(1)
Radiology Department Beijing You’an Hospital, Capital Medical University, Beijing, People’s Republic of China
Abstract
Innate immunity is the first line of defense in infection control. The key to control HIV infection and its progression lies in the host’s immune defense. The immunity against infections can be divided into two types, non-specific immunity, also referred to as the innate immunity, and specific immunity, also known as adaptive/acquired immunity.
Keywords
Non-specific immunitySpecific immunityCytokinesChemotactic factorsAllergic responseImmunity reconstruction2.1 Defense Mechanism of Organisms
2.1.1 Non-specific Immunity
Innate immunity is the first line of defense in infection control. The key to control HIV infection and its progression lies in the host’s immune defense. The immunity against infections can be divided into two types, non-specific immunity, also referred to as the innate immunity, and specific immunity, also known as adaptive/acquired immunity.
Innate immunity is already equipped at the time of birth. As the first line of defense, innate immunity has the ability to protect the body rapidly against infection and to trigger adaptive immune responses. The innate immunity consists of skin and mucosal epithelia, phagocytes and NK cells, as well as a series of soluble factors, such as cytokines, chemokines, and small molecular substances, like complements and Mannan-binding lectin (MBL) (see Table 2.1). All these factors constitute the rapid response system to protect human body against infections, whose functions to prevent the spreading of infections.
Table 2.1
Components of the innate immunity
Cellular component | Soluble factors |
|---|---|
Dentritic cells | Cytokines |
Macrophages | Chemokines |
Neutrophils | Definsins |
NK cells | APOBEC |
γδT cells | Complement |
NK-T cells | Lectin-binding proteins |
Plasmacytoid dentritic cells | Acute-phase reactants |
CD8+T cells | Mannan-binding lectin, MBL |
B-1 cells |
As antigen presenting cells, dentritic cells and mononuclear cells play roles in both innate and specific immunity. In addition to the natural barrier of skin mucosa, and cells components mentioned above, the innate immunity also includes some soluble factors. These factors either directly involve in fighting microorganisms, such as IFN-α/β or indirectly via reinforcing cell reactions, such as NK cells reaction. The innate immunity also enhances the expression of MHC in effector cells to strengthen the immunity of lymphocytes against pathogens. In addition, innate immunity participates in the activation of intracellular signal bypass, which is achieved by the recognition of the receptors at the surface of antigen presenting cells, namely TLRs (Toll-like receptors). As a result, the NFĸB (nuclear factor-activated T cells) are activated to induce the expression of cytokine with different immunologic functions [1].
Other differences between innate immunity and specific immunity include: (1) Innate immunity has a quick response (within minutes to days), while specific immunity is a delayed reaction (within days to weeks); (2) Innate immunity is a direct reaction against infection, while specific immunity targets at the specific antigen peptide of pathogens; (3) Innate immunity is non-specific, with no immunological memory, which can be repeatedly activated by appropriate factors, while specific immunity has immunological memory, with quicker and stronger responses to the recurrent infections.
2.1.2 Specific Immunity
The primary infection of HIV usually causes a strong specific immune response in human body. The specific immune response can be categorized into humoral immune response and cellular immune response.
2.1.2.1 Humoral Immune Response
Infections of HIV proteins can stimulate the human body to produce antibodies. Generally, after 2–12 weeks of HIV infection, B cells can produce a variety of specific antibodies against HIV proteins. Antibodies to IgM are firstly produced, targeting at the regions of gag and env, but with a brief persistence. Antibodies to IgG are produced slightly later, firstly those targeting at regions of p24, gpl20, gp160 and p41, with a longer persistence. Neutralizing antibodies are produced later on, with only antibodies against envelope proteins having the anti-viral neutralizing activity. With the emergence of large amount antibodies, the viruses are largely cleared; and others are captured by the dendritic cells in lymph nodes. With the decreased virus in blood, the primary HIV infection progresses into chronic infection. Generally, after neutralizing antibodies are produced in virus infections, they can bind to and eliminate the viruses. Therefore, this neutralizing activity is commonly used to assess the anti-viral immunity. But HIV-infected patients largely have low concentration and affinity of neutralizing antibody, which plays a protective role in early phase of infection or in long-term non-progressive infection. Enhancing antibodies are also produced in HIV-infected patients, which contribute to viral infections. And even some neutralizing antibodies have enhanced activity after diluted. In addition, neutralizing antibodies with the same specificity in different individuals may show a opposite activity to inhibit or enhance HIV infections. Therefore, simple use of antibodies or neutralizing antibodies production cannot sufficiently assess the immunity against HIV.
2.1.2.2 Cellular Immune Response
In HIV infected patients, cell-mediated immune response plays an important role in the control and removal of the infectious agents. Cellular immune responses mainly include CD4+ T lymphocytes immune response, the cytotoxic effect of cytotoxic T lymphocytes (CTL), natural killer cells (NK) immune response and CD8+ cell-mediated non-cytotoxic antiviral effects. Various HIV structures and regulatory proteins (ENV, GAG, POL, NFF and RT) can cause specific cellular immune response. But unlike antibody reactivity, the strength and range of the cellular immune response have obvious individual differences.
2.1.3 Cytokines
In addition to the direct regulation of HIV expression or latency in virus infected cells, cytokines play a more important role in the modulation of immune responses and maintenance of immune balance. Many cytokines can up-regulate the expression of HIV [2, 3]; some cytokines, such as GM-CSFB, can induce the expression of proviral HIV during the incubation period. TNF-α can up-regulate the expression of HIV by means of paracrine and exocrine. TNF-α and IL-6 have a synergistic and promoting effect on the expression of HIV in mononuclear cells. Among the cytokines down-regulating the expression of HIV, IFN-α, IFN-β and IL-10 play definitive roles. But IFN-γ, TCF-β, IL-4 and IL-13 demonstrate two different effects according to the differences in target cells, their activations and differentiations, status of virus infection, as well as interactions between different cytokines [4, 5]. Cytokines have multiple functions in the human body. In addition to increasing the expression of HIV, TNF-α is also an important inflammatory mediator and cachectin, which exerts an important role in pathogenesis of AIDS. In addition to promoting the expression of HIV and inducing cachectin, IL-6 is also related to the hyper-reaction of another immune characterized B cells in HIV infections. Additionally, IL-6 is related to the occurrence of B lymphocytes sarcoma.
2.1.4 Chemotactic Factors (CF)
Chemokine is a class of small molecules belonging to the super-family of cytokines. Chemokines and their receptors play important roles in the occurrence and development of many pathologic and physiologic processes, such as inflammation (including allergic inflammation), removal of the exterior pathogens, development of tissues and organs, the growth and metastasis of carcinomas, and immunodeficiency diseases. It was firstly discovered in 1996 that the chemokine receptor CXCR4 is the facilitative receptor for T-tropic HIV-1 invading T cells [6]. After that, it was discovered that CCR5 is the facilitative receptor for macrophage tropic HIV-1 infecting macrophage. Dean and his colleagues [7] have proved that the genetic mutation of CCR5 is associated with highly exposed and low-level infected populations. Studies in recent years have shown that some chemokines have an anti-HIV effect [8] (Fig. 2.1). HIV infection needs facilitations by chemokines receptors whose genetic mutation may change the susceptibility and the outcomes of HIV infection. On one hand, chemokines block the HIV-1 binding site by binding specifically with facilitative receptors. On the other hand, chemokines can down-regulate the expression of their facilitative receptors on the surface of HIV-1 susceptible cells. Therefore, chemokines and its derivatives are main components of facilitative receptor blockers.
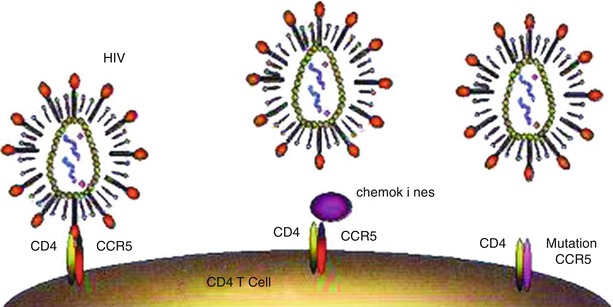

Fig. 2.1
Chemokine receptors and HIV infection. (a) T cells expressing receptors CD4 and CCR5 can be infected by HIV. (b) Chemokines can block the binding of HIV with CCR5. (c) Failed binding with mutated CCR5, no infection of T cells
2.2 Mechanism of Immune Response
2.2.1 Antiviral Immune Response
The innate immunity is the first line of defense for the human body to protect itself against HIV. Various innate immune cells and soluble factors play important roles in fighting against HIV infection. Most cytokines and substances from body fluids, such as saliva, tears and milk, are the components of innate immune system. Macrophages and dentritic cells bridge between the innate immunity and the acquired immunity. The non-cytotoxic anti-HIV effects of CD8+ cells have characteristics of innate immune response. Studies have demonstrated that astrocytes, DCs, macrophages, microglial cells and some soluble factors (like complements and chemokines) can interfere and impact on central nervous system diseases caused by HIV. Inducing the innate immune activities with specific cytokines and their complexes can effectively slow the progression of AIDS. The conventional reaction of the hosts to virus infection is to produce antibodies, which bind with viruses to inactivate them. The major viral proteins associated with neutralization of antibodies locate on the exterior membrane of gp120 and gp41. According to studies, anti-gp120 antibody has selective reactivity to B subtype of HIV-1 and anti-gp41 antibody has reactivity to a wider subtype of HIV-1. The mechanism of antibody-mediated HIV neutralization is not clear yet. The capability of antibody to cover the envelope or to make the envelope protein shed from virion may determine the neutralizing ability of the antibody. Some CD4+ cells have CTL activity specific to virus infected or uninfected CD4+ cells or MHC-II molecules related HIV expressions of polypeptide cells. But its antiviral reaction is at a relatively low level. A majority of cells capable of specifically removing virus-infected cells are CD8+ cells, whose toxicity killing reaction is characterized by HLA restrictions and antigenic specificity. In addition, its toxicity killing reaction requires intercellular interactions. The HIV specific cytotoxicity is mainly achieved by CD8+ effector cells secreted by IFN-γ and TNF-α, especially the cellular subsets expressing perforin and CD107.
2.2.2 Antibacterial Immune Response
Bacterial infection is the common opportunistic infection in patients with HIV/AIDS, and it is also one of the major causes of their clinic visits and death. Mycobacterial disease, bacterial pneumonia, skin and intestinal bacterial infections and tuberculous meningitis are commonly encountered bacterial infections. Neutrophils and phagocytosis of neutrophils, mononuclear cells and macrophages as well as activation of complement system play important roles in eliminating bacteria. Bacterial endotoxin stimulates macrophages and vascular endothelial cells to produce inflammatory factors such as TNF, IL-1, IL-6 and chemokines, which can promote inflammatory responses and facilitate eliminating bacteria. Exocellular bacterial antigen can directly stimulate B cells to induce protective humoral immunity. Responses to main T cells of extracellular bacteria include CD4+ cells’ response to MHC-II molecules binding proteins. The main immune responses to extracellular bacteria, such as mycobacterium, are mediated by CD4+ and CD8+ T cells. By producing IFN-γ, macrophages are activated to enhance their activities of phagocytosis, degradation and sterilization.
2.2.3 Antiparasitic Immune Response
CD4 T cell count obviously decreases in patients with HIV/AIDS. Some opportunistic parasites may cause serious and even fatal infections. Opportunistic parasites that cause intestinal infections in patients with AIDS include cryptosporidium, isospora, strongyloides stercoralis and microsporidia. Those cause pulmonary infections include pneumocystis and microsporidia. Those cause eyes infections include toxoplasma gondii, acanthamoeba and microsporidia. Those cause cerebral infections include toxoplasma gondii and acanthamoeba. In addition, toxoplasma gondii, strongyloides stercoralis and microsporidia can also cause general disseminated infections. Parasites gaining access to the blood circulation or tissue can adapt to and resist to the body’s innate immunity for their survival and replication. Specific immunity is the principal mechanism for anti-parasitic infection. Due to different structures and biochemical properties of different parasites, they induce different specific immune responses, including IgE medicated cytotoxicity depending on eosinophils, granuloma formation and fibrosis, specific CTL response and the effects of cytokines.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree