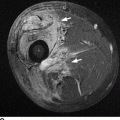
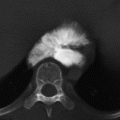
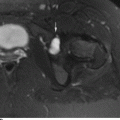
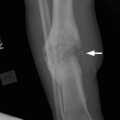
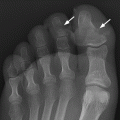
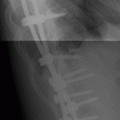
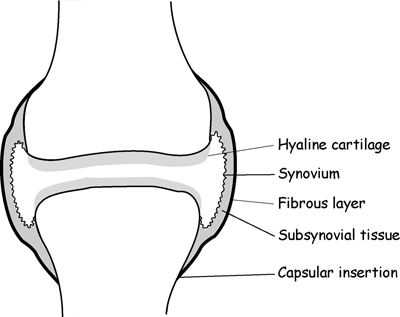
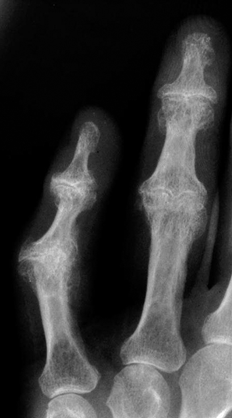
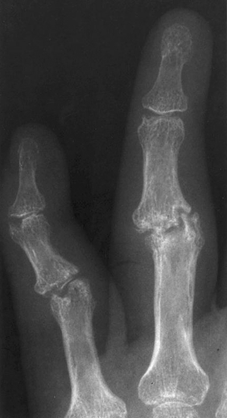
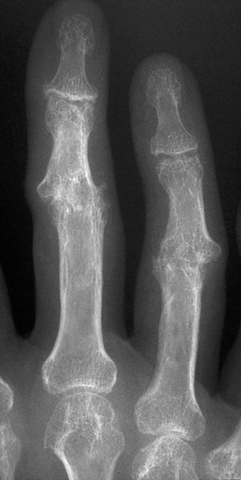
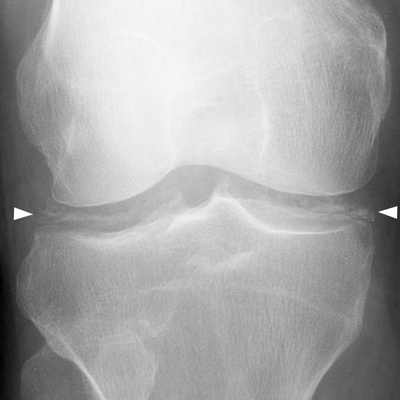
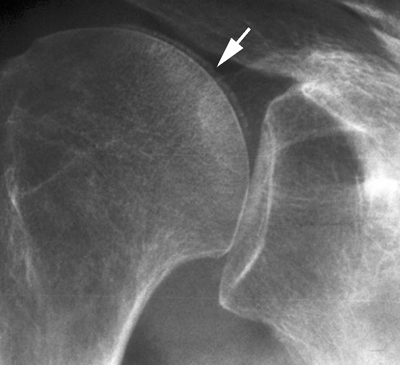
CHAPTER 11 This chapter describes a pragmatic approach to the radiology of joint disease, based on anatomy, pathophysiology, and radiographic analysis. This approach draws heavily on the work of Forrester, Brower, and Resnick (Table 11.1). Detailed discussions of specific clinical forms of arthritis are presented in Chapters 12 and 13. TABLE 11.1 Approach to Radiographic Analysis of Arthritic Changes in the Hand Source: Data from Brower AC. Arthritis in Black and White. 2nd Ed. Philadelphia, PA: WB Saunders; 1997 and Forrester DM, Brown JC. The Radiology of Joint Disease. 3rd Ed. Philadelphia, PA: WB Saunders; 1987. Radiographs mirror the pathologic processes that affect the joints and the functional adaptations that may follow. In general, the radiologic diagnosis of arthritis can be highly specific and reliable when classic changes are present in the expected distributions but much less specific in the early stages before the disease process has fully evolved. Regardless of the approach, however, several frustrations are unavoidable: A specific radiologic diagnosis is not always possible; many types of joint disease overlap in their radiologic and clinical features; two or more diseases may coexist in the same patient; and, finally, clinical disease may precede radiologic abnormalities and vice versa, sometimes by years. Diseases that affect joints do so by three broad pathophysiologic mechanisms, each with a distinctive radiographic appearance: degeneration, inflammation, and metabolic deposition. For practical purposes, one mechanism is usually predominant. Degeneration of a joint refers to mechanical damage and reparative adaptations; in essence, the joint is worn away. Inflammation of a joint may be acute, chronic, or both; the joint is dissolved by the inflammatory process. Metabolic deposition refers to the infiltration of a joint by aberrant metabolic products. Each of these mechanisms affects joints in radiographically distinctive ways (Table 11.2). TABLE 11.2 Characteristic Radiographic Signs of Arthritis Most articulations of the appendicular skeleton are synovial joints. In the axial skeleton, the facet joints of the spine, the atlantoaxial (C1–2) joint, the uncovertebral joints of the cervical spine, and the lower two thirds of the sacroiliac joints are synovial. Synovial joints have a joint cavity and are enclosed by a joint capsule consisting of an inner synovial layer (the synovium), a middle subsynovium, and an outer fibrous layer (Fig. 11.1). The synovium is a cellular secretory mucosa that produces synovial fluid. Synovial fluid is viscous because of a high concentration of hyaluronic acid. Joint capsules have an active blood supply with a large capillary surface area. The synovium has a mesenchymal rather than epithelial origin; therefore, no basement membrane or other structural barrier is present between the synovial fluid and the capillary bed. The change from synovium to fibrous capsule is gradual; there are no distinct boundaries between the layers. Joint capsules are densely innervated. Tendon sheaths invest tendons and reduce friction during motion. Bursae are located where complete freedom of motion between structures is necessary, for example, where a tendon passes directly over the periosteum. Because tendon sheaths and bursae are synovial structures, diseases that affect synovial joints may also involve them. FIGURE 11.1. Anatomy of a synovial joint. Soft-tissue swelling at a joint may reflect capsular distention from effusion, synovial hypertrophy, soft-tissue edema, or a mass. Symmetric, fusiform swelling suggests an inflammatory process with effusion, synovial edema, synovial hypertrophy, or some combination thereof (Fig. 11.2). Inflammatory distention of a tendon sheath may also produce soft-tissue swelling, but the swelling extends beyond the joint. In a digit, this kind of swelling produces an appearance that has been likened to a sausage (sausage digit). Generalized soft-tissue swelling may be caused by subcutaneous edema or hyperemia and suggests inflammation (Fig. 11.3). Lumpy-bumpy swelling that is not symmetric or centered near a joint suggests masses and may be caused by metabolic deposition disease with masslike deposits of metabolic products in the periarticular soft tissues (Fig. 11.4). Soft-tissue prominences at joints that are found on physical examination may actually result from bony or cartilaginous enlargements; the overlying soft tissues may be normal. Heberden and Bouchard nodes are swellings of this kind at the distal interphalangeal (DIP) and proximal interphalangeal (PIP) joints of the hand, respectively, and are characteristic of a degenerative process. Calcification in the soft tissues may affect cartilage, skin, muscles, tendons, or other connective tissues and is associated with connective tissue diseases. Soft-tissue atrophy or loss is present in various conditions. FIGURE 11.2. Fusiform soft-tissue swelling at the PIP joint (rheumatoid arthritis). FIGURE 11.3. “Sausage digit” soft-tissue swelling (psoriatic arthritis). FIGURE 11.4. Lumpy-bumpy soft-tissue swelling (tophaceous gout). The ends of the articulating bones, that is, the joint surfaces, are covered with hyaline articular cartilage. Hyaline cartilage is composed of a collagen fibril framework and a ground substance. One set of densely packed collagen fibrils is oriented parallel to the articular surface, forming an armor-plate layer with tiny surface pores that allow the passage of water and small electrolytes. A second, less densely packed set of collagen fibrils is oriented in arcades, linking the armor-plate layer to the subchondral bone (Fig. 11.5). The ground substance is a gel that consists of water and large proteoglycan aggregate macromolecules that are loosely fixed to the collagen framework. The proteoglycan macromolecules are too large to pass through the pores of the armor-plate layer. The physical and chemical properties of these macromolecules allow them to attract and bind water, providing sufficient swelling pressure beneath the armor-plate layer to “inflate” the articular cartilage, even during weight bearing. During motion, a thin layer of water is expressed through the small surface pores, providing a frictionless surface for a lifetime of mobility. Articular cartilage has a load-dampening ability that spreads transmitted loads over a greater area of the subchondral bone. Under rapid, transient loading, articular cartilage has elastic properties. Under a steady load, it creeps and deforms like a sponge. The portion of cartilage that is adjacent to the subchondral bone is calcified. Interdigitations between the calcified cartilage and the subchondral bone provide a strong mechanical coupling. Chondrocytes are the cells whose metabolic activity maintains the specialized structures of articular cartilage. Less than 1% of articular cartilage volume is composed of cells. Because cartilage is avascular and alymphatic, chondrocytes derive their nutrients by diffusion from the synovial fluid. Articular cartilage has only a limited ability to repair itself. Deep injuries may repair with cartilage that is densely fibrous. FIGURE 11.5. Structure of articular cartilage. Cartilage abnormalities are inferred from the radiolucent gap between articulating bones, the articular space, or the joint space. The articular cartilage fills this space. A potential space exists where the articulating surfaces meet. Loss of articular cartilage causes the joint space to narrow. Cartilage loss within a joint can be diffuse and concentric—indicating an inflammatory process (with enzymatic dissolution of cartilage)—or focal and uneven, indicating a mechanical one (Fig. 11.6). If there is complete cartilage loss, the ends of the bones may become eroded, making the joint space appear wider. The ends of the bone may form a pseudoarthrosis (Fig. 11.7), or fibrous or bony ankylosis (fusion) of the joint may occur (Fig. 11.8). Widening of the articular space may indicate abnormal cartilage proliferation or intra-articular fluid. Weight-bearing views may be necessary to assess accurately the degree of cartilage loss at the knee. Asymmetric cartilage loss may result in changes in radiographic joint space narrowing with changes in position. FIGURE 11.6. Asymmetric joint space narrowing, osteophytes, and subchondral sclerosis (osteoarthritis). FIGURE 11.7. Severe PIP joint subchondral bone erosions (psoriatic arthritis). FIGURE 11.8. Bony ankylosis (psoriatic arthritis). Calcification of cartilage is called chondrocalcinosis. Chondrocalcinosis may involve fibrocartilage structures such as the menisci of the knee (Fig. 11.9) or the triangular fibrocartilage complex of the wrist. Articular cartilage also may calcify (Fig. 11.10). FIGURE 11.9. Chondrocalcinosis of the menisci (arrowheads). FIGURE 11.10. Chondrocalcinosis of the articular cartilage (arrow).
Approach to Joint Disease

 GENERAL PRINCIPLES
GENERAL PRINCIPLES
 SYNOVIAL JOINTS
SYNOVIAL JOINTS
Soft Tissues
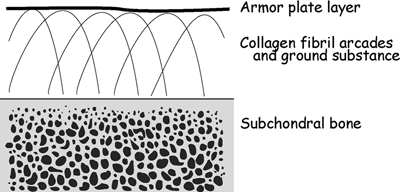
Cartilage
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree