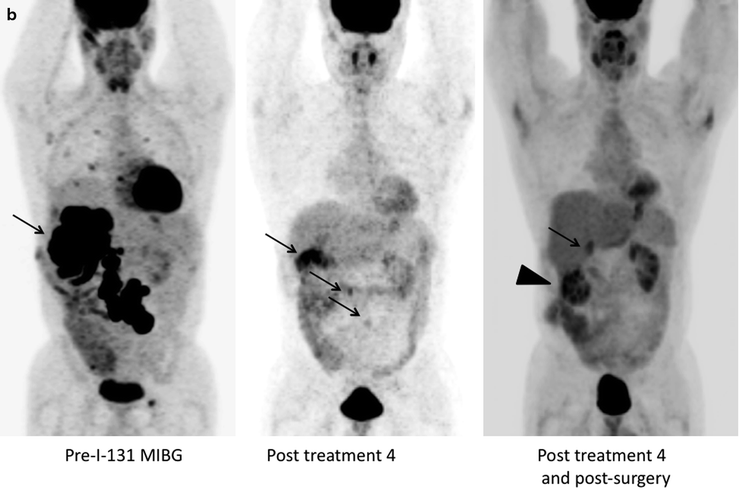
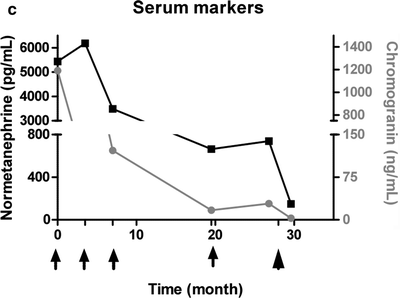
(a) Serial 123I-MIBG posterior whole body images from a patient with unresectable primary PHEO of the right adrenal (upper arrows) and metastatic disease to retroperitoneal nodes (lower arrows), spine, pelvis, ribs, and bilateral lung metastases are also present. He received four treatments consisting of 204, 211, 215, and 243 mCi of 131I-MIBG over a 14-month period. The initial posttreatment scan 1 showed some decrease in retroperitoneal nodes, and disappearance of some lung and bone lesions. Post treatment 4, all lung and bone lesions had disappeared, and retroperitoneal nodes had resolved or improved markedly. The adrenal mass had decreased in size and intensity and was Fig. 26.1 (continued) resectable for palliative reasons. (b) Anterior [18F]FDG PET/CT MIP images of the same patient as in Fig. 26.1a, showing extensive [18F]FDG avid PHEO uptake in primary tumor (arrow), retroperitoneal nodes, bone, and lungs. After four treatments (with cumulative activity of 873 mCi 131I-MIBG) (middle panel) only residual [18F]FDG activity was seen in right adrenal bed and retroperitoneal nodes (two lower arrows). The adrenal mass had shrunken considerably and became largely necrotic. Because persistent mild elevation of nor-metanephrines was observed and this was the site of largest disease, the patient had surgical resection of the residual adrenal mass. [18F]FDG PET 6 months postsurgery (right panel) showed small residual uptake in the surgical bed and two small hypermetabolic nodes in the abdomen (not clearly seen in this MIP image). Note that the activity in the right upper quadrant (arrowhead) represents kidney. (c) Biochemical parameters may be used to track response in PHEO. This is the same patient shown in Figs. 26.1a and 26.1b who had elevated chromogranin and normetanephrine levels pretreatment. The arrows represent the four 131I-MIBG treatments, totaling 873 mCi. Although chromogranins started to improve after the first treatment, it was not until after the second treatment that metanephrines started to improve. Chromogranin were in the normal range at the time of the fourth treatment, but normetanephrine levels did not normalize until after surgery
131I-MIBG Preparation
Procedure guidelines for the use of 131I-MIBG in therapy have been published [9]. The specific activity of the 131I-MIBG used for imaging typically is low (11 MBq/mg, or 3.33 mCi/mg), while the specific activity of the 131I-MIBG used for therapy is higher, ranging from 1.11 to 1.85 GBq/mg (30–50 mCi/mg) [10–15]. At these specific activities, approximately 1 in every 2,000 molecules of MIBG will be labeled with 131I. Therefore, for an activity of 18.5 GBq (500 mCi), a total of 10–17 mg of MIBG is administered. Even higher specific activity 131I-MIBG has been developed, in the range of 92.5 GBq/mg (2,500 mCi/mg) [16–18]. Theoretical and preclinical studies in small animals have shown that higher specific activity results in greater tumor uptake and higher tumor to normal tissue ratios than lower specific activity reagents [17 19]. However, a study with high specific activity MIBG resulted in higher activity in heart but not in tumor xenografts [20]. Limited studies in humans using high specific activity 123I-MIBG have not shown higher tumor targeting [21]. Phase I clinical trials with high specific activity 131I-MIBG have not clearly confirmed an advantage in terms of tumor targeting, although its safety has been documented [22]. Even if high specific activity 131I-MIBG is not shown to have a clear advantage over low specific activity material in terms of tumor targeting, administration of lower mass amounts of MIBG over a shorter period of time with a lower incidence of nausea, vomiting, and hypertension may be possible [23]. It should be noted that while 131I-MIBG was approved by the FDA for imaging, it has yet to be approved for therapy in the US. Thus, 131I-MIBG used for therapy trials is either made in accordance with USP regulations or used under an investigational new drug application (IND).
Patient Preparation
Preparation of patients prior to treatment with 131I-MIBG is not complex. A variety of drugs are known to interfere with uptake of MIBG by tumor cells and should be discontinued prior to therapy, including labetalol, tricyclic antidepressants, reserpine, and some sympathomimetics [24–28] (Table 26.1). A detailed list of drugs that may interfere has been complied [27]. When possible, these drugs are eliminated or substituted prior to therapy with 131I-MIBG. The amount of time of necessary for withdrawal is related to their half-life [27]. In patients with PHEO it is often not possible to withdraw from anti-hypertensive medications. Oral administration of phenoxybenzamine and atenolol is often used for the outpatient control of blood pressure and has not been shown to block 131I-MIBG uptake in vivo. In addition, nifedipine can also be added to antihypertensive regimens since interference has not been observed [29]. As a practical matter, patients generally undergo a diagnostic 123I-MIBG study on their current drug regimen to assess targeting of MIBG prior to considering therapy [30, 31].
Table 26.1
Class of drugs that interfere with MIBG uptakea
Documented in patients | Documented in vitro | |
|---|---|---|
Antihypertensives | Propranolol but not other beta-blocker [28], guanethidine, reserpine, nifedipine, verapamil | |
Opioids | Cocaine [30] | |
Tricyclic antidepressants | Imipramine [31] | Amitriptyline derivatives |
Sympathomimetics | Phenylpropanolamine, amphetamines, pseudoephedrine, phenylephrine | |
Antipsychotic | Phenothiazines, haloperidol |
Thyroid Blockade
Although USP release criteria require >95% 131I bound to MIBG, 131I-MIBG preparations contain a small amount of free radioiodide. In the interval between manufacture and dose administration, there is a time-dependent breakdown, with the release of free iodine [32] into the solution. In addition, a small amount of free iodide may be generated in vivo from metabolism [33]. Therefore, blocking the thyroid is necessary. A variety of regimens using potassium iodide (KI), saturated solution of potassium iodide (SSKI), or Lugol’s solution have been used. Several studies have shown the efficacy and timing of cold iodine administration to block thyroid uptake of radiolabeled iodine [34, 35]. The dose of KI recommended by the FDA for use during radiation emergencies is 130 mg of KI (100 mg iodine) for adults and is scaled down for children, (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm080542.pdf). This regimen is in line with what others have suggested for blockade prior to diagnostic MIBG scanning [36]. Typically, the dose of KI prior to 131I-MIBG therapy is higher than 130 mg. Thyroid blockade usually starts 1 day prior to administration of the 131I-MIBG [37–39], although some start up to 5–7 days prior [40, 41], but this appears to be unnecessary [34, 35]. The amount of KI has ranged from 120 to 200 mg with smaller amounts for children [9, 42–46]. The length of blockade has ranged from 7 to 30 days [10, 38, 40, 41, 43, 47]. Some have used more intensive regimens including thyroid suppression with thyroid hormone, in order to decrease TSH [41, 48, 49]. In pediatric populations some regimens add potassium perchlorate and T3 to lower the TSH [50]. The EANM guidelines suggest starting iodide blockade 24–48 h prior to treatment and continuing for 10–15 days and do not recommend thyroxine administration [9]. Pharmacokinetic studies of radioiodinated have shown that there is less than 10% in blood at 1 h post-administration [51] and that >80% of radiolabeled MIBG is released from the body over 4–5 days [22, 52, 53]. MIBG clears from the body with a terminal effective half life of 37 h [54]. Therefore, it is reasonable to stop administering iodide for thyroid blockade 14 days after treatment. In spite of these measures, it is not unusual to see some uptake of 131I in the normal thyroid [47].
SSKI and Lugol’s are well tolerated. Infrequent side effects include sialadenitis, gastrointestinal disturbances, allergic-like reactions, and minor rashes. Thyroidal side effects include iodine-induced thyrotoxicosis, which is more common in older people and in iodine deficient areas, and hypothyroidism, particularly in iodine-sufficient areas.
Because patients may have nausea and vomiting after administration of 131I-MIBG, antiemetics such as ondansetron are often administered prior to therapy and in the first 3 days post-administration. Since the major route of 131I-MIBG excretion is renal, the bladder is exposed to high concentrations of radioactivity. To minimize the radiation dose to the bladder, hydration is encouraged. Some investigators administer 3 L/m2 or 150 mL/h for several days [55, 56]. In small children, urinary catheterization is often performed to decrease both the radiation dose and the potential for contamination [37, 55, 57].
Candidates for 131I-MIBG therapy should have a reasonable performance status (Karnofsky >60) and a life expectancy of at least 3 months, unless they have intractable bone pain. Reasonable hematopoietic parameters (WBC >3,000/µL, Platelets >100 K/µL) are required prior to 131I-MIBG therapy, especially when stem cells are not available [9]. The degree of acceptable liver function required has not been well established, although some have used Bilirubin ≤2.5 and liver enzymes ≤2.5 times the upper limit of normal [14]. Kidney glomerular filtration rate >30 mL/min or serum creatinine ≤2.5 the upper limit of normal are also desirable [9, 14]. Recently it has been found that patients with nephrotic syndrome receiving large activities of 131I-MIBG may be prone to bronchiolitis obliterans organizing penumonia (BOOP) or acute distress respiratory distress syndrome (ARDS) and a recommendation has been made to limit the cumulative activity in these patients to less than 800 mCi of 131I-MIBG [14].
131I-MIBG is administered by pump i.v. through a plastic indwelling catheter or central line. A variety of infusion times have been suggested, ranging from 1 to 2 h [39, 46, 58–60]; 6 h and even 24 h infusions have been evaluated [50, 61, 62]. Slow administration is a precaution to minimize pharmacologic effects from the unlabeled MIBG, although some have administered the therapy over 30–60 min without sequela [46, 58, 63]. Our practice at MSKCC is to administer the drug over ∼45 min. When high specific activity material is given, the infusion rate has been shortened to 15–30 min, since the total mass of MIBG is reduced [22, 64]. Monitoring of vital signs during the administration is recommended since patients may develop a hypertensive response. This may be particularly significant in PHEO/PGL patients and require slowing of the infusion or pharmacologic intervention.
Administering therapy as an inpatient or outpatient is dependent on multiple considerations. In many countries, there are policies or regulations that define the limits of administered activity for outpatient treatment. In the USA, patients must be given oral and written instructions to ensure that there is less than 500 mrem exposure to caregivers or other household members when they return home. Several groups have confirmed that this exposure rate to caregivers can be achieved. Estimates of 30–125 mrem with activities up to 5.55 GBq (150 mCi) were observed by Hoefnagel et al. and a median of 60 mrem/3.7 GBq (60 mrem/100 mCi) was reported Garaventa et al. [41, 65]. At MSKCC, we perform most adult therapy as outpatients, although internal policies require that patients receiving >9.25 GBq(>250 mCi) of 131I-MIBG be inpatients. In children, most groups are more conservative and will hospitalize for ease of treatment, monitoring, and radiation safety considerations. Furthermore, in order to reduce bladder radiation, a Foley catheter is often placed for 3 days to minimize bladder dose and avoid contamination.
Patient Selection Criteria
There are no uniform guidelines for selecting or excluding patients from consideration for 131I-MIBG therapy. Most investigators have required tumor localization on a diagnostic MIBG scan, although it is not always indicated whether all known lesions were MIBG avid [14, 39, 46, 66–68]. Criteria for deciding how much tumor localization is required range from >20 cGy/37 MBq (>20 cGy/mCi) [66], 5 Gy/3.7 GBq (5 Gy/100 mCi) [45], visual localization above background, higher than two times background [14], and >1% of ID in tumor [42, 69]. Requiring a positive 131I- or 123I-MIBG diagnostic scan is very reasonable since not all tumor sites take up MIBG. Furthermore, this also helps to account for interfering medication.
Other selection criteria often include evidence of disease progression or symptoms [46, 68]. Patients need to follow radiation safety precautions; thus, a Karnofsky performance status of greater than 60% is advisable. Patients must not be pregnant or breast-feeding and must agree to birth control, although the exact period of time is not known. Sperm banking or ovum harvest may be considered for younger patients.
Activity/Dose Selection
Various approaches have been utilized to select the dose for 131I-MIBG therapy. Most studies have used fixed activities based on empirical evidence of limited toxicity [12, 41, 43, 48, 50, 59, 60, 65, 70–76]. Others have used fixed activities adjusted to body weight based on dose escalation studies [14, 57, 61, 77–82]. Others have performed dosimetry analysis using a diagnostic scan and have calculated activities to deliver less than 2–4 Gy to blood or whole body [55, 83, 84].
There is also no standardized practice in terms of frequency of 131I-MIBG therapy administration. If the patient responds to treatment or is stable and has no dose-limiting toxicity, the therapy is usually repeated. The frequency of retreatment is variable, ranging from a minimum of every 4 weeks to 6 months and requiring adequate recovery from toxicity [42, 43, 65, 71, 72, 75, 85]. Some investigators will tailor the frequency based on tumor growth rate, with fast growing tumors treated every 2.5–3 months and slower growing tumors every 6 months [58]. Others perform three treatments and then reassess whether to proceed [46, 58]. High-activity therapy often requires longer intervals between treatments to allow marrow recovery; lower activities are much better tolerated. Thus, there are no predefined limits in terms of repeated treatments (Table 26.2). Patients have received from 1 to 11 repeat treatments with 131I-MIBG [43, 45, 63]. Krempf et al. observed that 1–8 treatments were needed before a response was observed and no patient had a complete biochemical response until at least three treatments were given [45]. Similarly, Sisson et al. reported a response with 1–6 treatments and suggested a minimum tumor dose of 150 Gy [91]. Those utilizing very large doses have found that continued response may be seen many months after treatment and sometimes wait a year before considering retreatment [56].
Table 26.2
PHEO/PGL: treatment schema (administered activities are listed here in mCi; multiply by 37 for conversion to MBq)
Author | # patients | Single administration (mCi) | Cumulative (mCi) | # doses |
|---|---|---|---|---|
[59] | 28 | 97–301 (mean 181) | 111–916 (mean 479) | 1–6 |
[49] | 14 | 64–210 | 200–1,135 (median 559) | 1–6 |
13 | 100–200 | 96–711 (mean 200) | 1–6 (median 2) | |
[121] | 6 | 100–200 | 147–1,022 | 1–6 |
[91] | 5 | 97–197 (median 108) | 270–484 (median 373) | 1–4 |
[44] | 10 | 150 (mean 145) | 100–600 (mean 310) | 1–4 (median 2.1) |
[45] | 15 | 78.4–250 (mean 127) | 300–2,322 (mean 624) | 2–11 (median 3.5) |
[90] | *116 | 96–300 (mean 158) | 96–2,322 (mean 490) | 1–11 (mean 3.3) |
[69] | 15 | 100–300 (mean 188) | 200–1,592 (592 median) | 1–7 (mean 3.4) |
[76] | 12 | 124–149 (median 149) | 149–1,800 (median 1,065) | Up to 10 (median 7) |
16 | 200–350 (median 268) | 249–1,546 (median 651) | Up to 6 (median 2) | |
[75] | 6 | 200 | 200–1,200 (mean 640) | 1–6 (median 2.5) |
[43] | 19 | 100–700 (median 200) | 183–2,200 median (800) | 1–10 (median 3) |
[119] | 33 | 391 mean | 70–1,223 (mean 549) | 1–6 (median 1) |
[63] | 6 | 220–357 (mean 304) | 342–987 (mean 910) | 1–3 |
[80] | 12 | 5.6–18.3 mCi/kg (median 11.5 mCi/kg and 800) | 386–1,717 (median 1,015) | 1–3 (median 1.5) |
[14] | 50 | 6–19 mCi/kg (492–1,160), 500 if no stem cells (median 818) | 492–3,919 | 1–3 (median 1) |
Table 26.3
PHEO/PGL: response
Author | # patients | Objective CR (%) | Objective CR/PR (%) | Biochemical CR (%) | Biochemical CR/PR (%) | Symptomatic CR (%) | Symptomatic CR/PR (%) | Duration of response or survival |
|---|---|---|---|---|---|---|---|---|
[59] | 28 | 0 | 7 | 0 | 18 | NA | NA | Median PFS 18 months |
[49] | 14 | 0 | 14 | NA | NA | NA | 100 | NA |
[42] | 5 | 0 | 60 | 75 | 75 | 80 | 80 | Median OS > 50 months |
[91] | 5 | 0 | 40 | NA | 40 | NA | 40 | NA |
20 | 0 | 15 | 0 | 15 | 0 | 71 | Median OS 16 months | |
[44] | 10 | 0 | 30 | NA | 50 | NA | 50 | Median PFS 17.5 months |
[45] | 15 | 0 | 33 | 27 | 47 | NA | NA | Median TTP 36 months |
[90] | 116a | 4 | 30 | 13 | 45 | NA | 76 | Median PFS 19 months |
[69] | 15 | 0 | 62 | 22 | 89 | 7 | 93 | 5-year survival 85% |
[76] | Group 1 12/28 | 8.3 | 33 | 33 | 89 | NA | NA | Group 1 median response duration 1.9 years |
Group 2 16/28 | 12.5 | 25 | 29 | 67 | Group 2 median response duration 2.3 years | |||
[43] | 19 | 0 | 47 | 17 | 67 | 62 | 89 | Median PFS 24 months |
Median OS 42 months | ||||||||
[119] | 33 | NA | 38 | NA | 60 | NA | 86 | Median survival 4.7 years |
5-year survival 45% | ||||||||
Median OS 56 months | ||||||||
[63] | 6 | 0 | 83 | 25 | 75 | 50 | 83 | Median OS > 30.5 months |
[75] | 6 | 0 | 0 | 25 | 50 | NA | NA | Median TTP 12 months |
[80] | 12 | 18 | 36 | 22 | 56 | 50 | 90 | 15 months PFS |
Median symptomatic improvement | ||||||||
43 months | ||||||||
[14]a After first treatment | 50 | 9b | 27b | 19 | 35 | NA | NA | 64% 5-year OS |
17c | 50c | 47% 5 EFS |
The cumulative activity administered has ranged up to 85.9 GBq (2,322 mCi) in PHEO/PGL (Table 26.2), 78.1 GBq (2,112 mCi) in carcinoids (Table 26.4), and 89.3 GBq (2,414 mCi) or 31.325 GBq/kg (35.8 mCi/kg) in neuroblastoma (Tables 26.6 and 26.7).
Dosimetry
Several studies have reviewed the pharmacokinetics of 131I-MIBG. The pharmacokinetics of carrier-free 131I-MIBG in adults has been reported [22]—with alpha T1/2 of 3 h and beta T1/2 of 38.3 h. In adults the mean urinary excretion was 27.4% in the first 6 h, 49.8% in 24 h, and 80.3% in 120 h. Detailed pharmacokinetics in children with neuroblastoma has been published [52, 53, 86]. A terminal biologic T1/2 in serum of 45.8 h is reported. In addition, at 24 h there was a cumulative urinary excretion of 32%, 71% at 48 h and 65% at 6 days. MIRD dosimetry of 131I-MIBG [87, 88] identifies the main target organs as liver, myocardium, and gonads. Dosimetry of no-carrier added 131I-MIBG is somewhat different, with higher radiation dose per mCi to most normal organs than those described in the package insert for low specific activity 131I-MIBG [22]. While some of these dosimetry difference can be related to the specific activity, differences in methodology where self-irradiation is not considered likely plays a large role; dosimetry estimates performed from diagnostic scans are often significantly different from those estimated from the therapy dose. Fielding et al. found that estimates from the diagnostic scan would have resulted in under dosing 65% of the patients. Nonetheless, this was more reproducible than weight-based dosing [83, 89]. Using dosimetry from diagnostic scans, Lashford et al. predicted therapy doses to within −20–30% of that seen with the therapeutic dose [55]. It is not known what the required doses are to obtain biochemical or tumor responses. Estimates of radiation-absorbed dose to tumor vary considerably between patients. Tumor dose estimates of 10–40 cGy/37 MBq (10–40 cGy/mCi) have been reported [22 59], with cumulative doses ranging from 10 to 198 Gy [45, 65, 90, 91] in patients with PHEO/PGL. The impression has been that cumulative doses above 60 Gy are beneficial [45, 91]. Other studies suggested that doses of 150 Gy were necessary to obtain tumor response, but others believe 60–100 Gy is adequate [45, 70]. Because there is large variability in determining tumor dose due to inherent problems in quantification of single photon emitters, many studies use estimates of dose to whole body as a safe upper limit [59 83]. Whole body doses ranged from 50 to 231 Gy [12, 59].
Evaluation of Response
Response assessment may be performed as early as 2 months [63] but more frequently at 3–6-month intervals unless the patient is known to have fast growing disease [14, 37, 43, 44]. Follow-up typically involves anatomic imaging such as CT or MRI to evaluate changes in lesion size. Functional imaging is performed using 123I-MIBG and, in known [18F]FDG-avid lesions (Fig. 26.1b), changes in SUV may be an indication of response, although no such studies have yet been reported. In hormone secreting tumors, biochemical parameters can be used to assess response (Fig. 26.1c).
Following therapy, response is usually evaluated at 8–24 weeks. As in many other cancers, response is determined using size measurement criteria, most often using WHO criteria [46, 62, 68, 92]. WHO criteria for complete response include the disappearance of all lesions and negative 123I-MIBG scans; partial response is a 50% or greater reduction of all measurable disease; and progression is the appearance of new lesions or an increase of 25% or more in tumor size. In PHEO/PGL, because most patients have elevated catecholamines, levels of these and their metabolites are also used to gauge response. Biochemical CR is defined as complete normalization of biochemical markers, partial response typically by ≥50% drop but not normalization, and progression by ≥20% increase in these levels (Fig. 26.1c).
In neuroblastoma, posttreatment diagnostic 123I-MIBG scintigraphy is routinely used to assess response (Fig. 26.2). Patients undergoing high-dose therapy invariably have significant hematologic toxicity, and stem cell harvesting is routinely performed in these patients. The parameter that determines if the patient needs stem cell transplant is the duration and resistance of the hematologic toxicity to respond to supportive treatment. When transplantation is required it is usually not performed for at least 3 weeks post-131I-MIBG treatment [37, 57, 82].
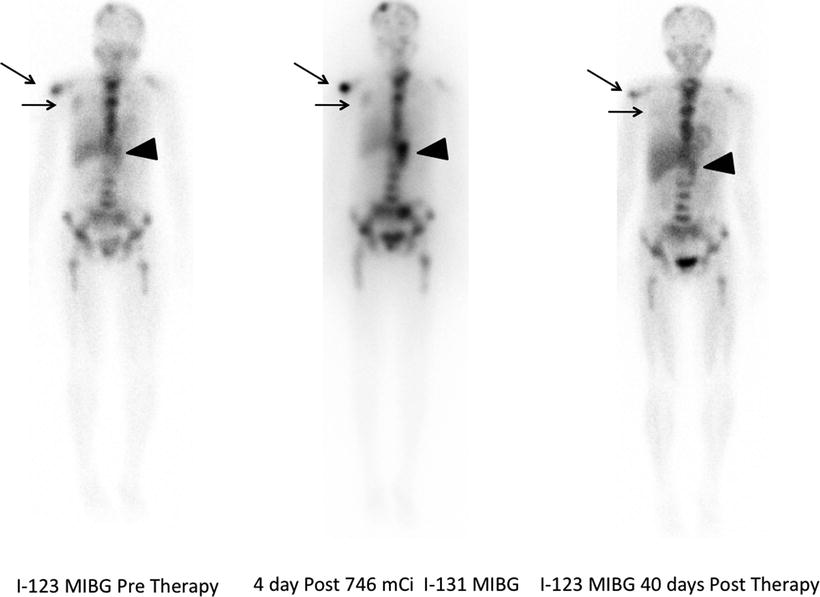

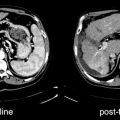
Fig. 26.2
A 26-year-old woman with neuroblastoma. 123I-MIBG diagnostic scan (left panel) pre-therapy shows extensive bone disease (arrows) and left adrenal mass (arrowhead). After receiving 27.6 GBq (746 MBq) 131I-MIBG, she was imaged at 4 days (middle panel). This showed excellent targeting of sites of disease. A follow-up 123I-MIBG scan (right panel) showed that, in spite of excellent tumor targeting, there was little response with only minor decreases in uptake in the left scapula and humerus compared to baseline
Pheochromocytoma/Paraganglioma
PHEO/PGL are tumors that originate in chromaffin tissue of the adrenal medulla or sympathetic nervous system. The incidence of PHEO/PGL is estimated to be ∼1–2 per million [93, 94], although in autopsy series an incidence of 1 in 2,031 cases has been reported [95]. These tumors are most often found in the adrenal and in this location are referred to as PHEO. If they occur outside of the adrenal they are referred to as PGL. Three percent of incidentalomas are PHEO [96]. These tumors often secrete catecholamines and consequently, patients present with hypertension and other symptoms of adrenergic excess. In some instances, however, they are nonfunctional and presentation is related to mass effect or incidental findings. While as many as 75% of PHEO/PGL are sporadic, some are related to genetic defects [97–99]. These familial diseases are usually associated with one of six susceptibility genes: RET, SDH (D, B, C), NF1, and VHL and several new genes. These genetic disorders have typical presentations and several are associated with an increased incidence of malignant and/or extra-adrenal disease. Although most PHEO/PGL are benign, 36% of extra-adrenal PGL are malignant [100]. Pathologically it is difficult to differentiate benign from malignant tumors because of lack of robust morphological and histological criteria [101]. Therefore, in order to classify PHEO/PGL as malignant, one must find disease in sites where this tissue is not normally present [102]. The most common sites of metastatic disease include lymph nodes, bone, liver, and lung [103].
The most sensitive tests to confirm the diagnosis of PHEO/PGL are plasma-free metanephrines or urinary fractionated metanephrines, with sensitivities of 97% and 99%, respectively [104, 105]. The diagnostic imaging modalities most often used for staging are CT and MRI; functional imaging modalities are also useful, including radiolabeled MIBG (Fig. 26.1a), 18F-fluorodopamine PET, 18F-DOPA PET, [18F]FDG PET (Fig. 26.1b), and 111In-pentetreotide scintigraphy [8, 106]. The optimal therapy for PHEO/PGL consists of surgical resection when feasible. Even in patients with metastatic disease, surgical debulking is often used with palliative intent, although no controlled studies have been performed to show an effect on survival. Patients with benign disease have normal overall survival, whereas patients with malignant disease typically have an overall 5-year survival of less than 50% [107–109]. Nonetheless, it is recognized that there is a subset of patients with malignant disease who have a more indolent course and may live >20 years with limited therapy [110]. Patients that are asymptomatic and have slow growth rates may opt for observation until symptoms require therapy.
Averbuch et al. evaluated the use of cyclophosphamide, vincristine, and dacarbazine (CVD) for metastatic disease [111]. In 14 patients with malignant PGL they observed a complete or partial response in 57%, with median duration of 21 months. A second analysis of this data expanded to 18 patients was performed with long-term follow-up of 22 years [112]. A CR was present in 11% of the patients; 44% had a partial response, and 72% had a biochemical response with a median duration of 20 months. Nonetheless, 16 of the 18 patients died of their disease and one was lost to follow-up. Patients that responded had a median survival of 3.8 years compared to 1.8 years for nonresponders. The common toxicities were myelosuppression, peripheral neuropathy, and gastrointestinal toxicity. Overall, the treatment was well tolerated as evidenced by the ability to administer a median of 23 cycles. These authors conclude that the treatment is not for all patients with metastatic disease but can be considered when patients are symptomatic or tumor shrinkage is desirable. Results in other small series or case reports have shown similar findings, with remissions typically lasting less than 2 years [113]. CVD have become the most often-used first-line chemotherapy regimen for metastatic disease, although other regimens have also been utilized [114, 115]. Given the suboptimal response to chemotherapy, newer therapeutic modalities such as 131I-MIBG therapy are being applied in patients with PHEO/PGL. Patients with disease limited to the liver have also been treated successfully with RFA ablation [116]. In patients with rapidly progressive disease, chemotherapy with the CVD regimen is often used first.
There has not been a systematic evaluation comparing regimens using low, mid, or high doses of 131I-MIBG in PHEO/PGL. Initial reports, first by Vetter et al. [117] and followed shortly by a report of treatment of five patients at the University of Michigan [91] appeared in the early 1980s. In the latter report, patients were treated with total activities ranging from 9.99 to 17.91 GBq (270-484 mCi), resulting in 13–120 Gy to tumor [91]. A significant reduction in tumor size was observed. Since then, a variety of dose ranges have been used. Individual doses ranging from low activity (2.96–≤7.4 GBq, or 80–200 mCi), intermediate (up to 18.5 GBq, or 500 mCi), to high activities that are often weight-based (444–666 GBq/kg, or 12–18 mCi/kg) have been used (Table 26.2). Strategies using low–intermediate activity rely on repeat treatment, with which cumulative activities as high as 85.1 GBq (2.3 Ci) have been reported, although median cumulative activities are generally in the 18.5-37 GBq (500–1,000 mCi) range (Table 26.2). The UCSF group has been a proponent of high doses with stem cell support, based on a phase I dose escalation trial [14, 80, 118]. Activities >12 mCi/kg usually require having stem cells available for transplantation.
A meta-analysis of publications from 1983 to 1997 on 116 patients treated with 131I-MIBG has been reported [90]. These studies used a mean single activity of 5.85 GB (range 3.55-11.1 GBq; mean 158 mCi, range 96-300 mCi) with a mean of 3.3 (1–11) repeated treatments, resulting in cumulative activities of 3.55-8.59 GBq (mean of 18.11 GBq; range 96-2,322 mCi, mean of 490 mCi). Although these retrospective studies had different methodologies, clear therapeutic results were observed using what are now regarded as relatively low activities. Of the 116 patients, 4% had an objective CR and 26% had partial response (PR). Biochemical catecholamine response demonstrated complete normalization in 13% of 96 patients, 32% had >50% improvement, and 76% of 99 patients had improvement in symptoms.
Other reports describe results with low (median below 7.4 GBq, or 200 mCi) [43, 44, 76], intermediate (median above ≥7.4 GBq, or above 200 mCi) [63, 75, 76, 119], and high activities (>44.4 MBq/kg, or >12 mCi/kg) (Table 26.3) [14, 80]. A retrospective review of the Duke experience in 33 patients treated with 131I-MIBG with cumulative activities ranging from 2.59 to 42.25 GBq, or 70-1,223 mCi (mean individual activity 14.47 GBq, or 391 mCi demonstrated an objective response rate of 38%, a symptomatic response rate of 86%, and a biochemical response rate of 60%. Responders had a median survival of 4.7 years compared to 1.7 years for nonresponders. Patients receiving high-activity therapy (>18.5 GBq, or 500 mCi) had a survival advantage with median survival of 3.8 years versus 2.6 years for the low-activity group [119].
A prospective study using high-dose therapy, median individual activity of 30.27 GBq (818 mCi), showed a CR of 8% and objective response in 36%. In this group, the majority of the patients only received a single administration, with a small proportion receiving two or three treatments. Because of the expected myelotoxicity, peripheral stem cells were harvested, although some patients recovered adequate marrow function and did not require stem cell infusion [14].
The use of high-specific activity 131I-MIBG for therapy was recently evaluated in a Phase I clinical trial [22]. In a group of 11 patients receiving no carrier added 131I-MIBG, no side effects were observed related to the injection. Estimated tumor doses ranged from 11 to 40 rad/37 MBq (11-40 rad/mCi). In this phase I study, activities ranged from 222 to 333 MBq/kg (6-9 mCi/kg) with a maximum activity of 24.98 GBq (675 mCi).
When comparing objective responses in different studies where patients received median activity of approximately 7.4, 14.47, or 30.27 GBq (20, 391, or 818 mCi), the observed response rates overlap, in the range of 20–40% (Table 26.3) [14, 43, 119]. Within a single institution, Castellani et al. reviewed patients treated with a median activity of 5.51 GBq (149 mCi) versus 9.92 GBq (268 mCi). Their conclusion was that in these ranges, higher activities could deliver the desired dose faster, with a modest increase in toxicity and similar overall response rates [76].
Several protocols have combined chemotherapy with 131I-MIBG therapy in neuroblastoma, but there has been only one such prospective report in patients with PHEO [15]. In this small series, six patients underwent dosimetry and were scheduled to receive three 131I-MIBG treatments at 3-month intervals with activities estimated to deliver 85–90 cGy to the whole body per single administration. The patients would then start the CVD chemotherapy regimen [111]. Three of the six patients had improvement in tumor following 131I-MIBG therapy. Two patients completed 9 months of sequential chemotherapy with further improvement and were considered to have partial response. Toxicity from 131I-MIBG was minimal but forced reduction of chemotherapy doses in some patients.
The most frequent sites of metastatic disease include lymph nodes, bone, lung, and liver. There is suggestion that soft tissue disease response to 131I-MIBG therapy is superior to that of bone [45, 91, 120]. It also appears that patients with smaller volume disease following surgical resection are more likely to respond.
In summary, high-quality data on the use of 131I-MIBG therapy in PHEO/PGL is limited, with few prospective trials, and there is currently no consensus on an optimal dosing strategy. Differences in patient selection and follow-up may account for differences in response rates and survival. It is clear that many patients will benefit from treatment, and objective responses are often observed, but complete response rates are low, ranging from 0 to 18%. The duration of responses is also variable, with 5-year survivals between 45 and 85% [69, 119]. There is some suggestion that higher activities >18.5 GBq (500 mCi) or myeloablative regimens may be associated with higher response rates. However, there are no direct comparisons to determine if fractionated smaller activities, that are associated with lesser toxicity, result in different overall survival. Nonetheless, the ease of performing this therapy, the evidence of objective responses, symptomatic improvement, biochemical improvement, and the limited toxicity and few late effects compared to other chemotherapy and external beam regimens make this an acceptable strategy in patients with metastatic PHEO/PGL (Tables 26.2 and 26.3).
Carcinoid
131I-MIBG is used most frequently to treat neuroblastoma and PHEO/PGL. The third largest experience is with carcinoid tumors (Tables 26.4 and 26.5) [46, 58]. Carcinoids are slow-growing NETs that often secrete substances leading to hormonal syndromes. These tumors are rare, with incidence in the range of two cases per 100,000 [122, 123]. The most frequent sites for carcinoids are the gastrointestinal (GI) tract (73.7%) and the bronchopulmonary system (25.1%). Within the GI tract, most occur in the small bowel (28.7%), appendix (18.9%), rectum (12.6%), and stomach (6%). Although 45% exhibit metastases at the time of diagnosis, the overall 5-year survival is 50–67% [123, 124]. Higher incidences of carcinoids are found in patients with MEN1, VHL, and neurofibromatosis. In 2000, recognizing the heterogeneity of presentation, prognosis, and outcome, the WHO developed a classification system for NET reflecting a better understanding of cell biology, therapeutic implications, and pathology [125]. Three types of NET were defined: (1) well-differentiated NET (benign behavior, low grade), (2) well-differentiated neuroendocrine carcinoma (low-grade malignant), and (3) poorly differentiated neuroendocrine carcinoma (high-grade malignant). Because this classification is relatively new, most reports of 131I-MIBG therapy in carcinoid have not incorporated this classification.
Table 26.4
Carcinoid: treatment schema (administered activities are listed here in mCi; multiply by 37 for conversion to MBq)
Author | # patients | Single administration (median) (mCi) | Cumulative (mCi) | # doses |
|---|---|---|---|---|
[58] | 25a | 54–91 | 54–635 (median 300) | 1–11 |
[68] | 32a | 157–554 (median 381) | 157–554 (median 381) | 1 |
[135] | 58 | 2.5 mCi/kg capped at 150 | Median 182 mCi | Mean 2.8 |
[69] | 18 | 70–220 (median 190) | 200–1,592 mCi (median 600) | 1–8 (median 3.2) |
[133] | 98 | 77–500 (median 306) | 77–1,076 mCi (median 401) | 1–3 (mean 1.2) |
[46] | 13a | 2.5 mCi/kg (median 149) | 85–2,112 (median 594) | 1–7 |
[39] | 12 | 200 Initial single dose | 1 | |
[134] | 48a | 200 Each cycle | 200–800 mCi (mean ∼362) | 1–4 (mean 1.8) |
[67] | 30 | 200 | 200–600 | 1–3 (mean 1.8) |
Table 26.5
Carcinoid: response
Author | # patients | Objective CR (%) | Objective CR/PR (%) | Biochemical CR (%) | Biochemical CR/PR (%) | Symptomatic CR (%) | Symptomatic CR/PR (%) | Duration of response or survival |
|---|---|---|---|---|---|---|---|---|
[39] | 12 | 0 | 17 | NA | NA | 58 | 83 | Mean symptom response 15.4 months |
[135] | 58 | NA | NA | NA | NA | NA | NA | 5-year survival 63% |
Median OS 8.3 years | ||||||||
[69] | 18 | 0 | 11 | 15 | 38 | 20 | 73 | 5-year survival 78% |
[133] | 98 | 0 | 15 | 6 | 37 | NA | 49 | 5-year survival 22% |
Median OS 2.3 years | ||||||||
[67] | 30 | 0 | 0 | 0 | 13 | 0 | 60 | 3-year survival 22% |
Median OS 29 months | ||||||||
Median symptom response 8 months | ||||||||
[58] | 25a | 0 | 35 | NA | NA | NA | NA | Median PFS 14.5 months |
Median OS 18 months | ||||||||
[46] | 13b | 8 | 15 | 0 | 15 | 0 | 92% | Mean symptom response 17 months |
[134] | 48c | 0 | 28 | NA | 37 | NA | 56% | Median survival of symptomatic responder 59 months |
Nonresponder 22 months | ||||||||
[127] | 52d | 4 | 15
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|