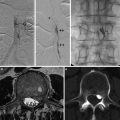
Fig. 1
Different patterns of intracranial hemorrhage related to aneurysmal rupture. (a) Typical subarachnoid hemorrhage in a patient with an AComm aneurysm. Spontaneous hyperdensities are seen within the cortical sulci. Note a thickener amount of blood in the interhemispheric fissure, close to the ruptured aneurysm (arrow). (b) Intraparenchymal hemorrhage related to a right MCA aneurysm rupture. (c) Acute subdural hematoma related to a calcified left MCA aneurysm rupture
Table 1
World Federation of Neurological Surgeons (WFNS) scale
Grade | Glasgow Coma Scale | Motor deficit |
|---|---|---|
1 | 15 | Absent |
2 | 13–14 | Absent |
3 | 13–14 | Present |
4 | 7–12 | Present or absent |
5 | 3–6 | Present or absent |
Table 2
Hunt and Hess scale
I | Asymptomatic or minimal headache and slight nuchal rigidity |
II | Moderate to severe headache, nuchal rigidity, no neurological deficit (except cranial nerve palsy) |
III | Drowsiness, confusion, or mild focal deficit |
IV | Stupor, moderate or severe hemiparesis, possible early decerebrate rigidity, and vegetative disturbance |
V | Deep coma, decerebrate rigidity, moribund |
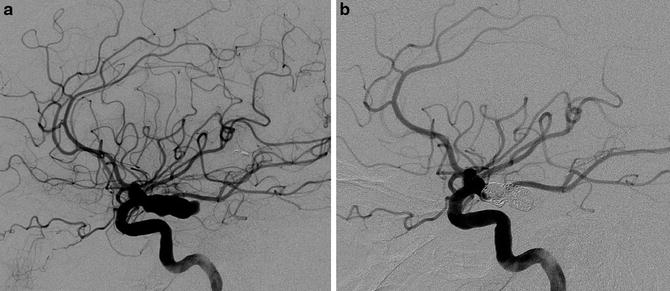
Fig. 2
A 59-year-old female presenting with severe headache and acute CN III palsy. (a) Left ICA digital subtraction angiography in lateral projection showing a 17 mm PComm aneurysm. (b) Control DSA in lateral projection after embolization showing a satisfactory occlusion of the aneurysm’s sac and the patency of the PComm. The CN III palsy completely recovered 5 weeks after the symptoms’ onset
In case of large or giant aneurysms, especially on the carotid siphon, IC aneurysms may be revealed by progressive CN palsy (oculomotor palsy or trigeminal neuralgia) [26]. These symptoms are related to the mass effect and the edema that may compress/irritate the CNs of the cavernous sinus (Fig. 3) [27]. ICA giant/large aneurysms may also be revealed by epistaxis when an erosion of the wall of the sphenoid sinus is present – the aneurysm presenting a risk of rupture in the nasal cavities, leading to a potentially cataclysmic and life-threatening nosebleeding [28]. Finally, headache may reveal intracranial aneurysms. However, the relationship between the pain and the aneurysm is often difficult to establish.
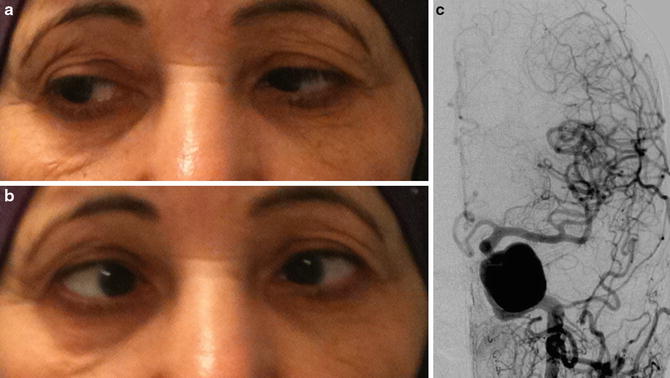

Fig. 3
Giant left cavernous ICA aneurysm revealed by a CN VI palsy in a 68-year-old female. (a) and (b) photographs showing the left CN VI palsy: (a) right position gaze and (b) left position gaze. Abduction deficit of the left eye is seen in left position gaze. (c) Left ICA DSA in AP projection showing the giant cavernous ICA aneurysm
Diagnostic Imaging
Diagnosis of SAH
Unenhanced computed tomography (CT) is the best imaging option at the acute phase of the SAH. Indeed, the sensitivity of CT scan to detect SAH is over 98 % within the first 48 h following the hemorrhage [29]. It appears with a typical pattern of spontaneous hyperdensity in the subarachnoid space that insinuates inside the cortical sulci. In case of massive hemorrhage, intraventricular inundation may also be seen. It is noteworthy that the thickest amount of blood may orientate to the location of the aneurysm. Finally, CT scan may also help to depict associated hydrocephalus. However, this sensitivity of CT for SAH progressively decreases with time (58 % at 5 days, 50 % at 1 week) [30]. Brain magnetic resonance imaging (MRI) may increase the sensitivity of CT scan for the detection of SAH, especially few days after the rupture.
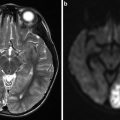
MRI is rarely performed in suspected SAH. Indeed, the accessibility of MRI is far lower than that of CT scan. The best sequence to depict SAH is the T2*WI. SAH will appear as a hypointense signal within the cortical sulci (Fig. 4a). The interest of this sequence is also to depict SAH at the late phase [31]. Fluid-attenuated inversion recovery (FLAIR) sequence is also helpful do depict SAH showing a hyperintense signal within the cortical sulci (Fig. 4b). However, even if FLAIR-WI may be positive for SAH at the acute phase whereas CT scan is negative, this sequence is not the panacea for SAH diagnosis. Indeed, the FLAIR-WI will be positive in case of negative CT scan in only less than 20 % of the cases [32]. A recent study has nonetheless showed that an MRI protocol combining FLAIR-WI and susceptibility-WI (SWI) had a high sensitivity for the depiction of SAH [33].
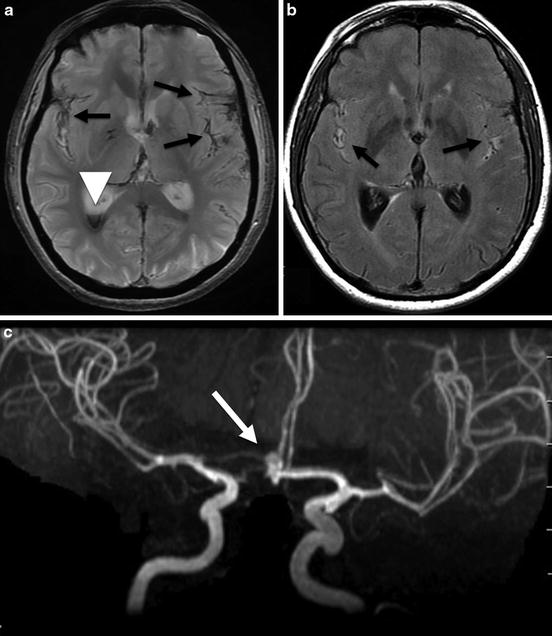

Fig. 4
Brain MRI in a 69-year-old female presenting with an aneurysmal subarachnoid hemorrhage. (a) Axial T2*WI showing hypointense signal within the subarachnoid space (black arrows) corresponding to subarachnoid hemorrhage and hypointense signal in the occipital horn of the right lateral ventricle (white arrow head). (b) Axial fluid-attenuated inversion recovery-WI showing hyperintense signal in the subarachnoid space of both sylvian fissures (black arrows). (c) MR angiography; 3D TOF showing an AComm aneurysm (arrow)
Despite the high sensitivity of these various imaging techniques, the gold standard exam for the positive diagnosis of SAH remains the lumbar puncture (LP) [34, 35].
Indeed, LP will find incoagulable blood in the CSF and products of hemoglobin breakdown, confirming the diagnosis of SAH [36].
Finally, even if aneurysmal rupture is the most frequent cause of spontaneous SAH, one should keep in mind that the most frequent cause of SAH is trauma. Other causes of SAH should not be missed when the presence of an aneurysm has been ruled out, like cerebral vein thrombosis, amyloid angiopathy, or rupture of an intracranial vascular malformation [37]. It is noteworthy that in case of brain arteriovenous malformation (bAVM) rupture, the SAH is often associated with an intraparenchymal hemorrhage [38].
Imaging Modalities for the Depiction of IC Aneurysms
The aim of vascular diagnostic imaging is to depict the IC aneurysm responsible for the SAH. The presence of other IC aneurysms on the circle of Willis should always be looked for, since IC aneurysms are multiple in more than 25 % of the cases. Additionally, cerebral vasospasm should be assessed on vascular imaging, especially for patients admitted few days after the SAH. Finally, differential diagnosis, like brain AVM or cerebral vein thrombosis, will be searched on vascular imaging if no IC aneurysm is found.
CT angiography (CTA) is a valuable tool for the detection of IC aneurysms [39–41]. This exam can be performed just after the CT scan that authenticated the SAH. It requires an iodinated contrast media injection via a venous access (most of time antebrachial). Its sensitivity for the detection of IC aneurysms is about 95 % [39]. However, very small aneurysms (<3 mm) and aneurysms close to the skull base may be missed by CTA [42]. It is noteworthy that the continuous improvement of CT technologies increases the sensitivity of CTA for the depiction of IC aneurysms [43]. The other limitations for CTA are due to the fact that it delivers X-rays, which limits its use for pregnant women or children, and due to the potential risk of allergy to the iodinated contrast media.
Three-dimensional (3D) time-of-flight (TOF) magnetic resonance angiography (MRA) has a lower spatial resolution than CTA. However, due to the fact that it does not require contrast media injection, this sequence may be interesting for patients with renal insufficiency or in pregnant patients [44].
Finally, digital subtraction angiography (DSA) is the gold standard exam for the visualization of IC aneurysms. Indeed, its spatial resolution is very high: 200 μm. Moreover, 3D rotational angiography developed in the early 2000s, by providing a manipulable volume, increased dramatically the understanding of IC aneurysms’ anatomy and is very helpful to tailor their treatment [45]. Thus, DSA is the best imaging modality to depict very small aneurysms and to see accurately and precisely small branches originating from the neck or from the parent artery close to the aneurysm (Fig. 5). Except in case of extreme emergency (e.g., ruptured MCA aneurysm with clinically non-tolerated hematoma requiring surgical evacuation), DSA should always be performed before treatment (either endovascular or surgical) of an IC aneurysm. Indeed, DSA helps to clearly demonstrate the shape, size, neck, and relationships of the aneurysm with the parent artery. DSA should always be performed via the four supra-aortic arterial vessels (i.e., both ICAs and both vertebral arteries) in order not to miss multiple aneurysms on the circle of Willis.
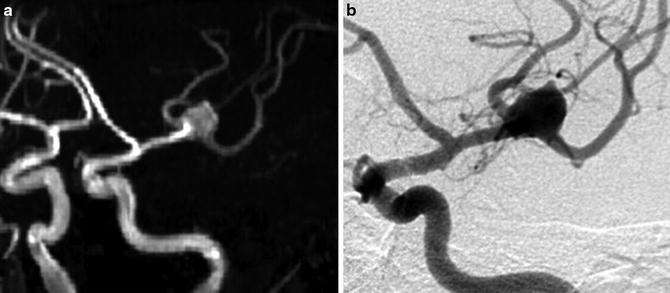

Fig. 5
Comparison of 3D time-of-flight (TOF) MR angiography (a) and digital subtraction angiography (DSA) (b) in left oblique anterior projection for visualization of a left bifurcation MCA aneurysm. Note that on DSA, the small branches close to the aneurysm are more precisely seen than on 3D TOF MRA
DSA-Negative SAH
DSA-negative SAHs are defined as proven SAHs with negative initial DSA for the diagnosis of IC aneurysm or other IC vascular malformations [46]. The frequency of such SAH is as high as about 20 % of the cases [47]. This subgroup of SAH is divided into perimesencephalic and non-perimesencephalic (i.e., diffuse, cortical, or xanthochromia only) SAHs. In perimesencephalic SAH overall diagnostic yield of repeat angiography is 0 %, while it reaches 12.5 % in non-perimesencephalic ones [48]. Even if DSA-negative SAHs have a better prognosis than aSAH, and despite the very low rate of rebleeding, in such SAH delayed complications may be observed like hydrocephalus or arterial vasospasm [47].
Treatment
General Considerations on Ruptured Intracranial Aneurysms
The first-line treatment for ruptured IC aneurysms, since the publication of the ISAT study [49], became the endovascular one. Indeed, when eligible for both treatments, a patient with ruptured aneurysm is less likely to have poor clinical outcome (death or severe disability) at 1-year follow-up when treated by endovascular means than by surgical clipping (23.7 % vs. 30.6 %). However, the treatment option is largely dependent on the experience of the operator of each specialty in a given center. Moreover, neurosurgical clipping has still some consensual indications like very large-necked aneurysms or aneurysms associated with clinically non-tolerated parenchymal hematoma, for which hematoma evacuation and aneurysm clipping will be performed during the same treatment.
Endovascular Treatment
The aim of the endovascular treatment is to “secure” the aneurysm sac by avoiding blood circulation in the sac. The treatment should be performed in emergency due to the potential risk of acute rebleeding of the ruptured IC aneurysm within the first days following the SAH [50].
Selective Coiling
Since they have been developed by Guglielmi et al. in the early 1990s [51], detachable coils have revolutionized the treatment of IC aneurysms. Most of these coils are made of platinum, and all of them are detachable. They are deployed inside the aneurysm via a microcatheter positioned in the sac and detached from a stainless-steel microguidewire by an electrical current (Fig. 6). The objective is to fill the aneurysm by means of decreasing size coils until total angiographic occlusion, while preserving the patency of the parent artery and other adjacent branches. Even if safe and efficient for the treatment of most IC aneurysms, large-necked aneurysms may be difficult to treat by regular coiling due to the risk of coil protrusion in the parent artery that may be responsible for thromboembolic complication.
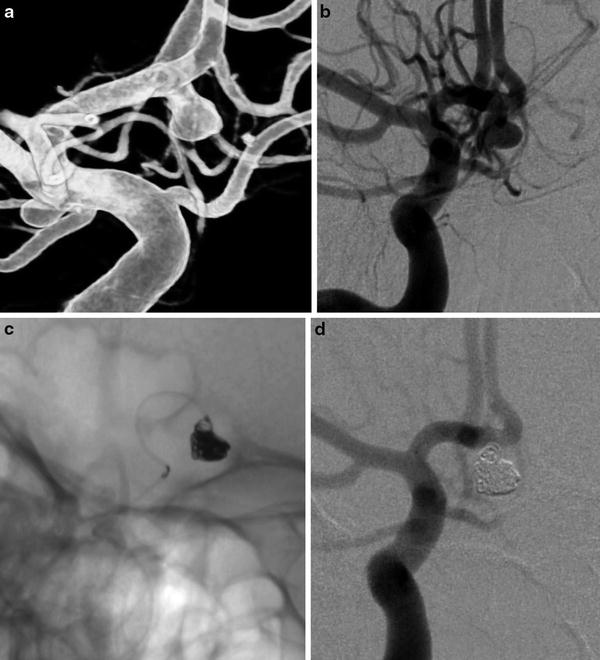

Fig. 6
Regular coiling in an AComm ruptured aneurysm. (a) and (b) right ICA DSA: (a) 3D rotational angiography, (b) working projection showing the saccular AComm aneurysm. (c) Unsubtracted snapshot during the aneurysm sac coiling. (d) Post-coiling DSA in working projection showing the complete occlusion of the aneurysm
“Remodeling” Technique
The remodeling technique has been developed in the mid-1990s to overcome the limitation of the presence of a large neck for the endovascular treatment of IC aneurysms [52]. The principle of this technique consists in inflating temporarily a balloon in front of the aneurysm’s neck during the coil deployment. This balloon helps to constrain the positioning of the coil’s loops in the aneurysm’s sac during its delivery (Fig. 7). With this technique, almost all IC aneurysms are now eligible for endovascular treatment. Despite some controversies [53], there are no evidences that the balloon remodeling technique increases the thromboembolic complication rate [54, 55].
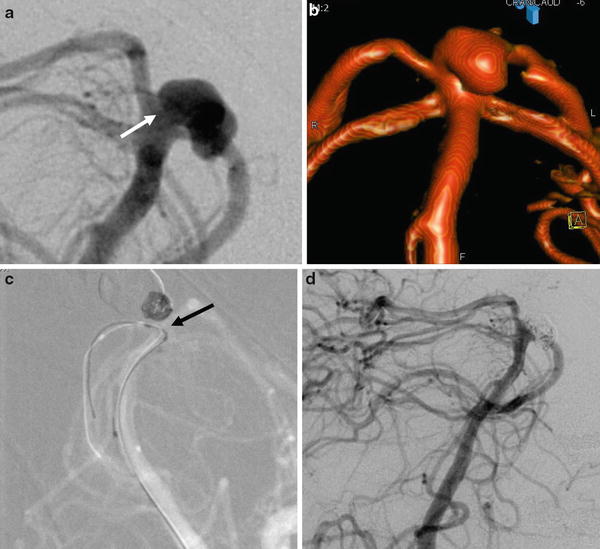

Fig. 7
Ruptured basilar tip aneurysm treated by remodeling technique in a 49-year-old female. (a) Left vertebral artery DSA showing the basilar tip aneurysm with a relatively large neck (arrow). (b) 3D rotational angiography reconstruction showing more precisely the anatomy of the aneurysm. (c) Snapshot of the road map during the coiling under balloon protection (remodeling technique). Note the positioning of the balloon in front of the aneurysm’s neck to constrain the coils in the aneurysm’s sac (arrow). (d) Final DSA control showing the total angiographic exclusion of the aneurysm
Parent Artery Occlusion
Parent artery occlusion is a potential therapeutic option for ruptured aneurysms, but can only be performed in case of sufficient collaterals, especially for large parent artery [26]. Moreover, the potential risks of delayed vasospasm that can lead to ischemic complications in patients with ruptured IC aneurysms may prevent from occluding a large vessel to preserve the circle of Willis.
Treatment with Biological Glue
Biological glue (n-butyl-cyanoacrylate [NBCA]) may be used in ruptured IC aneurysms for parent artery occlusion in dissecting aneurysms (e.g., posterior inferior cerebellar artery [PICA] dissecting aneurysm). The aim of this treatment is to perform a segmental occlusion by means of glue of the portion of the parent artery harboring the dissecting aneurysm [56]. The other main indication for the use of glue in aSAH is the presence of infectious IC aneurysms (aka mycotic aneurysms). As for dissecting IC aneurysms, the aim of the treatment is to obtain a segmental occlusion of the vessel harboring the aneurysm [57].
Intracranial Stenting
The use of intracranial stents is not recommended for the treatment of ruptured IC aneurysms at the acute phase. Indeed, these devices require a dual anti-aggregation platelet therapy that may be responsible for hemorrhagic complications. However, in some selected cases, IC stents may be used for the ruptured IC aneurysms. Indeed, in case of coil protrusion in the parent artery, the deployment of a stent in front of the aneurysm’s neck may be on option to avoid thromboembolic complication. This technique is called the “bailout technique” [58]. In case of uncoilable blood blister-like aneurysms, the use of flow diverter stents may be a valuable option, either at the acute phase or few days after the SAH (Fig. 8) [59].
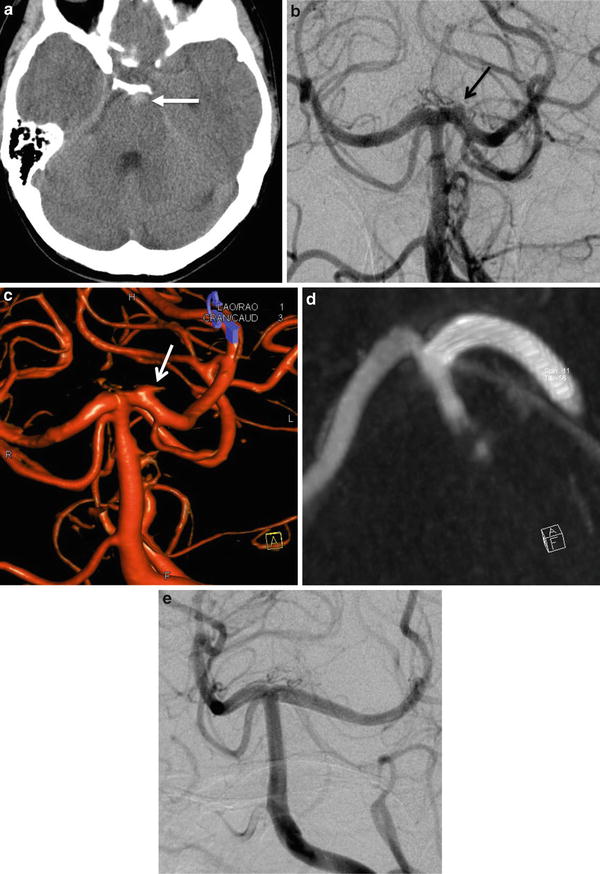

Fig. 8
Aneurysmal subarachnoid hemorrhage in a 39-year-old female. (a) Unenhanced CT scan demonstrating a prepontine spontaneous hyperdensity (arrow). (b) DSA in AP projection showing a “blood blister-like” aneurysm located on the left P1 segment (arrow). (c) 3D rotational angiography, confirming the presence of a “blood blister-like” aneurysm of the P1 segment of the left PCA (arrow). (d) Flat-panel CT-like acquisition after the stent deployment showing its satisfactory apposition on the parent artery wall. (e) DSA performed at 6 months showing the complete occlusion of the aneurysm covered by the flow diverter stent
Surgical Treatment
The aim of the surgical treatment is the same as that for the endovascular one: to avoid aneurysm sac circulation. The main technique used is the surgical clipping.
Walter Dandy first reported the surgical clipping of an aneurysm in 1937.
After having performed a craniotomy and a microsurgical dissection around the aneurysm’s sac and the parent artery, this technique consists in excluding the aneurysm from the intracranial circulation by positioning a surgical clip at the neck of the aneurysm (Fig. 9). In case of complex shape aneurysms, few clips may be necessary to exclude the aneurysm and reconstruct the parent artery. Most of IC aneurysms can be treated by surgical means. However, IC aneurysms located in the posterior circulation are more difficult to treat, leading to a higher complication rate [60].
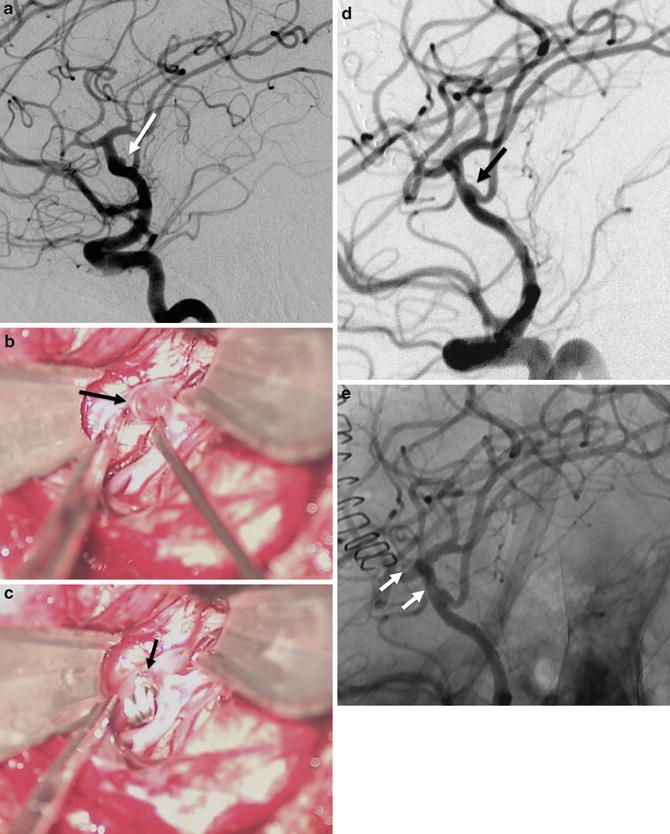

Fig. 9
A 60-year-old female with a ruptured left MCA bifurcation treated by surgical clipping. (a) Left ICA DSA in working projection showing a small, large-necked, MCA bifurcation aneurysm (arrow). (b) Photographs during the surgical clipping. Exposition of the aneurysm (arrow) by microsurgical dissection. (c) Photograph after the positioning of the clip at the aneurysm’s neck. (d) DSA in working projection after the clipping showing a satisfactory exclusion of the aneurysm (arrow). (e) On non-subtracted image, the clip can be seen at the aneurysm’s neck (arrows)
In complex aneurysms, for which a clipping seems too risky, or for blood blister-like aneurysms, the “wrapping technique” may be an option to prevent rebleeding. This technique consists in wrapping the aneurysm and the parent artery by surgical gauze or plastic sheeting in order to prevent further rebleeding [61]. Finally, bypass associated with parent artery sacrifice may be an option for the treatment of some very complex IC aneurysms, especially for giant/large aneurysms of the MCA [62].
Conservative Ma nagement
Conservative management and imaging follow-up may be a valuable option for unruptured intracranial aneurysms, especially for small ones [63]. In ruptured IC aneurysms, conservative management should not be proposed, since rebleeding rate is high. However, for untreatable aneurysms in patients in poor clinical condition, a “wait and see” strategy could be proposed, the treatment being to be discussed few weeks later, if the patient recovers from the aSAH and its complications. It is noteworthy that, in aSAH, the spontaneous rebleeding rate within 2 weeks ranges from 15 % to 25 % and is about 50 % within 6 months. Six months after the SAH, the cumulative annual risk for rebleeding is 2–3 % per year [11, 64–66].
Complications
Complications that may be observed in aneurysmal SAH are divided into those related to the SAH and those related to the treatment.
SAH-Related Complications
SAH-related complications may be severe and life threatening. Their consequences should thus not be underestimated. Among these complications, hydrocephalus, arterial vasospasm, and neurological/neuropsychological deficits may be seen at the acute phase of the SAH or with a delay [67]. Their management requires a close coordination between neurosurgical, interventional neuroradiology, and intensive care teams.
Spontaneous Rebleeding
Spontaneous rebleeding is the most severe complication in aSAH. Indeed, the clinical consequences of the rebleeding are frequently worse than the ones from the first bleeding [68]. The rebleeding is related to the lysis of the clot that stopped the first hemorrhage. The early rebleeding has been reported with a rate around 14 % [69] and is higher within the first 6 h [68]. This high rate of spontaneous rebleeding and the poor clinical consequences of such rebleeding are the rationale for an early treatment of ruptured IC aneurysms.
Hydrocephalus
Hydrocephalus may be observed at the acute phase of the hemorrhage or can be delayed. The mechanisms that may lead to hydrocephalus are acute obstruction of the cerebrospinal fluid pathway by a clot and abnormal blood resorption due to the SAH. Hydrocephalus may occur in the following weeks after the SAH. It is related to a fibrosis of the subarachnoid spaces and an inflammatory proliferation of arachnoid villosities’ cells. Such delayed hydrocephalus is more frequent in patients in poor initial grade (WFNS). Clinically, consciousness disorders and psychiatric disorders may be observed. Communicant hydrocephalus may require a ventriculoatrial or ventriculoperitoneal shunting and can be temporarily treated by serial lumbar punctures [70].
Arterial Vasospasm
Arterial vasospasm may occur few days after the hemorrhage (> day 3) until day 21. Its frequency and severity increase with the volume of blood in the subarachnoid space (see Fisher score; Table 3). Arterial vasospasm is seen on angiographic exams in about 70 % of the cases in aSAH. In 30 % of the cases, arterial vasospasm may lead to ischemic lesions [71]. Thus, vasospasm should be prevented by aggressive therapies and treated, when present, to prevent potential severe complications. The clear pathophysiology of arterial vasospasm related to aSAH remains unclear, some data suggesting an inflammatory vasculitis rather than a true vasospasm [72].
1 | No hemorrhage evident |
2 | Subarachnoid hemorrhage less than 1 mm thick |
3 | Subarachnoid hemorrhage more than 1 mm thick |
4 | Subarachnoid hemorrhage of any thickness with intraventricular hemorrhage (IVH) or parenchymal extension |
The “H therapy” is one of the first-line therapies in the armamentarium of the treatment of aSAH-related arterial vasospasm. This therapy associates hypertension while keeping normovolemia and normalized cardiac flow. Vasoconstrictor therapy with noradrenalin is administered in an intensive care unit, with continuous monitoring comprising, at least, an invasive measurement of the blood pressure. The “triple-H therapy” that associates hemodilution, hypervolemia, and arterial hypertension is no longer used to prevent arterial vasospasm because it did not prove its efficacy for the treatment of cerebral vasospasm. The use of a vasodilator with cerebral tropism like nimodipine is systematically performed during the first 3 weeks after the hemorrhage either per os or intravenously. Daily low doses of statins have proven their effectiveness in preventing cerebral vasospasm [73]. The diagnosis of vasospasm should be suspected in case of fever, disorders of consciousness, and motor or sensitive deficit or on ultrasonographic findings (accelerated mean velocities on arterial vessels). Such signs should incite to perform brain CT scan to rule out ischemic infarct, hydrocephalus, parenchymal hematoma, or rebleeding and a vascular imaging (CTA or DSA). On vascular imaging, arterial vasospasm typically appears as narrowing of the ICA termination, A1 and M1 segments (Fig. 10). Cortical defect may also be seen on capillary phase on DSA.
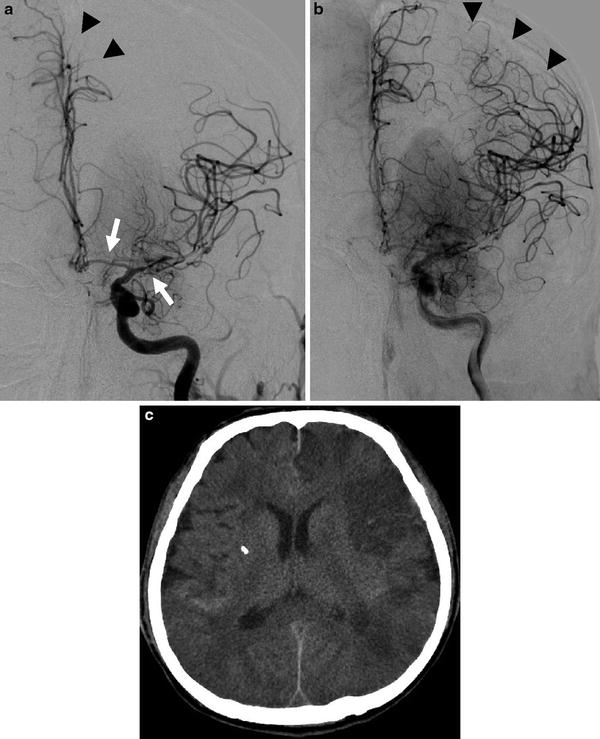

Fig. 10




A 53-year-old male who presented an AComm aneurysm rupture. Increasing of blood velocities on Doppler sonography at day 5. Left ICA DSA in anteroposterior projection: arterial phase (a) and capillary phase (b). Narrowing of A1 and M1 segments is seen (a, arrows) consistent with a proximal arterial vasospasm. Note the delayed opacification of the left ACA (a, arrow head). On capillary phase, extensive parenchymal defect is seen in the left ACA and MCA territories, corresponding to a severe distal vasospasm (b, arrow heads). (c) Unenhanced CT scan performed the same day, showing multiple vasospasm-related ischemic lesions in both hemispheres, predominating in the left side
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree