Cord signal changes are often encountered in the clinical setting of myelopathy, a neurologic deficit related to the spinal cord pathology (1). The most common causes of myelopathy include disc herniations, metastases, and trauma. Therefore, the primary role of the radiologist in the setting of myelopathy is to ascertain any potential source of cord compression (3). If no source of extrinsic mass is identified, then the intrinsic cord signal should be carefully scrutinized to evaluate potential causes of noncompressive myelopathy.
Normally, a pattern based approach is employed to identify intramedullary cord lesions on MRI (12,13). Seemingly specific manifestations of cord signal changes are not always straightforward. Subacute combined degeneration (SCD) is a particular form of demyelination secondary to vitamin B12 deficiency with involvement of the dorsal and lateral columns. MRI demonstrates a characteristic “inverted V” sign of T2 signal hyperintensity usually involving the cervical and upper thoracic cord (Fig. 17.1). Clinical manifestations include paresthesias of the hands and feet, which may progress to ataxic paraplegia if left untreated (6). An indistinguishable pattern of abnormal signal can also be seen in the setting of chronic nitrous oxide toxicity since there is a convergence of the same pathophysiologic pathway from inactivation of vitamin B12 (14). Prompt diagnosis is critical since early treatment can improve symptoms and partially reverse MRI findings. Other diseases that may present with imaging features radiologically similar to SCD include copper deficiency, AIDS-related myelopathy, and the now rarely encountered tabes dorsalis, a neurologic form of tertiary syphilis (5,6).
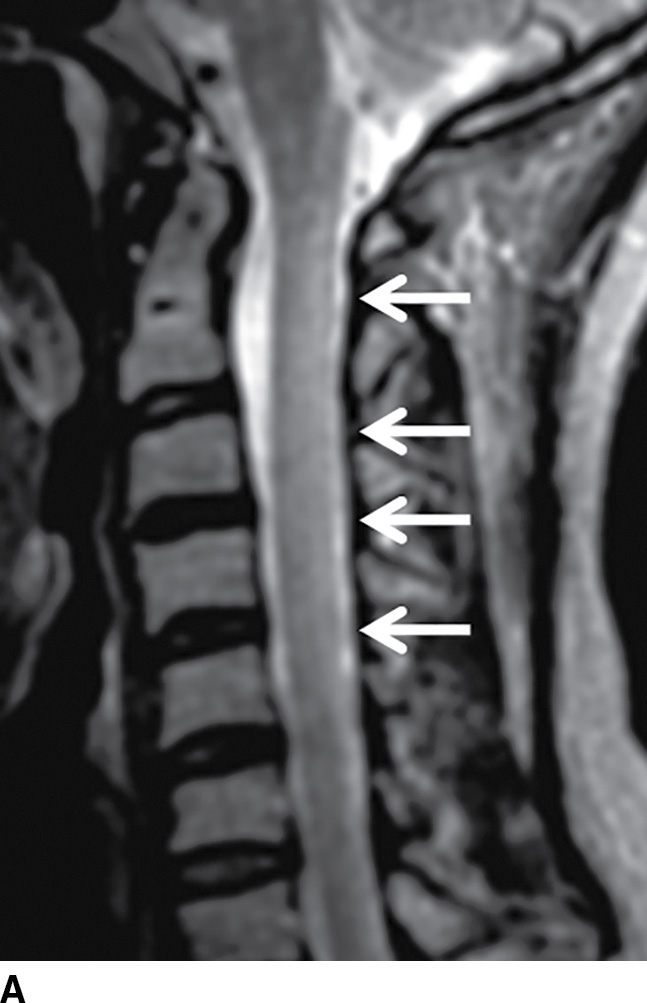
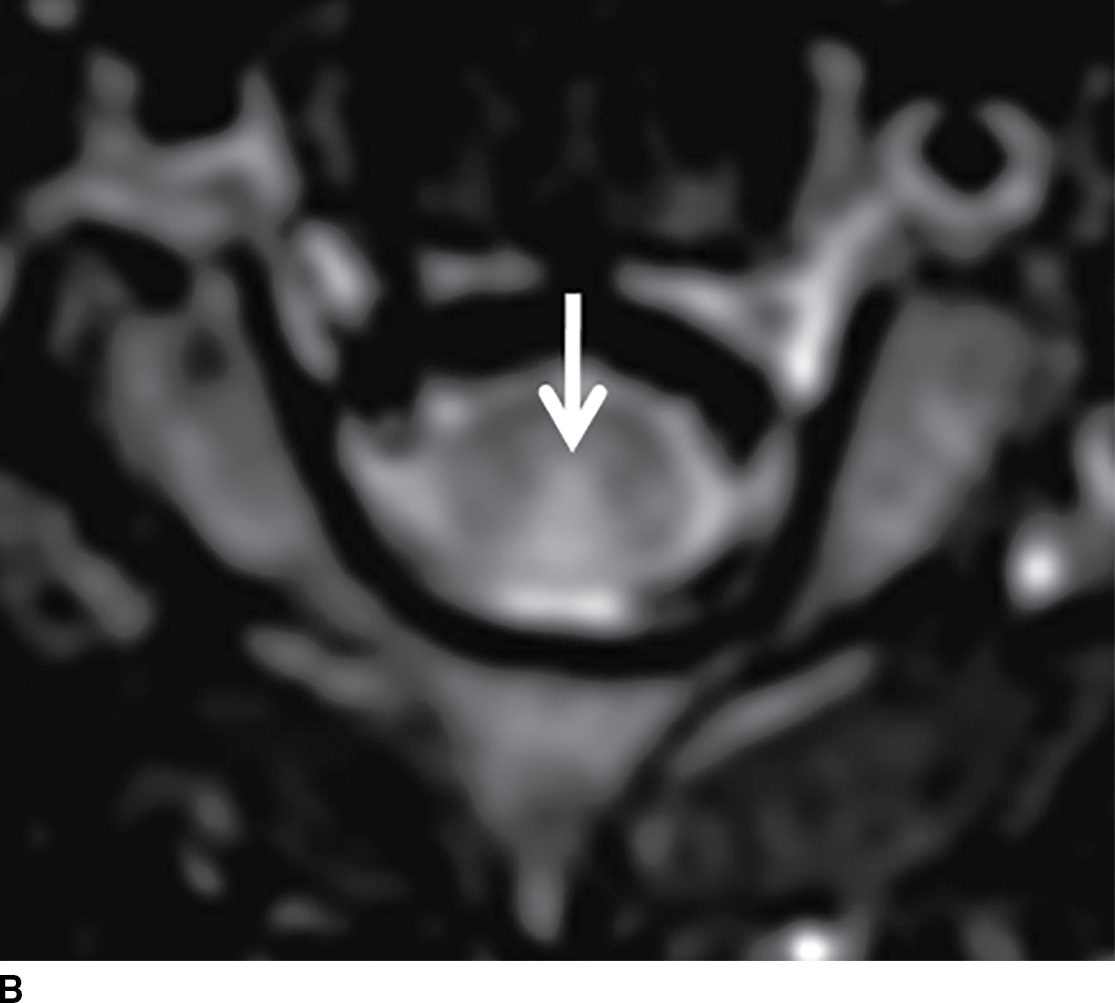
FIG. 17.1 Sagittal (A) and axial (B) T2 MR images of the cervical spine illustrate characteristic features of SCD. Arrows demarcate the posterior distribution of T2 hyperintense signal changes within the cervical spinal cord and the classic inverted V pattern on axial imaging.
Fusiform cord expansion is commonly associated with intramedullary neoplasms such as an ependymoma or astrocytoma (Fig. 17.2). Cord expansion should not, however, be solely attributed to the presence of a neoplasm since nonneoplastic cord lesions can often exhibit both expansion and enhancement (15,16). For instance, neurocysticercosis affecting the cervical spinal cord can mimic a neoplasm with a peripherally enhancing cystic mass causing perilesional cord edema and cord expansion (Fig. 17.3). The presentation of spinal cysticercosis can be variable with lesions appearing in intramedullary, leptomeningeal, or epidural spaces, with leptomeningeal lesions being most common. Isolated intramedullary cysticercosis is rare. Surgery is favored, when feasible, for both diagnosis and treatment since removal of the scolex eliminates the possibility of an intense immune reaction that could exacerbate myelopathic symptoms (17–19).
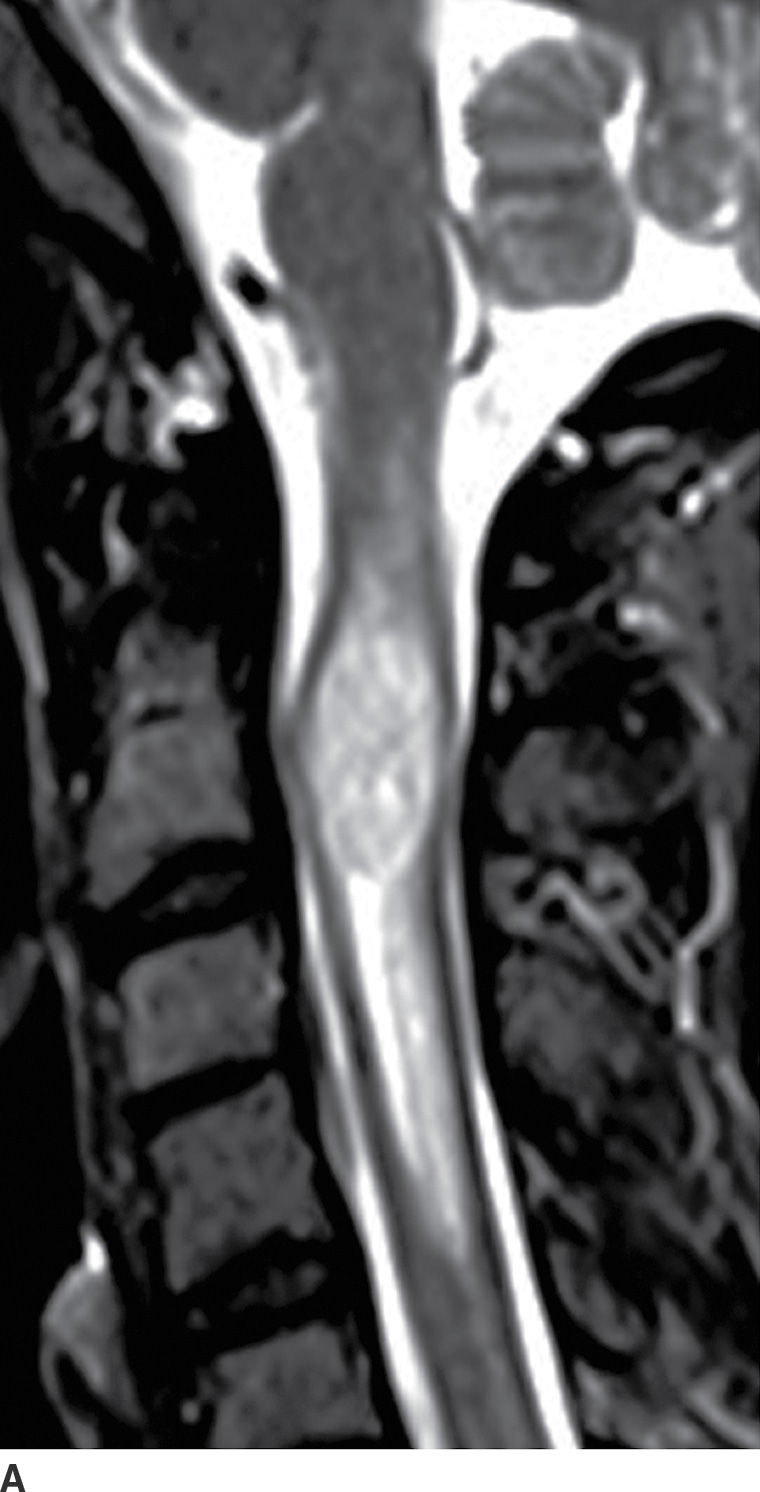
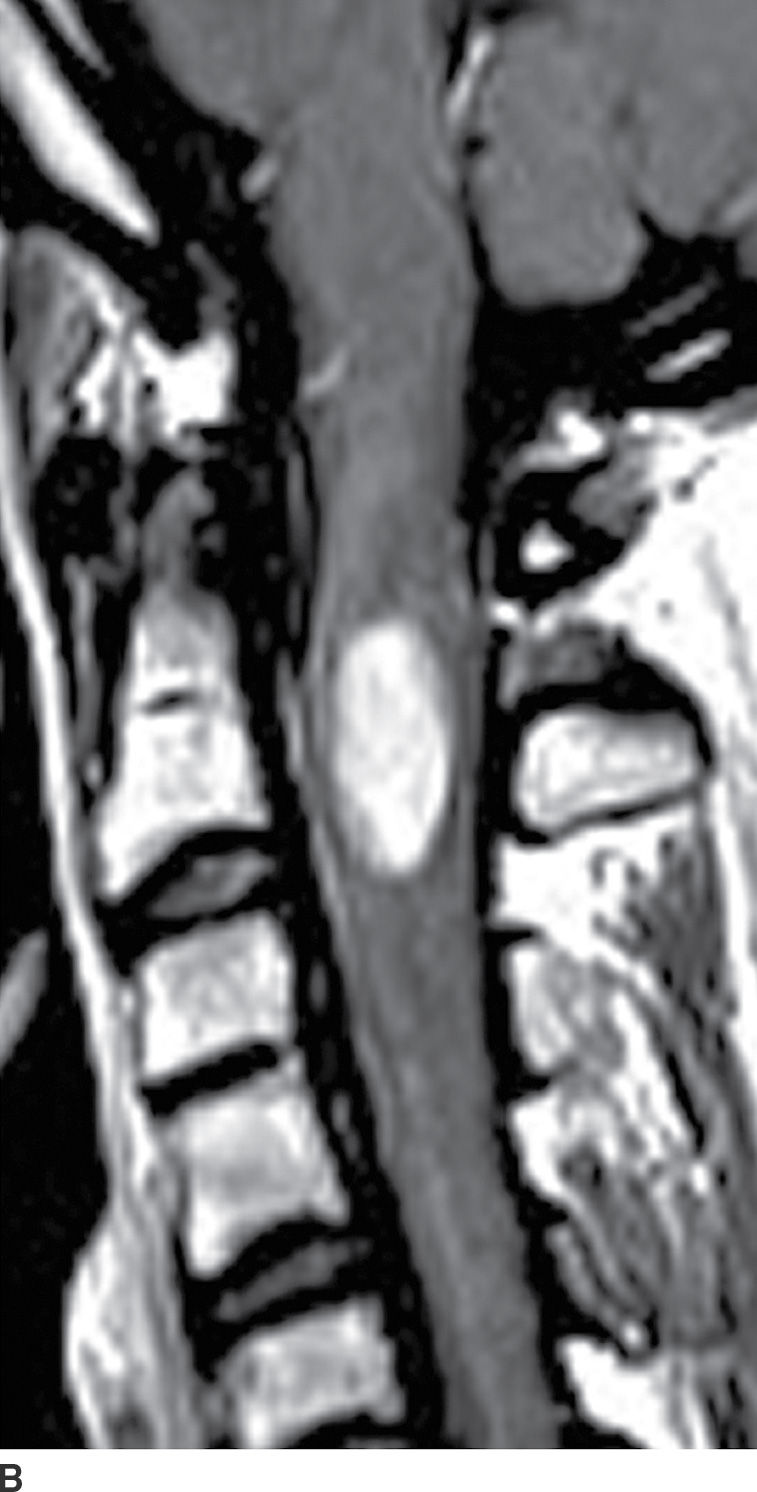
FIG. 17.2 Sagittal T2 (A) and contrast-enhanced T1 (B) images reveal a homogeneously enhancing intramedullary mass, representing an ependymoma, with perilesional edema and fusiform cord expansion. In the pediatric age group, ependymomas are most commonly encountered in patients with neurofibromatosis type II.
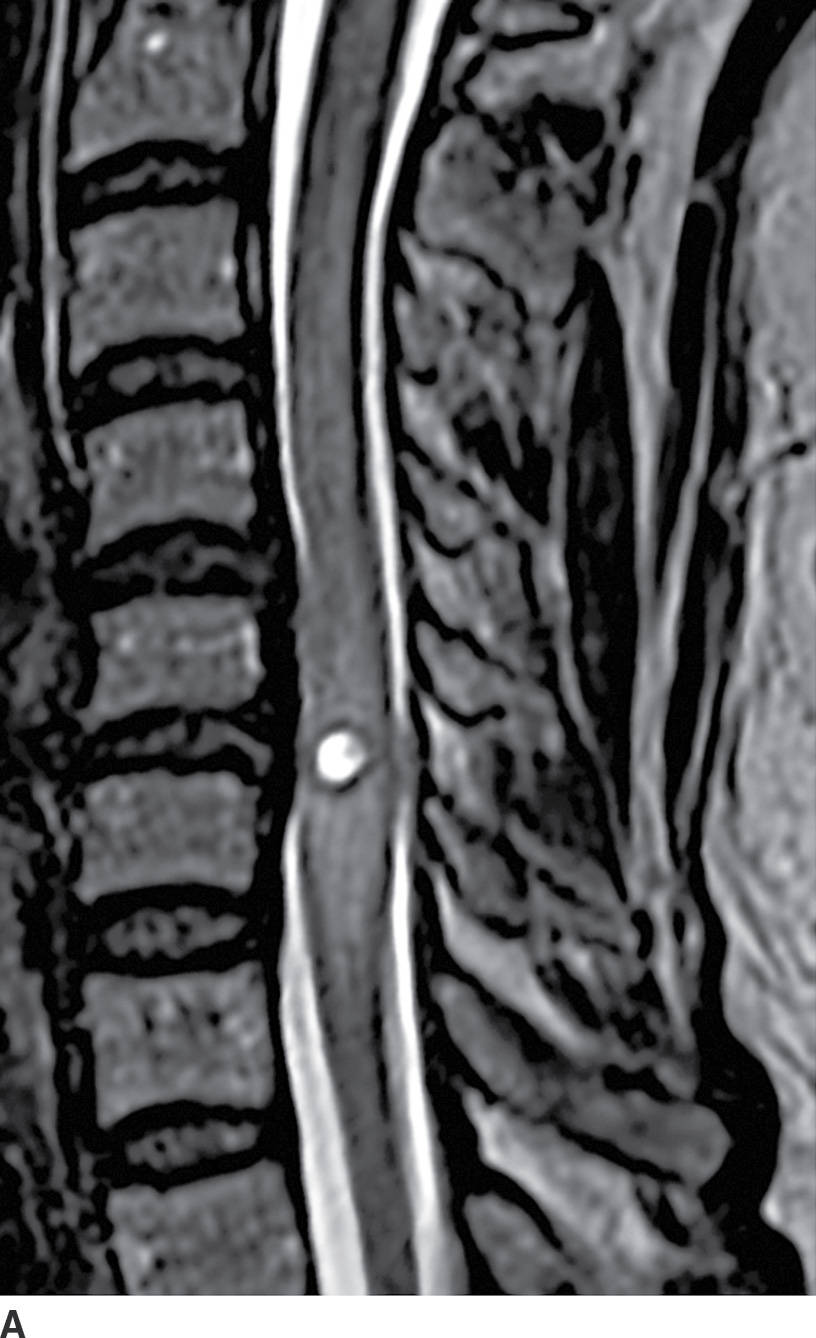
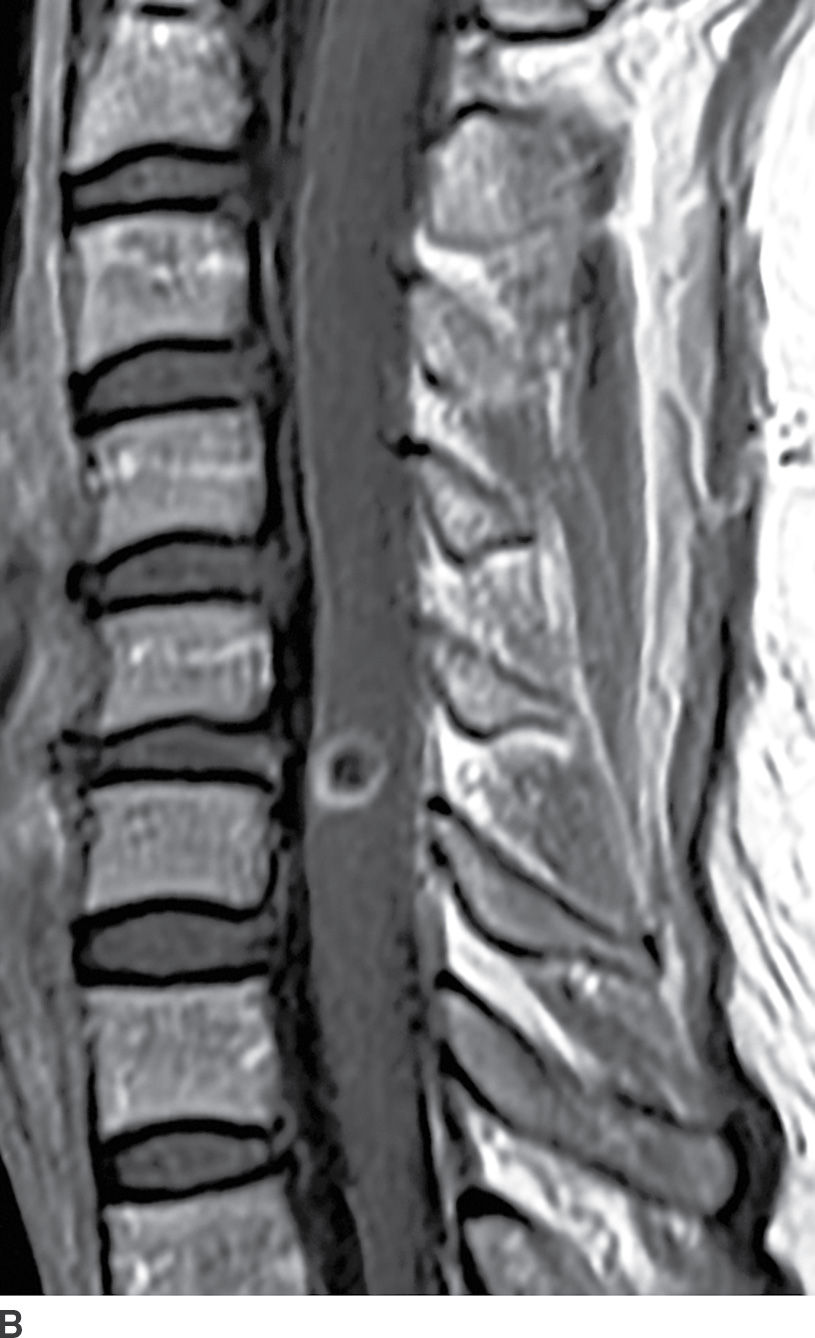
FIG. 17.3 Neurocysticercosis. Sagittal T2 (A) and contrast-enhanced T1 (B) images demonstrate a ring-enhancing, cystic mass within the cervical spinal cord. Perilesional cord edema contributes to local fusiform expansion of the cord.
Active demyelination (Fig. 17.4) can also present with cord expansion (15). For example, neuromyelitis optica, a severe inflammatory demyelinating disorder that is distinct from multiple sclerosis, is classically characterized by the presence of longitudinally extensive cervical spinal cord lesions that are frequently associated with cord expansion related to vasogenic and/or cytotoxic edema (11). This entity is characteristically preceded by optic neuritis. Acute disseminated encephalomyelitis (ADEM) and transverse myelitis can have similar findings but are usually less extensive, and ADEM has associated intracerebral white matter lesions. Caution should be exercised since these features are nonspecific and can be seen with other inflammatory diseases such as Sjogren disease or systemic lupus erythematosus. Venous hypertension in the setting of a dural arteriovenous fistula (AVF) (Fig. 17.5) can result in a long segment of cord expansion, increased T2 signal in the central aspect of the cord, and occasionally patchy intramedullary enhancement (20). The identification of intradural flow voids representing dilated spinal veins should indicate the correct diagnosis of AVF. Radiation-induced myelopathy commonly presents with a long segment of cord expansion with increased T2 signal and patchy enhancement. The coexistence of radiation-related fatty marrow replacement in the adjacent vertebrae can help establish the correct diagnosis (Fig. 17.6). Multiple sclerosis, by contrast, tends to have short-segment T2 hyperintense lesions in the periphery of the spinal cord and usually has associated classic intracerebral white matter lesions (see Fig. 17.4).
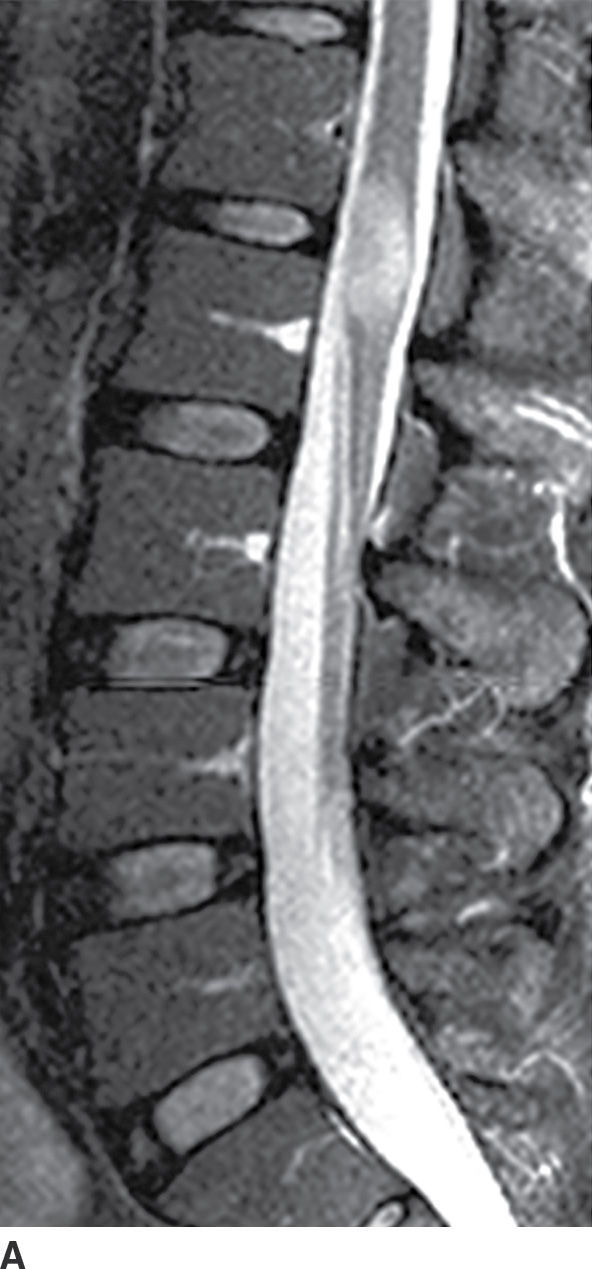
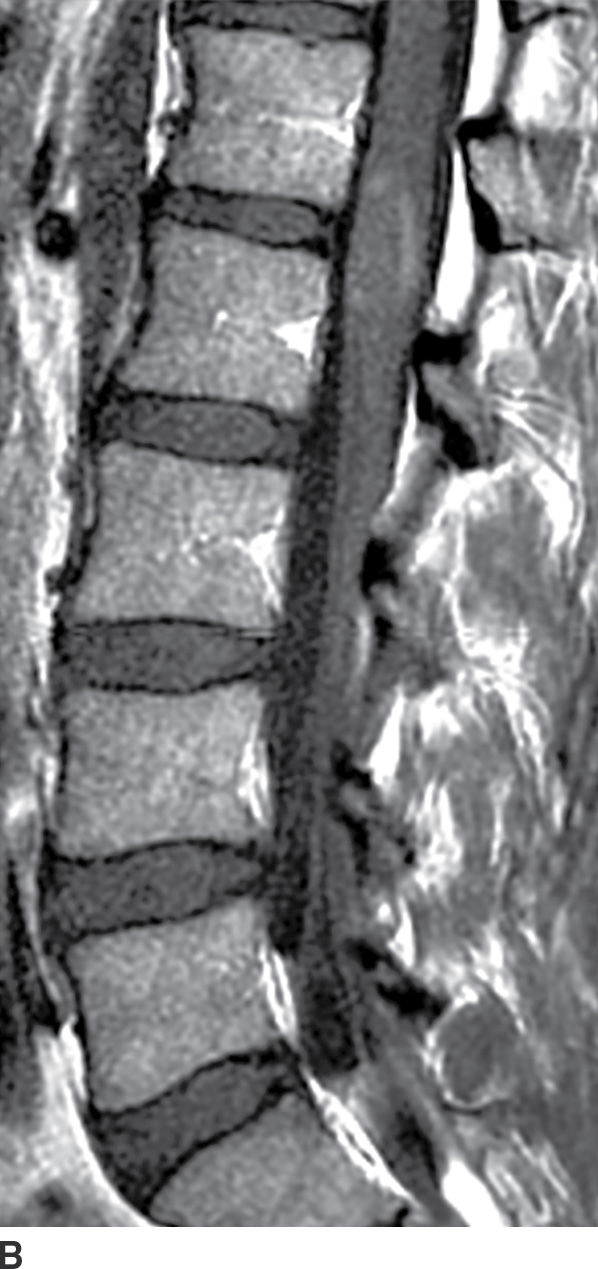
FIG. 17.4 In a patient with multiple sclerosis presenting with subacute myelopathy, a discrete intramedullary demyelinating lesion causes focal cord expansion, increased T2 signal, and incomplete rim enhancement on sagittal STIR (A) and sagittal contrast-enhanced T1 (B) images.
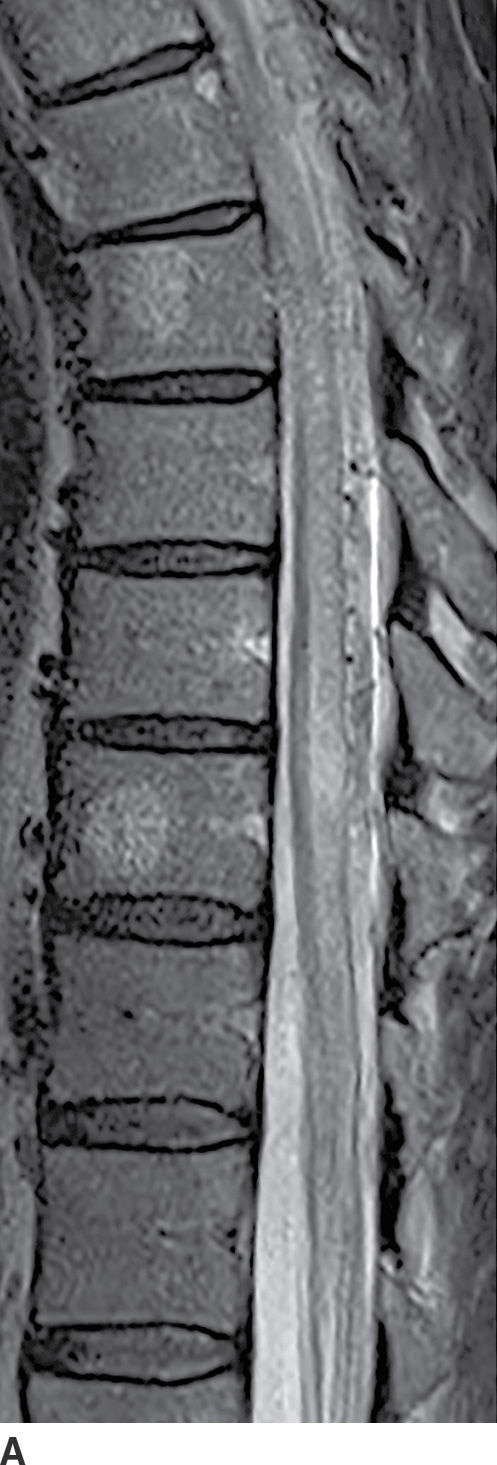

FIG. 17.5 A characteristic appearance of a dural AVF is illustrated with a sagittal T2 image (A) showing cord edema and serpentine flow voids filling the dorsal subarachnoid space and a contrast-enhanced T1 image (B) showing patchy enhancement of the cord as well as enhancement of the dorsal vessels.
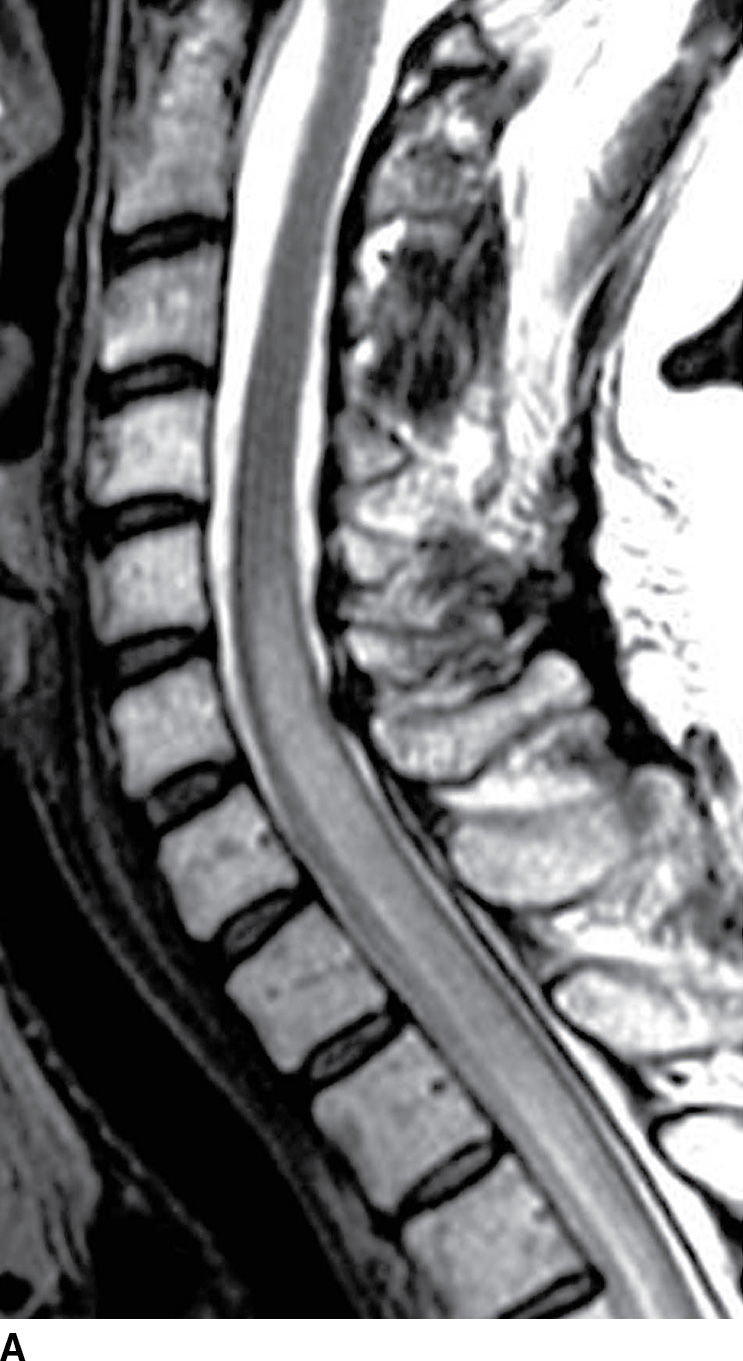
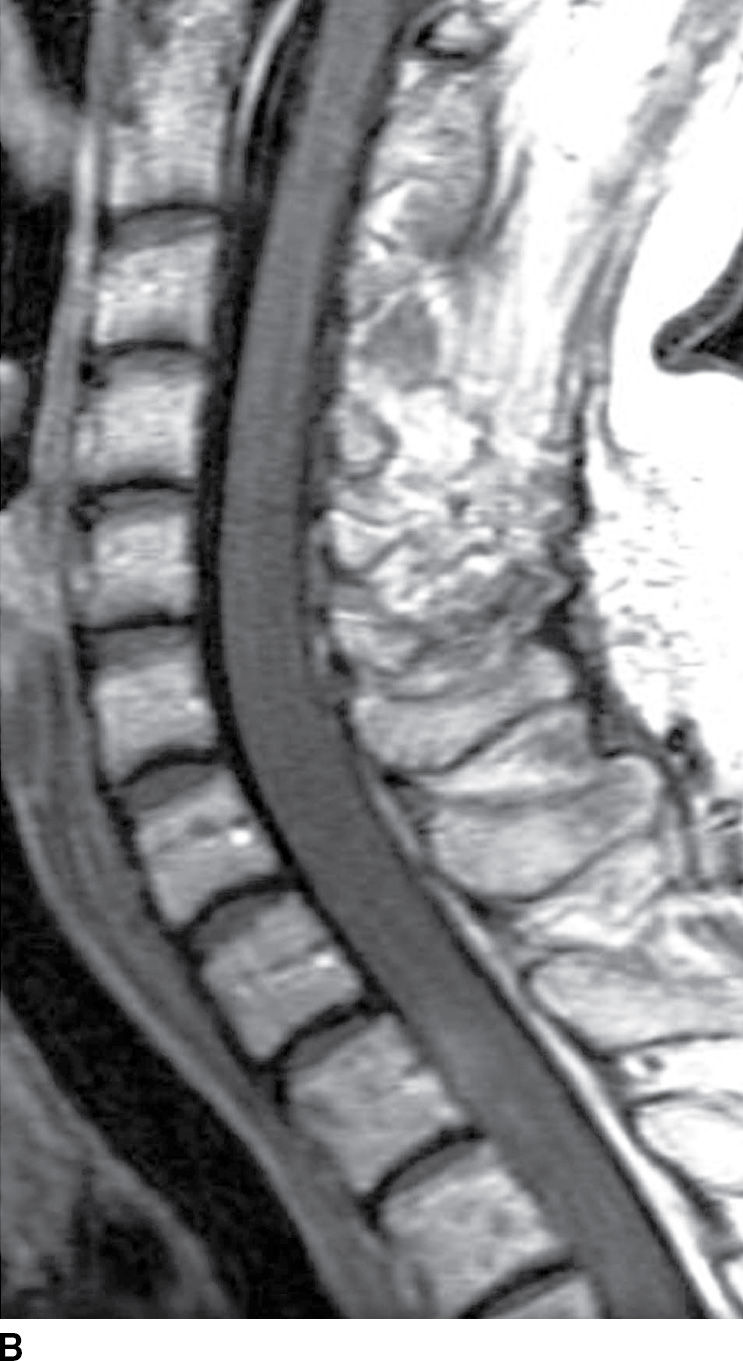
FIG. 17.6 Sagittal T2 (A) and contrast-enhanced T1 (B) images of the cervical spine obtained after radiation treatment for nodular sclerosing Hodgkin lymphoma reveal extensive cord edema and a small patchy focus of residual intramedullary enhancement. The patient presented with myelopathic symptoms, which raised the concern for disease recurrence and prompted the scan.
Rarely, a lumbar spine MRI may show mass-like cystic dilation of the ventriculus terminalis (VT) (Fig. 17.7). Under normal circumstances, the VT is a narrow, ependymal-lined prominence of the central canal within the conus medullaris, commonly described as the “fifth ventricle.” Mild fusiform prominence of the terminal ventricle can be a common developmental feature in the pediatric population, visible in approximately 2.6% of MRIs of normal children under the age of five (21). When present, the size of the pediatric VT can vary, ranging from 1.5 to 6 mm in diameter. Occasionally in the adult population, the VT can exhibit severe dilation, which may result in symptoms of bladder dysfunction, back pain, and/or sciatica necessitating surgical decompression. This cystic lesion should exhibit signal characteristics identical to cerebrospinal fluid (CSF) and should not enhance following the administration of gadolinium.
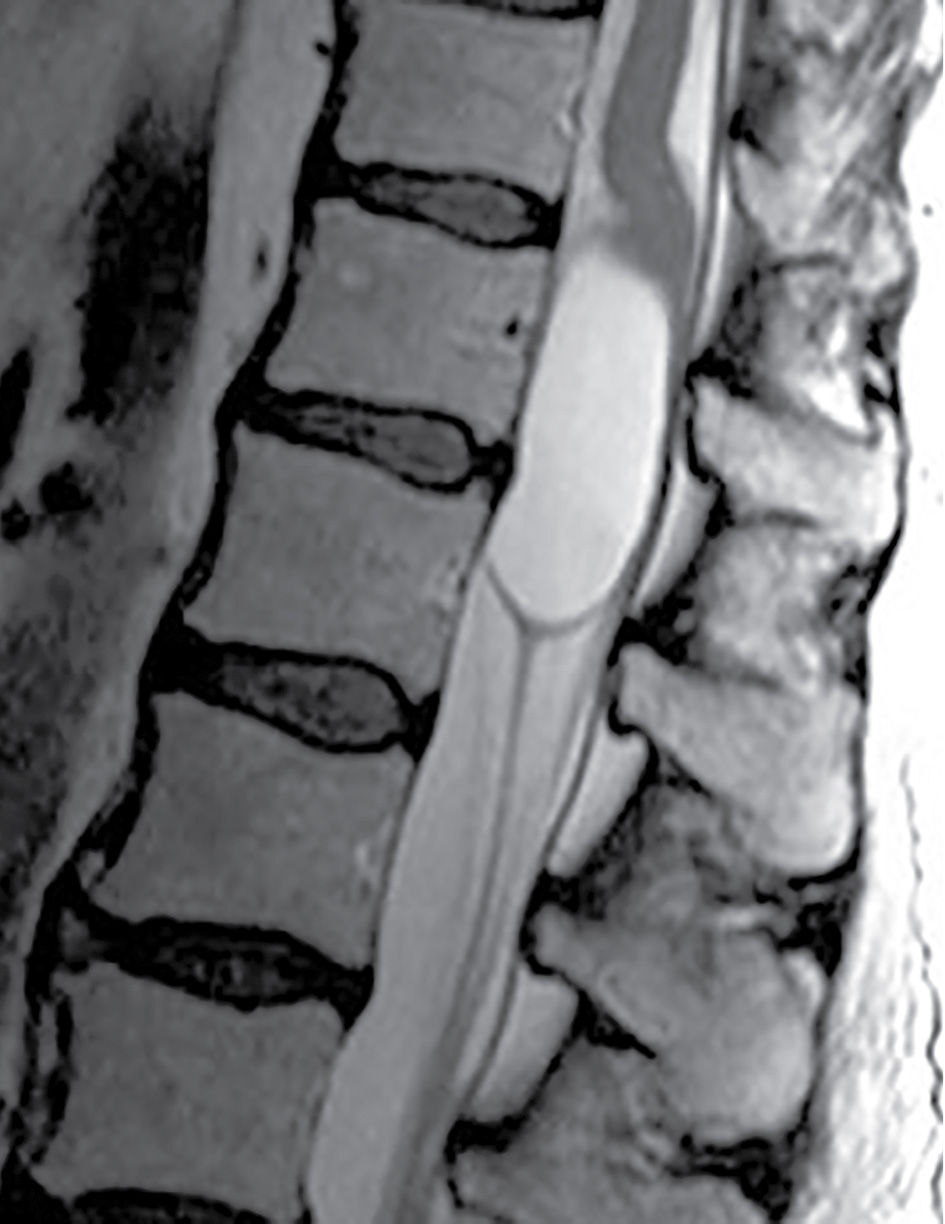
FIG. 17.7 Massive cystic dilatation of the terminal ventricle is demonstrated on a sagittal T2 image of the thoracolumbar junction. The cyst fills the canal, and there is buckling of the cord proximal to the lesion.
Extramedullary Intradural Compartment
Discrete masses that occur within the subarachnoid compartment will displace the cord or nerve roots away from the mass, expand the sac, and exhibit a “meniscus” above and below the lesion (22). A common differential diagnosis for enhancing extramedullary intradural masses (Table 17.2) includes meningiomas (Fig. 17.8), nerve sheath tumors (Fig. 17.9), drop metastases, and myxopapillary ependymomas (22). Meningiomas are usually isointense to the spinal cord on all sequences, homogeneously enhance, and frequently have associated dural enhancement. Nerve sheath tumors are typically hyperintense on T2-weighted images and most frequently extend into the neural foramina with intradural and extradural components. Drop metastases usually have associated intracranial leptomeningeal disease. Myxopapillary ependymomas typically have a “sausage” or “dripping candle wax” appearance and extend inferiorly from the conus medullaris. If an extramedullary intradural lesion has associated hemorrhage and/or abnormal flow voids, a hemangioblastoma or paraganglioma should be considered.
Table 17.2 Lesions of the Extramedullary Intradural Compartment
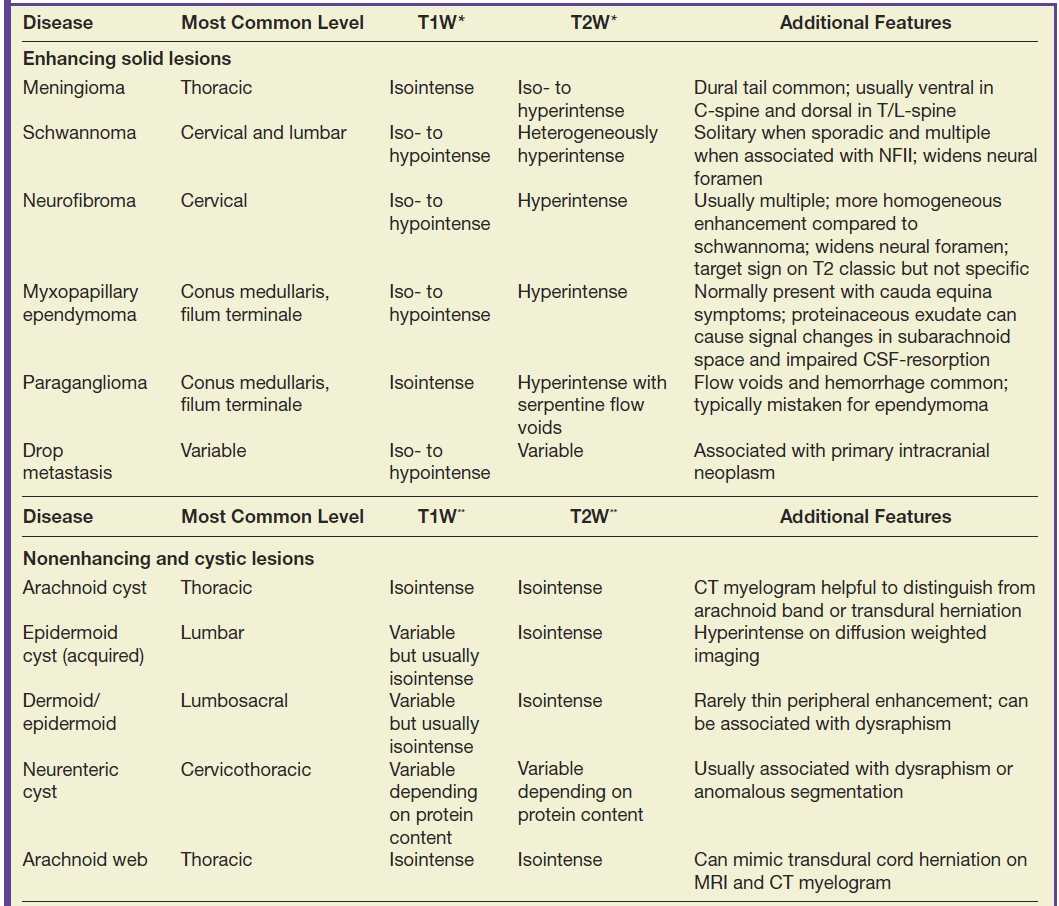
*Intensity of T1/T2 signal of solid lesions is relative to cord.
**Intensity of T1/T2 signal is relative to cerebrospinal fluid.
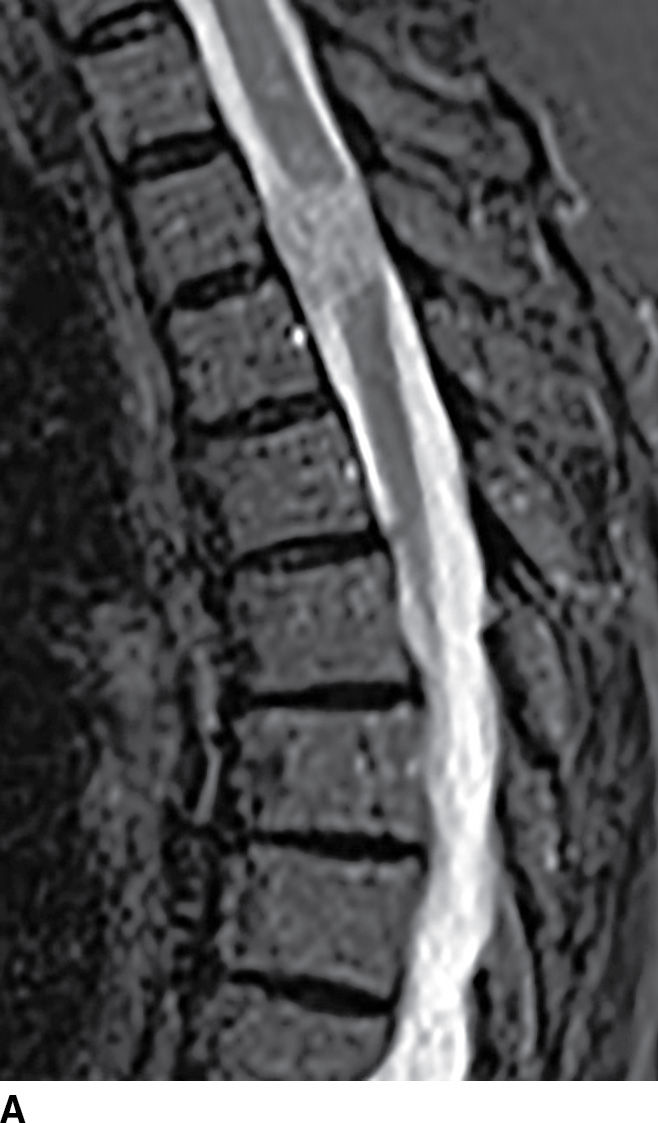
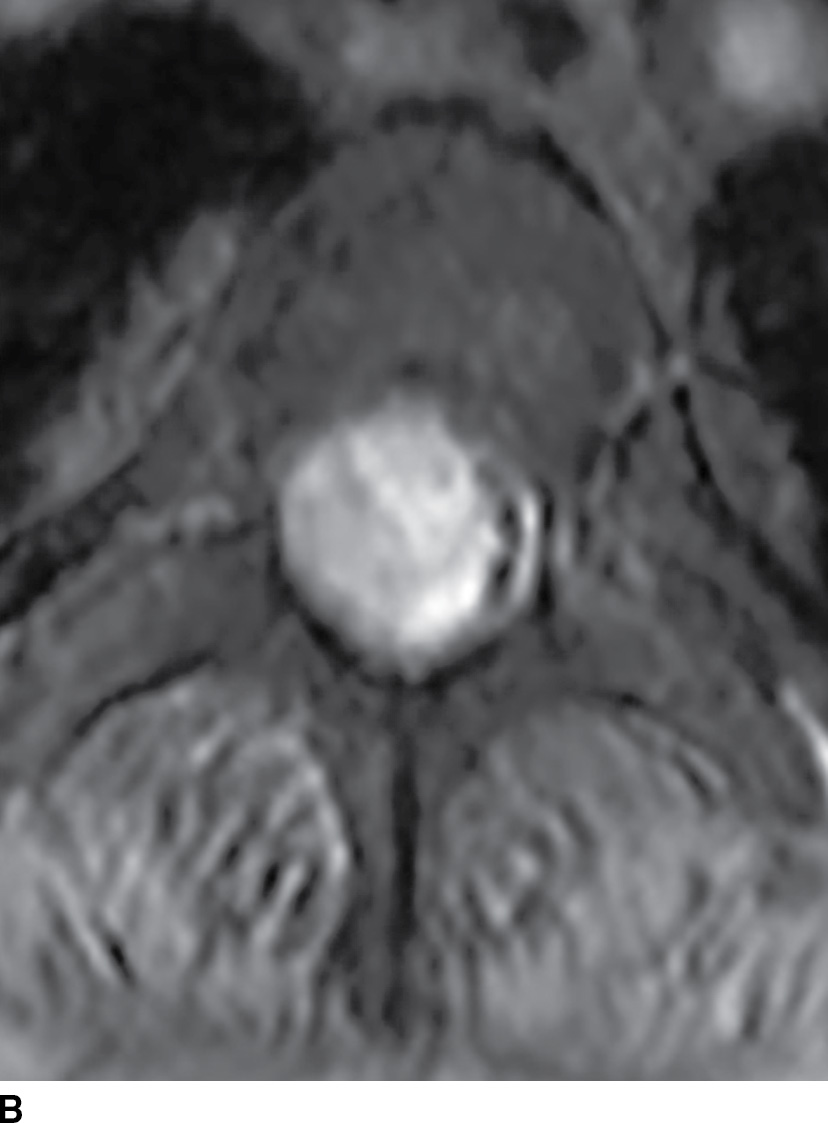
FIG. 17.8 Sagittal STIR (A) and axial contrast-enhanced T1 with fat suppression (B) reveal a homogeneously enhancing extramedullary intradural mass that nearly completely fills the canal resulting in severe cord compression. This lesion was ultimately confirmed to represent a meningioma.
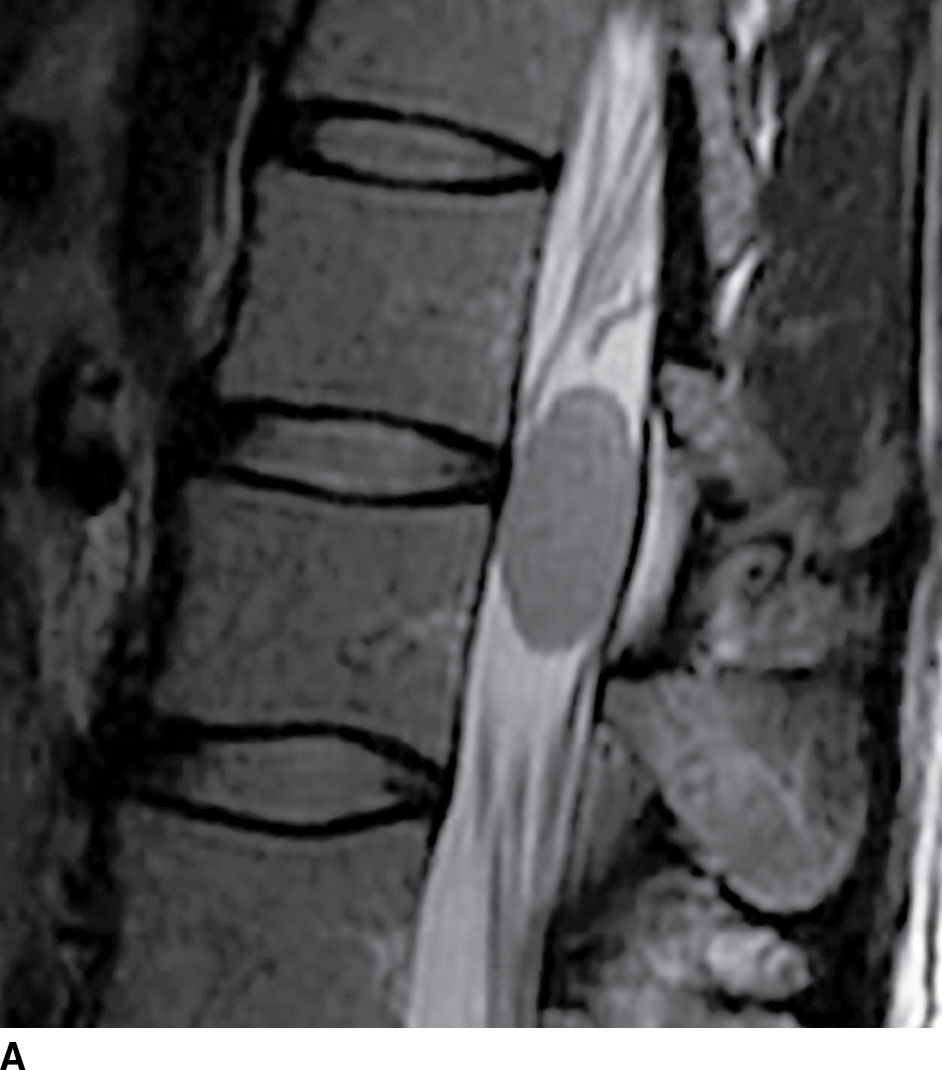
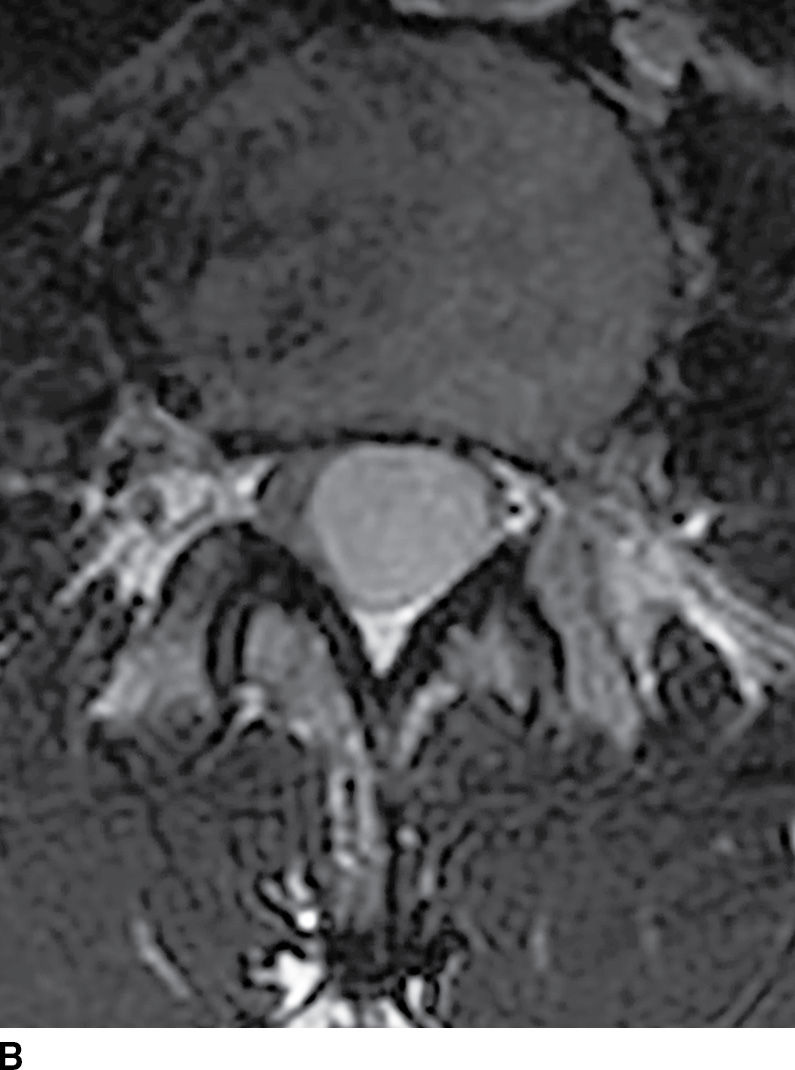
FIG. 17.8 Sagittal T2 (A) and contrast-enhanced axial T1 (B) images reveal a homogeneously enhancing ovoid extramedullary intradural mass representing a schwannoma.
Extramedullary intradural masses that do not enhance (Table 17.2) include arachnoid cysts, neurenteric cysts, and epidermoid cysts. Arachnoid cysts are CSF-filled cavities bounded by arachnoid matter usually located dorsal to the cord. These lesions are most frequently identified incidentally but can become symptomatic when they are large enough to cause cord compression (23). Smaller lesions often appear as a subtle focal contour deformity along the dorsal surface of the cord. This may be difficult to differentiate from the natural ventral deflection of the thoracic cord in the setting of an accentuated thoracic kyphosis; however, this should not exhibit a focal contour deformity. The vast majority of arachnoid cysts occur in the thoracic spine (80%), followed by cervical (15%) and lumbar (5%) locations (24). The signal characteristics within the cyst are normally isointense to CSF on all sequences.
A rare mimic of the arachnoid cyst is the neurenteric cyst, also known as an endodermal cyst or enterogenous cyst (25) (Fig. 17.10). These lesions represent a distinct pathologic entity derived from remnants of the primitive streak of early embryonic development. Endodermal cysts have a predilection for the cervicothoracic region and, in contrast to arachnoid cysts, normally arise within the ventral subarachnoid space, displacing or compressing the cord posteriorly. Intensity within these lesions is variable depending on the amount of protein content but is commonly similar to CSF signal (23). The inclusion of this lesion within the preoperative differential diagnosis may be critical since the surgical approach requires complete removal of the cyst wall to minimize recurrence. This lesion is also frequently associated with vertebral anomalies.
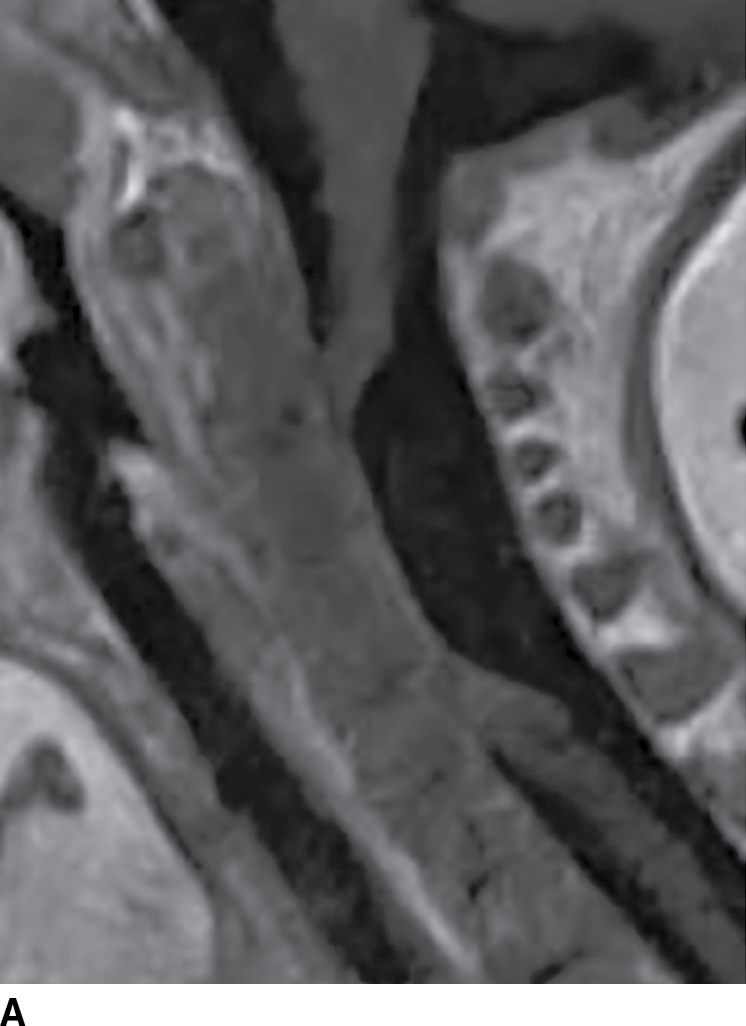
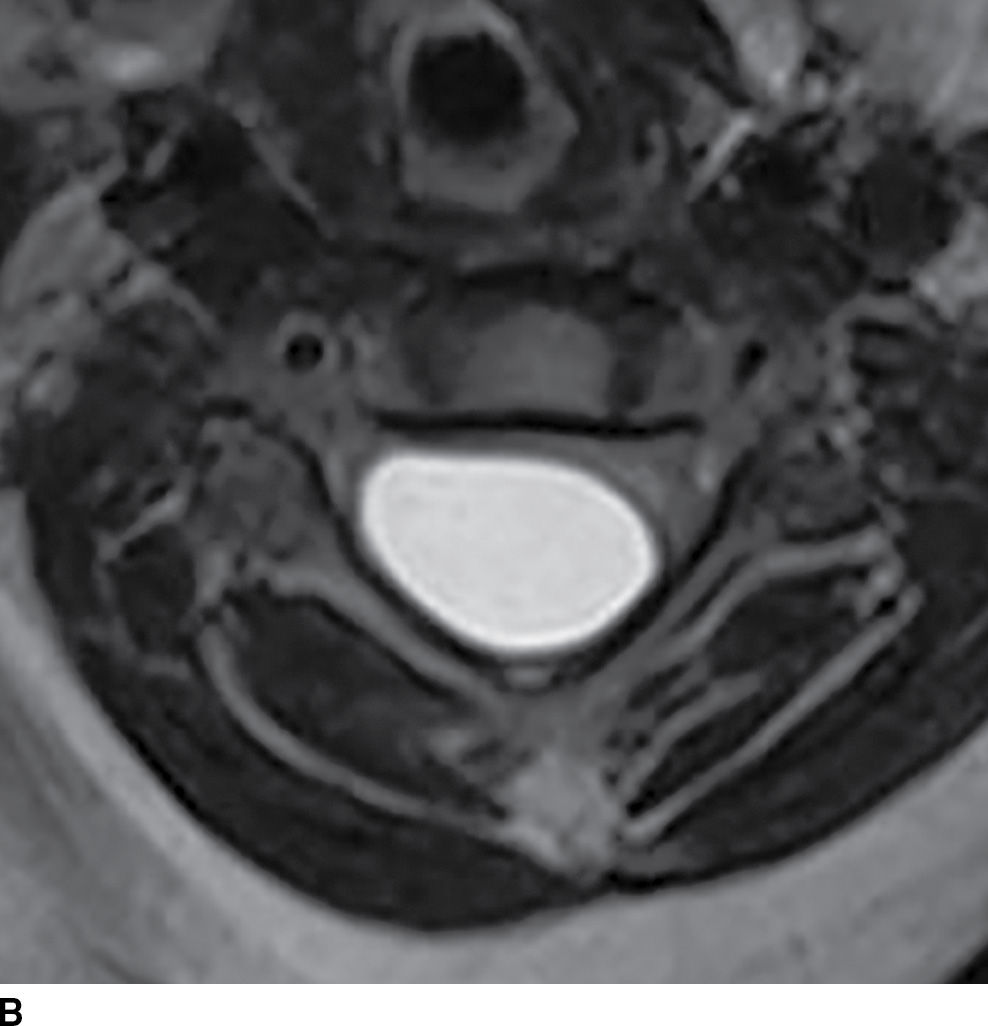
FIG. 17.10 Contrast-enhanced sagittal T1 (A) and axial T2 (B) images reveal a large nonenhancing right dorsolateral extramedullary intradural cystic mass that was ultimately confirmed to represent an endodermal cyst in this pediatric patient. Although endodermal cysts are most frequently encountered in the ventral epidural space, this lesion presented dorsally, mimicking an arachnoid cyst.
Spinal epidermoids (Fig. 17.11) can also be mistaken for other nonenhancing cystic masses. Epidermoids are inclusions of stratified squamous epithelium that lack dermal appendages such as glands or hair follicles. These lesions are rare and can be either congenital or acquired. The MRI characteristics can be nearly identical to those of CSF, and this lesion may be easily confused with an arachnoid cyst. On myelography, this solid lesion does not fill with contrast. A dynamic CT myelogram can distinguish an arachnoid cyst from an epidermoid since arachnoid cysts can exhibit progressive filling on delayed imaging (22,23). Furthermore, unlike arachnoid cysts, these lesions are hyperintense on diffusion-weighted images.
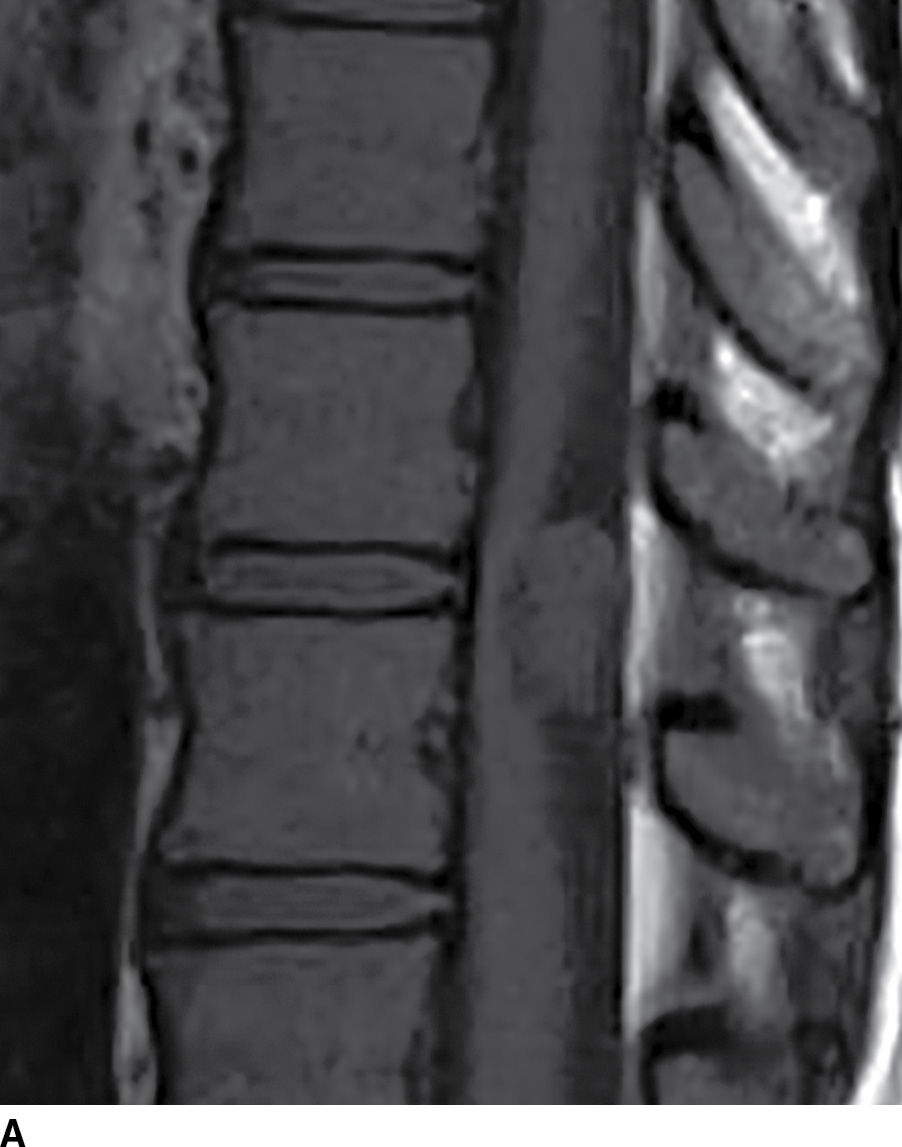
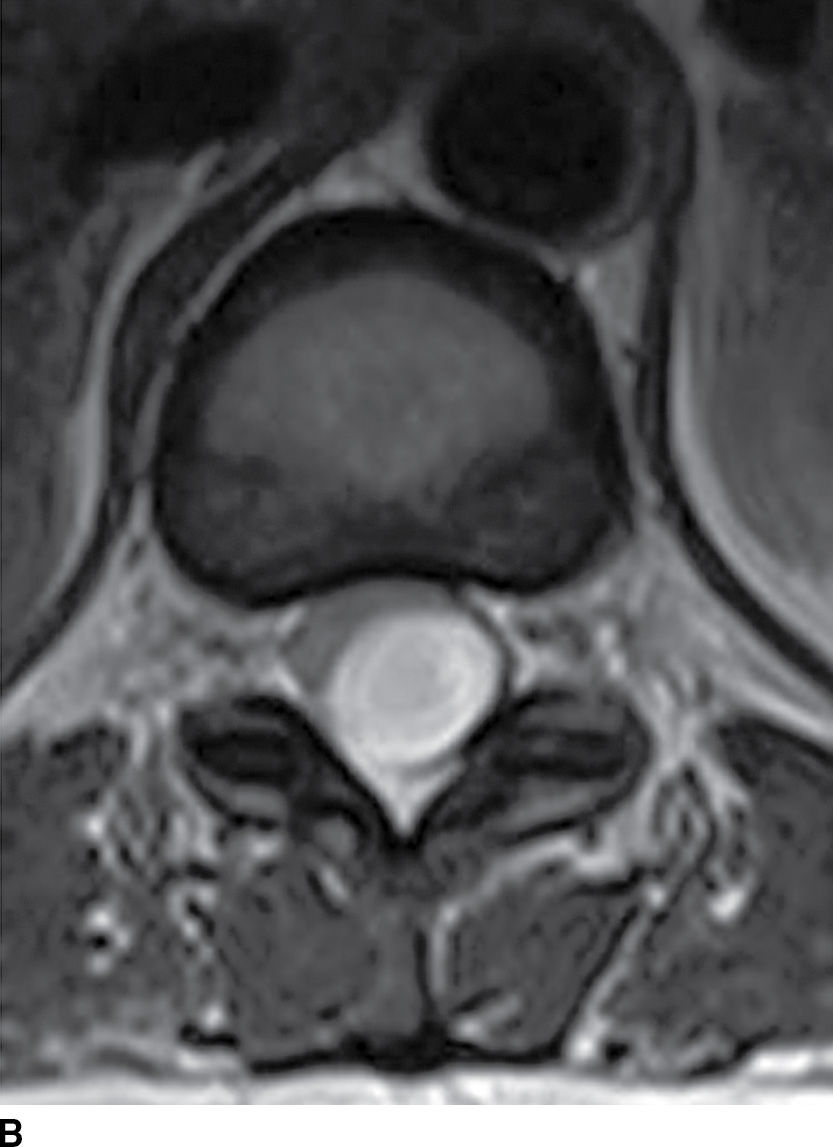
FIG. 17.11 Sagittal T1 (A) and axial T2 (B) images reveal a nonenhancing extramedullary intradural mass located dorsal to and compressing the thoracic spinal cord. This lesion was ultimately diagnosed as an epidermoid, but it could have been mistaken for an arachnoid cyst due to the similar imaging characteristics.
Transdural cord herniations are unique lesions that can mimic a nonenhancing extramedullary mass (26). In the presence of an acquired or spontaneous dural injury, the cord may herniate into the resulting defect, leaving a CSF-filled void opposite the dural injury (Fig. 17.12). This prominent space can be mistaken for a nonenhancing cystic mass. Axial images can be helpful in the diagnosis of a herniated cord since the cord will often appear small, rotated, or displaced (26). Similar to transdural cord herniations, arachnoid webs or bands, which are often invisible on MRI, can deform and tether the cord and create a focally enlarged CSF space that mimics an underlying arachnoid cyst. Imaging features that are associated with arachnoid webs include the “scalpel sign” on sagittal imaging and flattening of the cord visible on axial imaging (27). Myelography can be an effective tool to distinguish transdural cord herniation or arachnoid web from an intradural arachnoid cyst (Fig. 17.13) or other mass since real-time fluoroscopy should demonstrate prompt filling of the CSF space with no evidence of spinal block. High-resolution, heavily T2-weighted steady-state sequences (CISS, FIESTA) are also very useful for identifying cyst walls, webs, and dural defects.

FIG. 17.12 A sagittal image from a CT myelogram demonstrates features of a transdural thoracic cord herniation. Although the initial suspicion based on MRI features included an arachnoid cyst, the myelogram clearly illustrates prompt filling of the subarachnoid space with no evidence of an intradural mass.
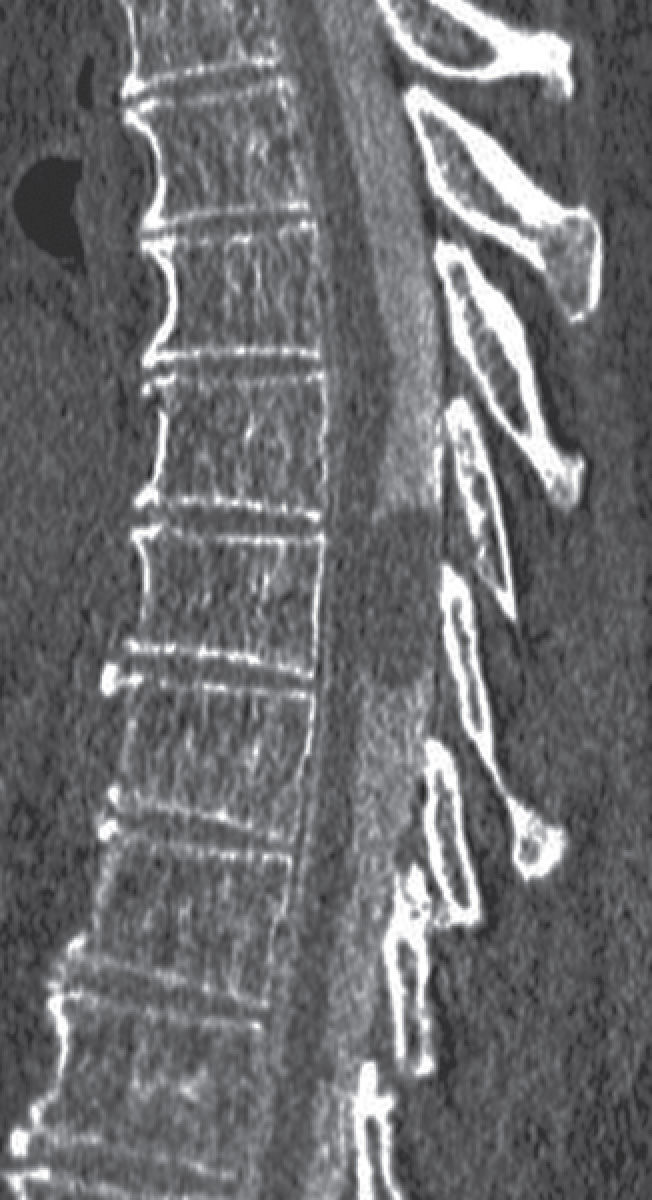
FIG. 17.13 A sagittal image from a CT myelogram demonstrates an oval filling defect within the thecal sac caused by an arachnoid cyst.
Nerve Roots
Intradural nerve root enhancement can be either localized or diffuse. Isolated nerve root enhancement is most commonly encountered in the setting of spinal stenosis or disc herniation (28,29). The precise cause of enhancement in this setting is not well understood, but it is felt to be due to blood–brain barrier breakdown or enhancement of the radicular veins (30). The presence of nerve root enhancement in the setting of stenosis or nerve root compression does not accurately predict radiculopathic symptoms, but it can often be helpful for the confirmation of clinically relevant disease (28). By contrast, diffuse nerve root enhancement can be seen in a broad variety of clinical conditions; therefore, establishing a firm diagnosis on the basis of imaging alone is difficult. Factors contributing to nerve root enhancement can include demyelinating, infectious, inflammatory, and malignant conditions. A list of disease entities that result in nerve root enhancement is provided in Table 17.3.
Table 17.3 Nerve Root Enhancement
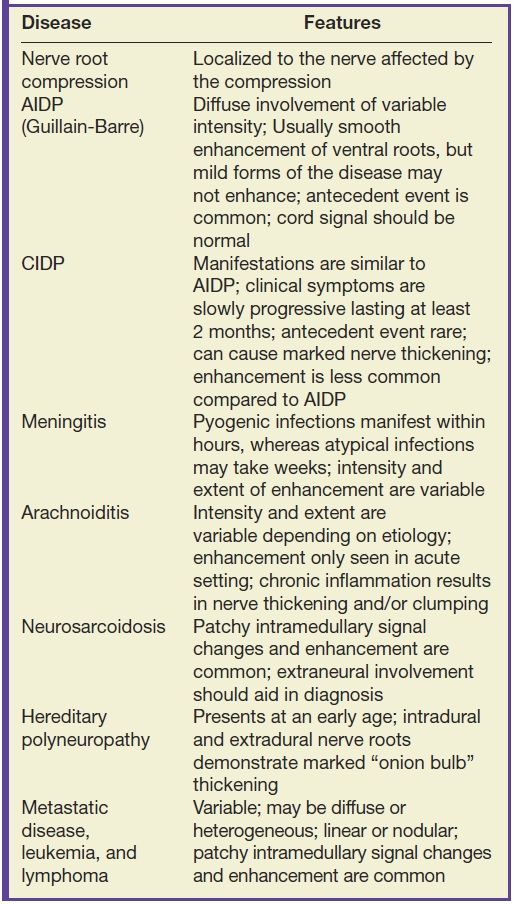
Acute inflammatory demyelinating polyneuropathy (AIDP), the prototypical form of Guillain-Barre syndrome, is a monophasic demyelinating syndrome that can present with diffuse, usually smooth, intradural nerve root enhancement of variable degrees. Mild presentations of the disease can demonstrate little or no enhancement. When nerve root enhancement is present, there is preferential involvement of the ventral roots. Severe presentations can exhibit profound enhancement involving dorsal and ventral nerve roots and even cranial nerve enhancement (31) (Fig. 17.14). In the setting of AIDP, there should be no associated intramedullary signal changes. The majority of patients presenting with this disease report an antecedent event such as infection, vaccination, or surgery, and the initial symptoms should occur within 3 weeks of the precipitating factor (31). Chronic inflammatory demyelinating polyneuropathy is similar to AIDP in many ways except an antecedent event such as infection or immunization is rarely identified and symptoms progress over a period of at least 2 months. Imaging features are similar, although enhancement is less common.
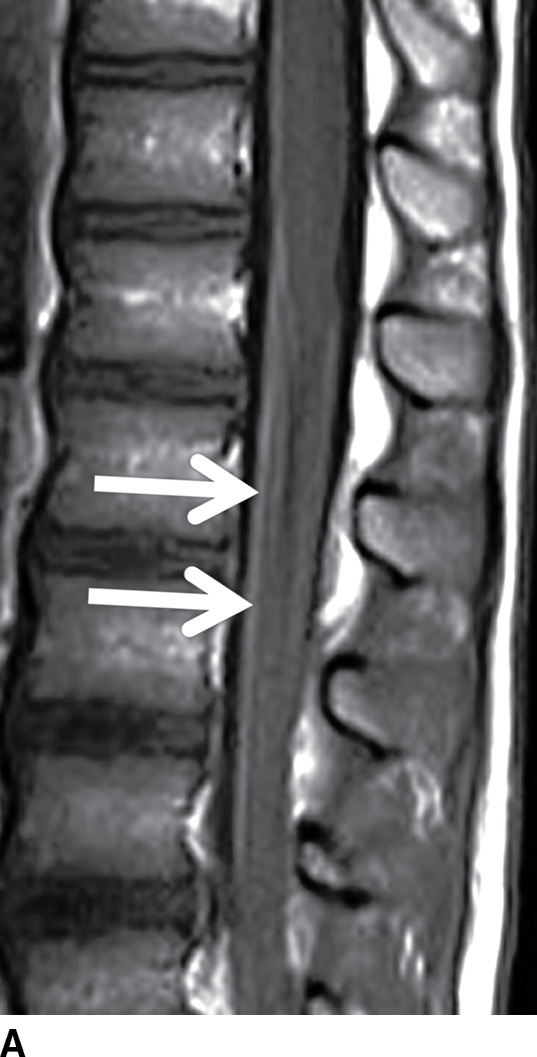
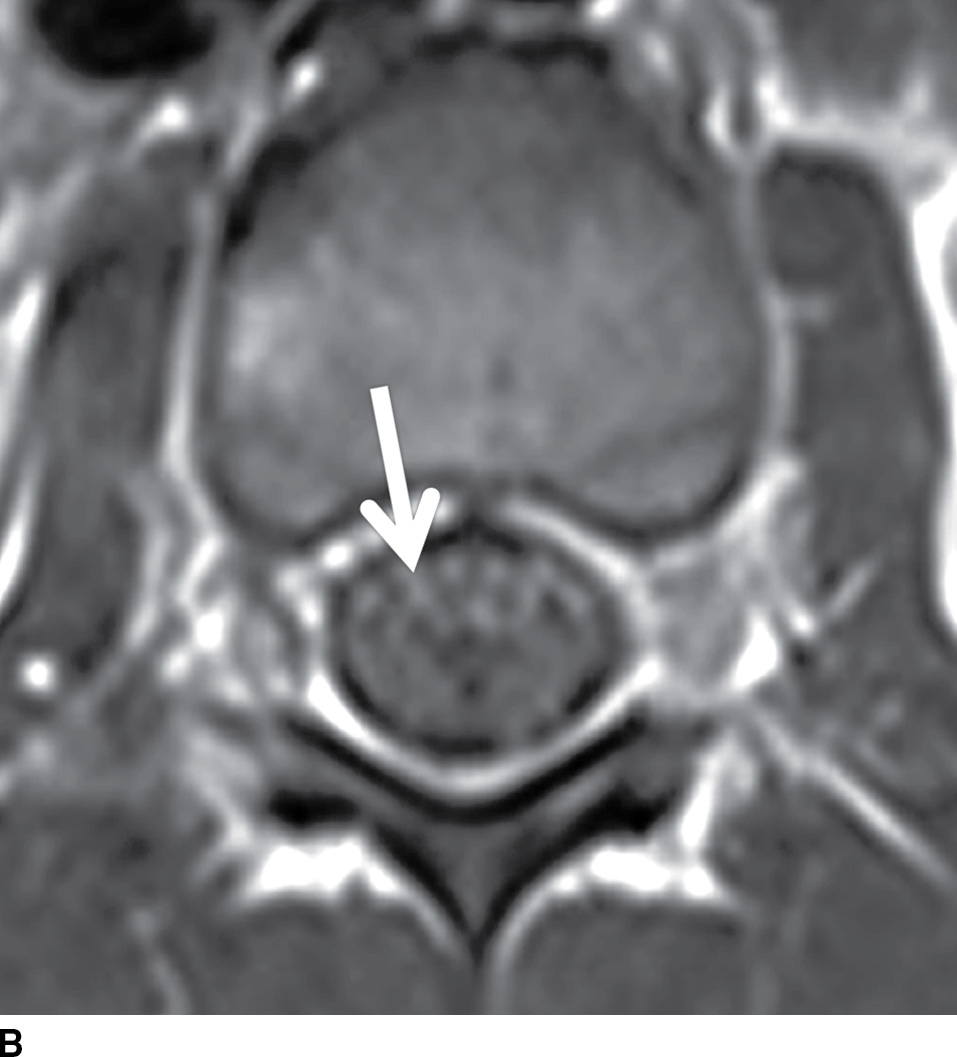
FIG. 17.14 Contrast-enhanced sagittal and axial T1 images (A,B) reveal extensive enhancement (double arrows) of the cauda equina caused by acute inflammatory demyelination polyneuropathy (Guillain-Barre). The axial image demonstrates relatively greater enhancement of the ventral nerve roots (single arrow).
Diffuse nerve root enhancement can also be seen in the setting of leptomeningeal metastatic disease (Fig. 17.15). The most common primary CNS neoplasms that cause drop metastases include glioblastoma, anaplastic astrocytoma, medulloblastoma, ependymoma, and germinoma. The most common systemic tumors that metastasize to the leptomeningeal surfaces include adenocarcinoma of the breast and lung, melanoma, lymphoma, and leukemia. The extent of leptomeningeal enhancement can be variable depending on the burden of disease. Early manifestations of leptomeningeal disease can be subtle or undetectable. The absence of enhancement should not dissuade a lumbar puncture since CSF cytology remains more sensitive than MRI. When disease burden is heavy, nerve root enhancement may be accompanied by intramedullary signal changes and nodular enhancement, which should allow the radiologist to distinguish metastatic disease from AIDP.
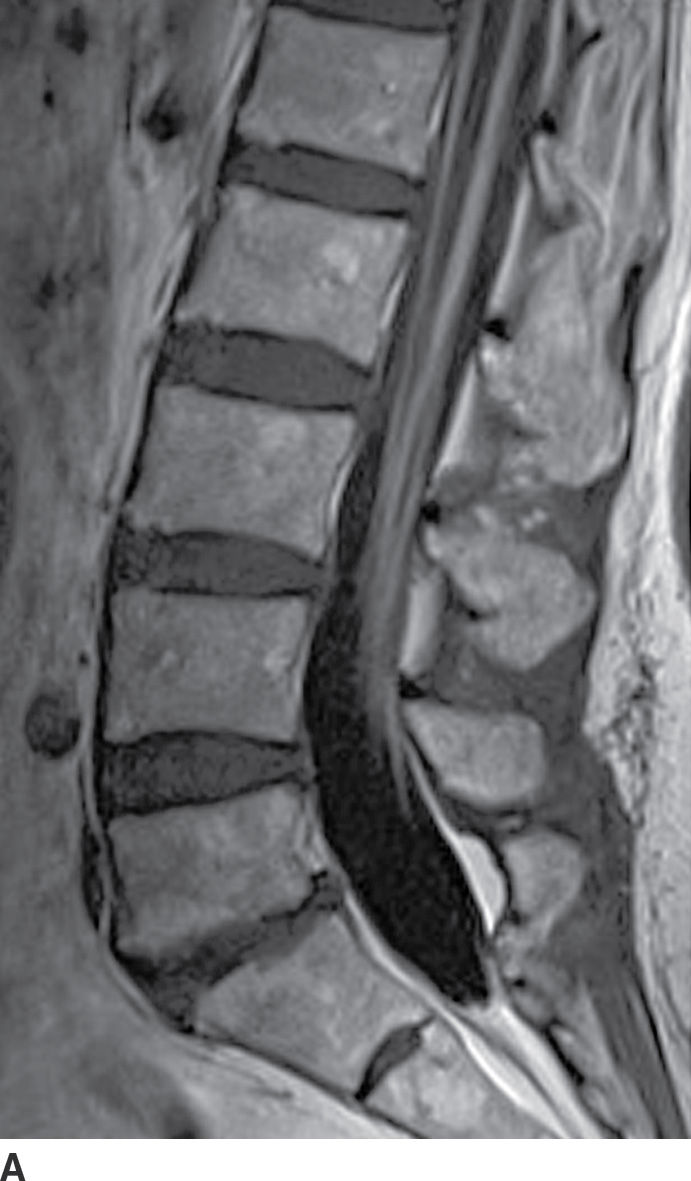
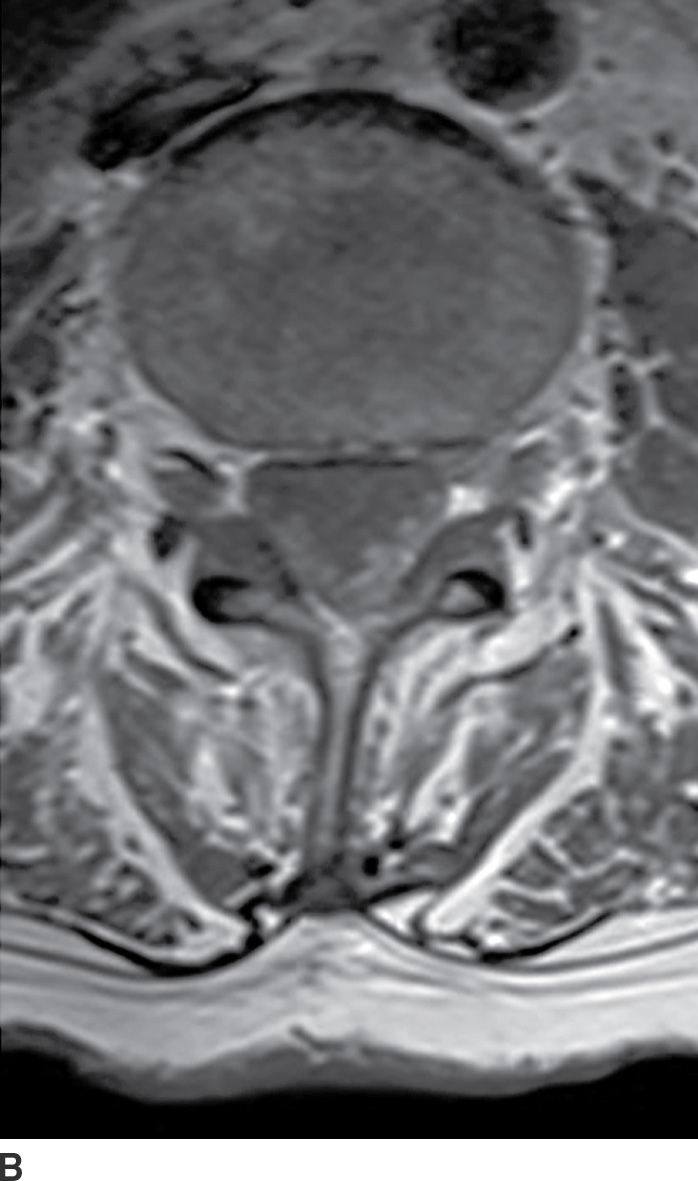
FIG. 17.15 Contrast-enhanced sagittal and axial T1 images (A,B) illustrate diffuse thickening and heterogeneous enhancement of the cauda equina in a patient with glioblastoma and leptomeningeal dissemination.
Other conditions that can result in diffuse smooth and/or nodular nerve root enhancement include spinal meningitis, sarcoidosis, and hereditary polyneuropathies. Some organisms such as tuberculosis, syphilis, and fungi typically produce symptoms over several days or weeks, whereas pyogenic and viral infections typically produce symptoms within hours. Inflammatory conditions such as sarcoidosis can also produce pathologic enhancement of the nerve roots that may be indistinguishable from meningitis or leptomeningeal metastatic disease; however, neurosarcoidosis frequently occurs after the development of mediastinal adenopathy. Chronic idiopathic demyelinating polyneuropathy and some forms of hereditary polyneuropathies such as Dejerine-Sottas and Charcot-Marie-Tooth disease usually present with more thickening of the intradural and/or extradural nerve roots compared to other diseases.
Arachnoiditis is a chronic manifestation following any intrathecal inflammatory condition such as meningitis, surgery, intrathecal hemorrhage, or myelography. There is no specific clinical syndrome for this disease, and symptoms may vary. Imaging manifestations are nonspecific and are characterized by the presence of clumped and adherent nerve roots that can produce what is known as an empty thecal sac sign in the advanced stages (Fig. 17.16).
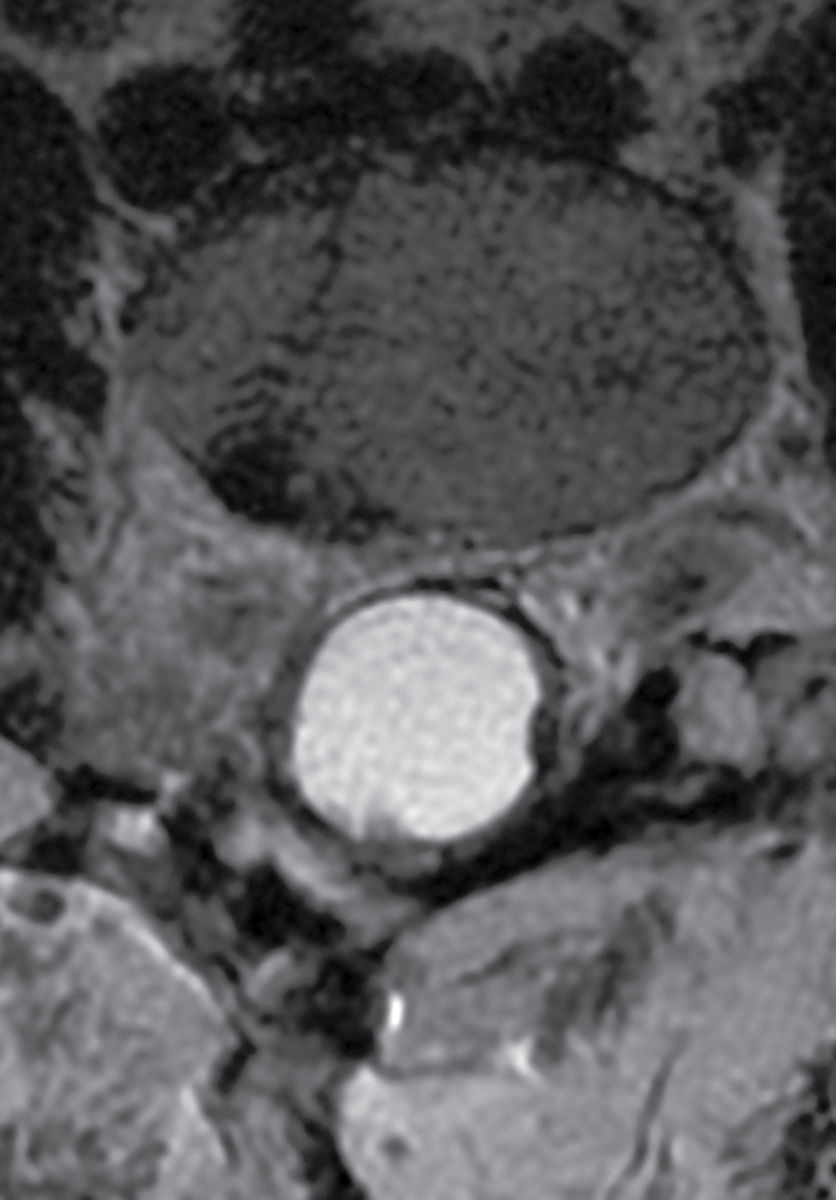
FIG. 17.16 An axial T2 image from the lumbar spine reveals late manifestations of arachnoiditis with clumping and thickening of the nerve roots that adhere to the arachnoid membrane creating the classic “empty sac” sign.
Extradural Compartment
Masses and some collections that form in the extradural compartment often compress the cord resulting in myelopathy, while other types of extradural collections produce a more benign or indolent clinical course. It is important to recognize characteristics that differentiate the types of extradural collections so that prompt and accurate diagnosis can facilitate the implementation of appropriate therapy and avoid unnecessary intervention. Table 17.4 highlights the characteristic features of various extradural collections.
Table 17.4 Extradural Collections
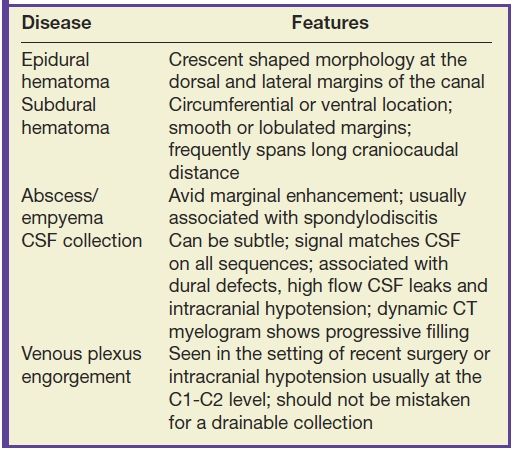
Hematomas that form in the epidural space can rapidly expand causing cord compression or ischemia if unrecognized. Epidural hematomas are encountered in up to 40% of cases of acute spinal trauma (Fig. 17.17), but they can also appear spontaneously in patients with predisposing factors such as anticoagulation or hemophilia (32,33). CT or MRI is usually effective in demonstrating epidural hematomas, with MRI being favored due to superior contrast resolution, superior detection of hemorrhage with gradient echo sequences, and superior evaluation of the spinal cord. Epidural hematomas are typically crescent-shaped collections located along the dorsal or lateral margins of the canal. The signal characteristics depend on the stage of hemorrhage (typically T1 isointense and T2 hyperintense oxyhemoglobin in the first few hours and T1 isointense and T2 hypointense deoxyhemoglobin after the first few hours to several days). The early clinical manifestations of epidural hematoma can include pain, headache, myelopathy, or radiculopathy (33). Occasionally, subacute hematomas can show enhancement and mimic inflammatory or neoplastic masses. For example, hematomas can exhibit peripheral enhancement, which is thought to represent hyperemia. Also, in the setting of active hemorrhage, hematomas can demonstrate a central point of enhancement that is analogous to the “spot sign” in CT angiography. Recognizing these phenomena is critical to prevent misdiagnosis and delay of treatment (34,35). Subdural hematomas (Fig. 17.18) behave similar to their epidural counterparts from a clinical standpoint, but their imaging manifestations are slightly different. Subdural hematomas are typically circumferential or ventral to the cord with a more lobulated internal contour and frequently extend along a greater craniocaudal distance (36).
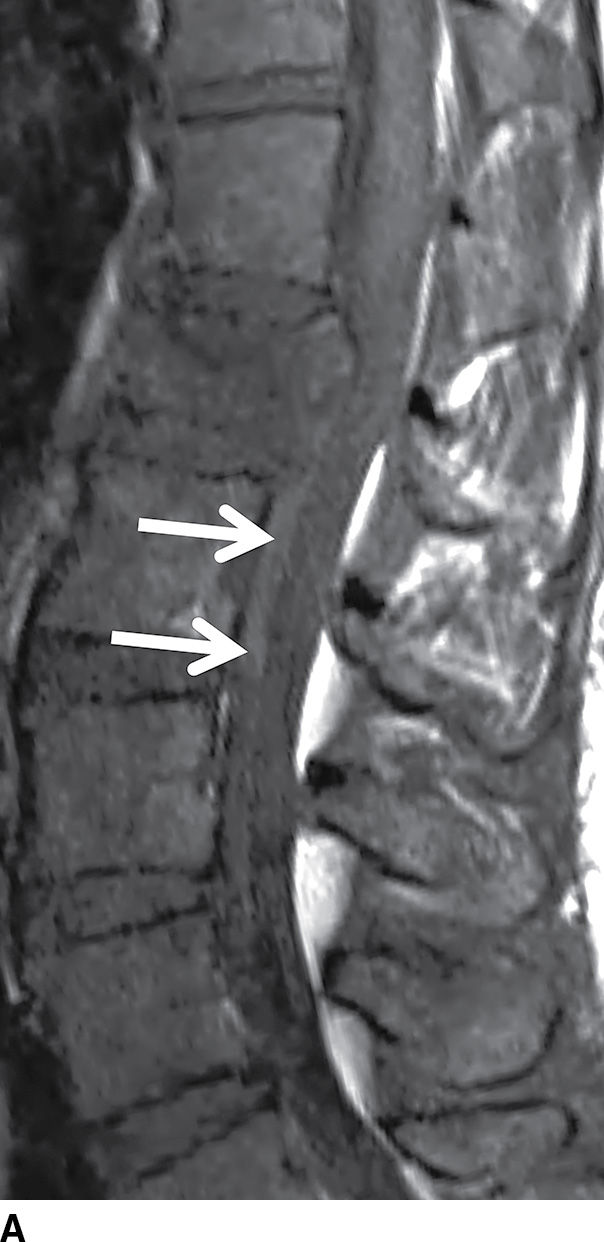
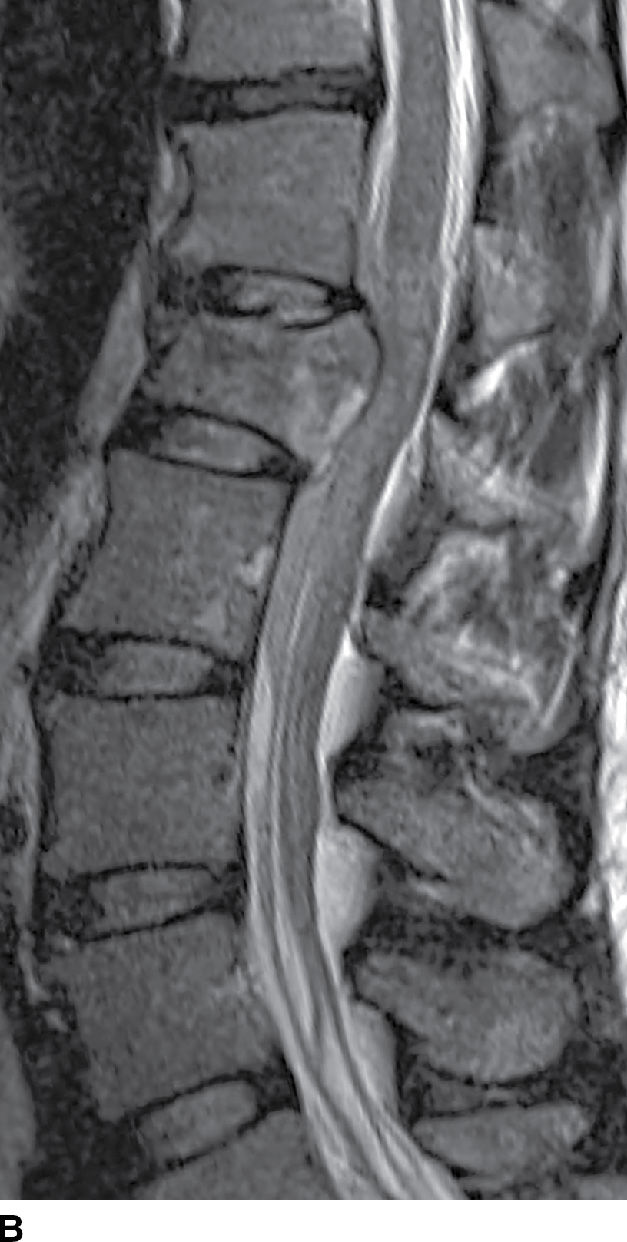
FIG. 17.17 An acute traumatic compression fracture of the L1 vertebral body compresses the conus medullaris and is accompanied by a hyperacute ventral epidural hematoma (arrows) that is isointense to cord on sagittal T1 (A) and hyperintense to cord on sagittal T2 (B) images. The collection displaces the intrathecal contents posteriorly. The fracture lines are also delineated on the sagittal T2 images.
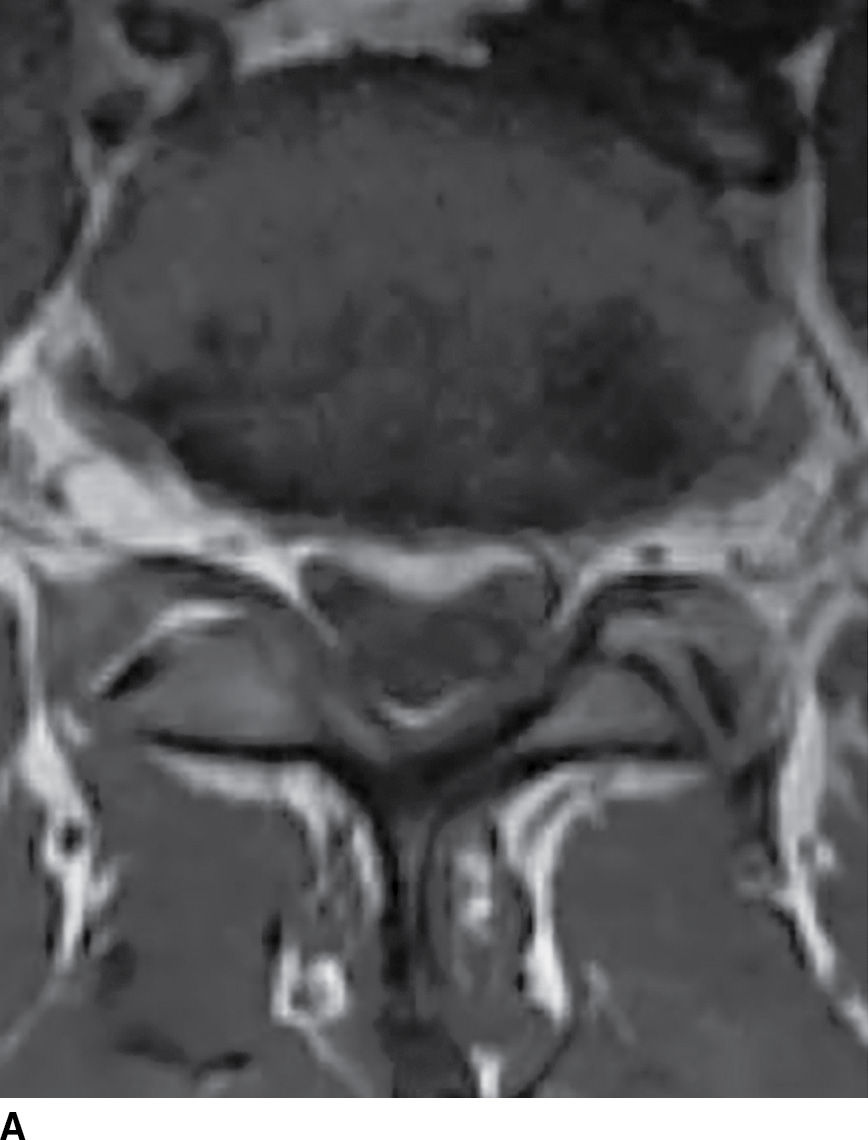
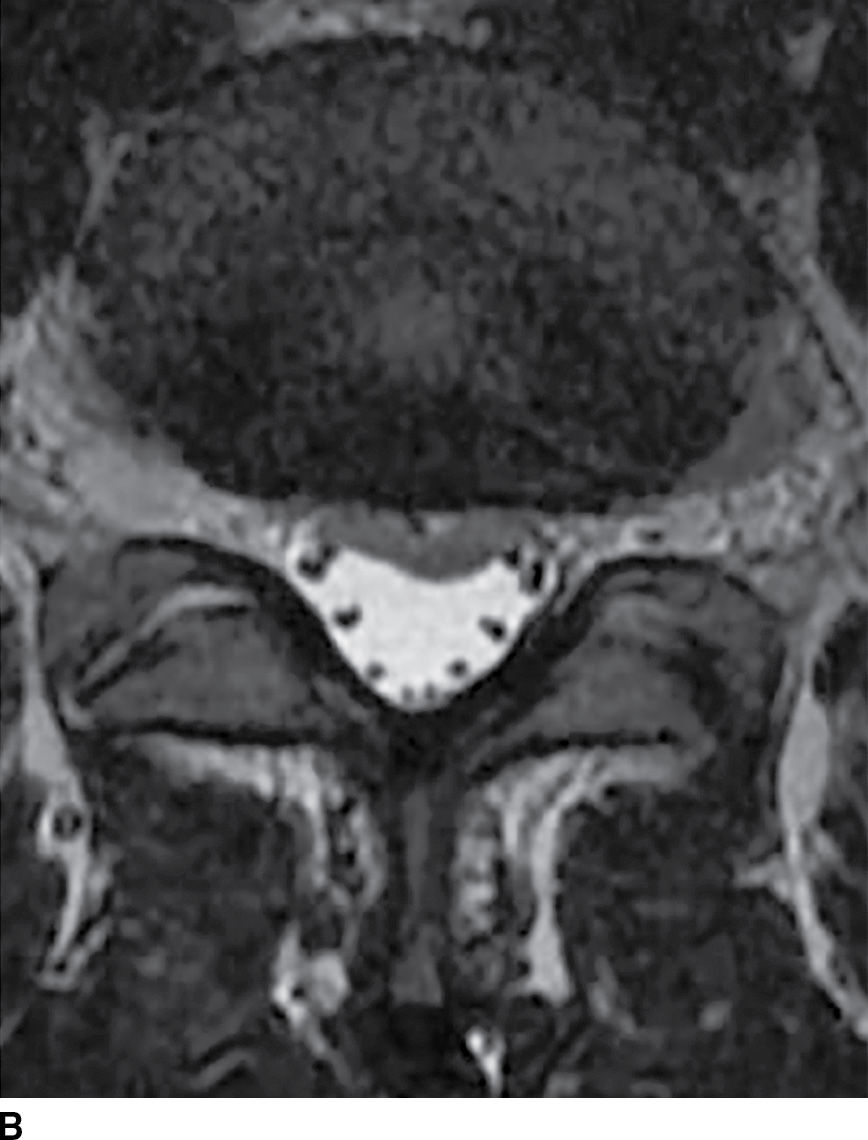
FIG. 17.18 Axial T1 (A) and axial T2 (B) images reveal a small acute subdural hematoma. There is a lobulated eccentric T1 hyperintense and T2 hypointense collection along the ventral surface of the canal.
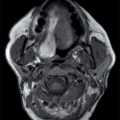
CSF collections in the epidural space are often seen in the setting of spinal headache and are usually associated with high-flow CSF leaks. These extradural collections may be iatrogenic or spontaneous. The location is most commonly ventral in the cervicothoracic region, and it can extend along several vertebral segments, often remote from the site of the dural defect. MRI features may be subtle but are usually effective in identifying these lesions. Dynamic CT myelogram or high-resolution MRI may be necessary to better characterize the findings or assist in the identification of the precise location of the dural tear (Fig. 17.19). Neurologic symptoms may or may not be present. Imaging the brain can frequently provide clues to the presence of a CSF leak with characteristic features including brain stem sagging, smooth pachymeningeal enhancement, and subdural collections. It is important to recognize however that brain imaging may be normal.
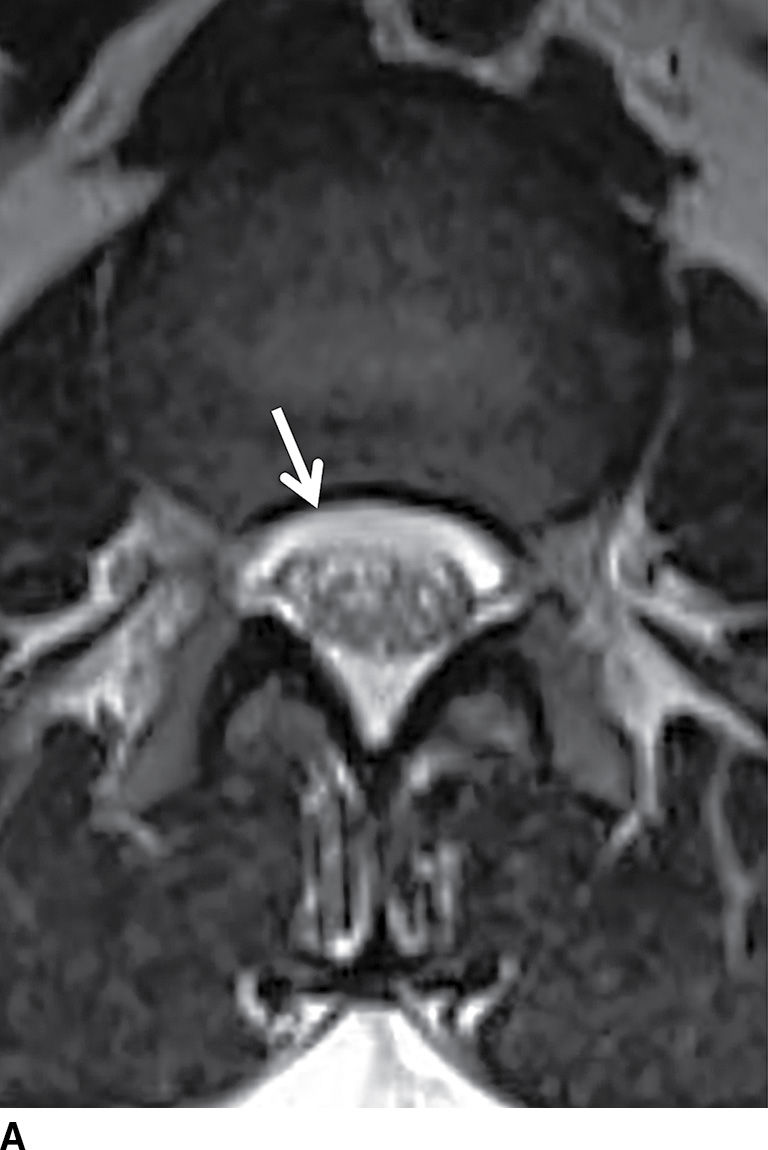
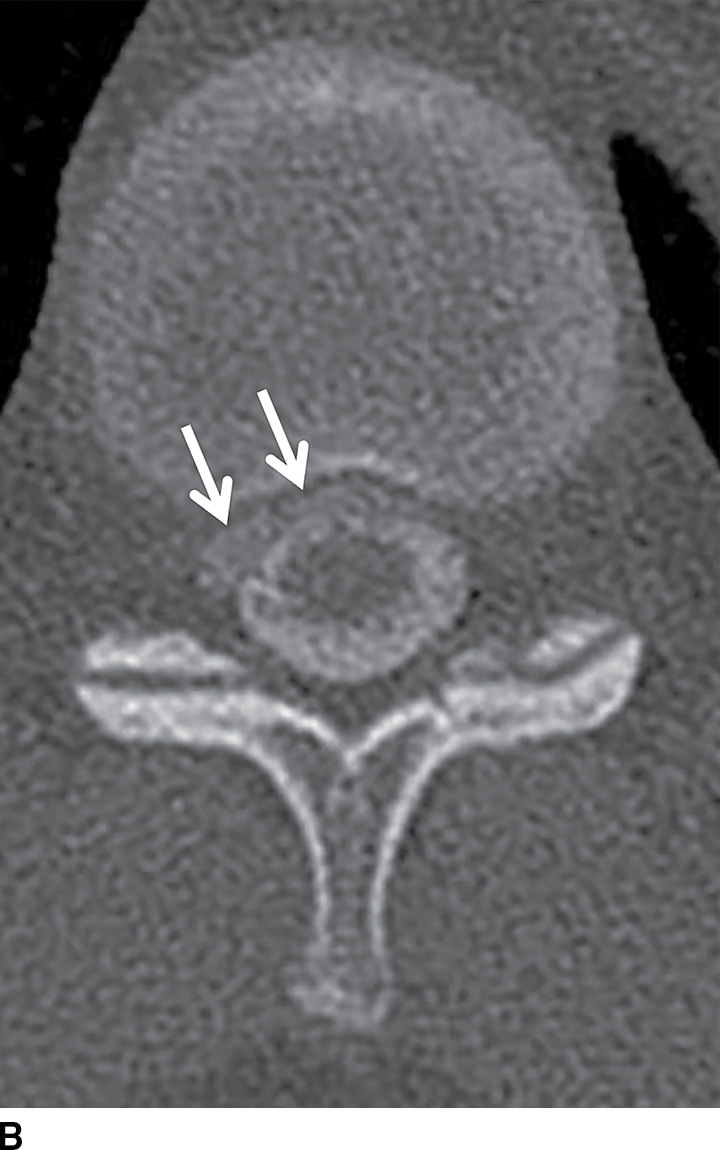
FIG. 17.19 An adolescent with neurofibromatosis type II struggling with a long-standing CSF leak that manifested as a ventral extra-arachnoid collection, matching CSF signal (single arrow) on the axial T2 image (A). An axial CT image (B) from the delayed phase of a dynamic CT myelogram illustrates the epidural collection (double arrows), which was not visible on the early images (not shown).
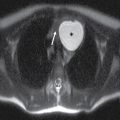
In contrast to the intracranial compartment where the dura closely adheres to the skull, in the upper cervical spine, the dura is loose and surrounded by a rich venous plexus that can engorge dramatically with fluctuations in CSF pressure. This phenomenon can be seen with recent surgery or in cases of overshunting (Fig. 17.20). These epidural varices should not be mistaken for an epidural hematoma, but caution should be exercised since rare instances of cord compression by a dilated varix have been documented (37). Epidural varices are a visual reminder of the Monro-Kellie hypothesis, which states that within the closed intracranial compartment, the sum volume of brain, CSF, and blood is constant so that a decrease in CSF volume will typically result in vascular engorgement causing meningeal enhancement, enlargement of the pituitary gland, and prominence of the cerebral venous sinuses and spinal epidural venous plexus (38).
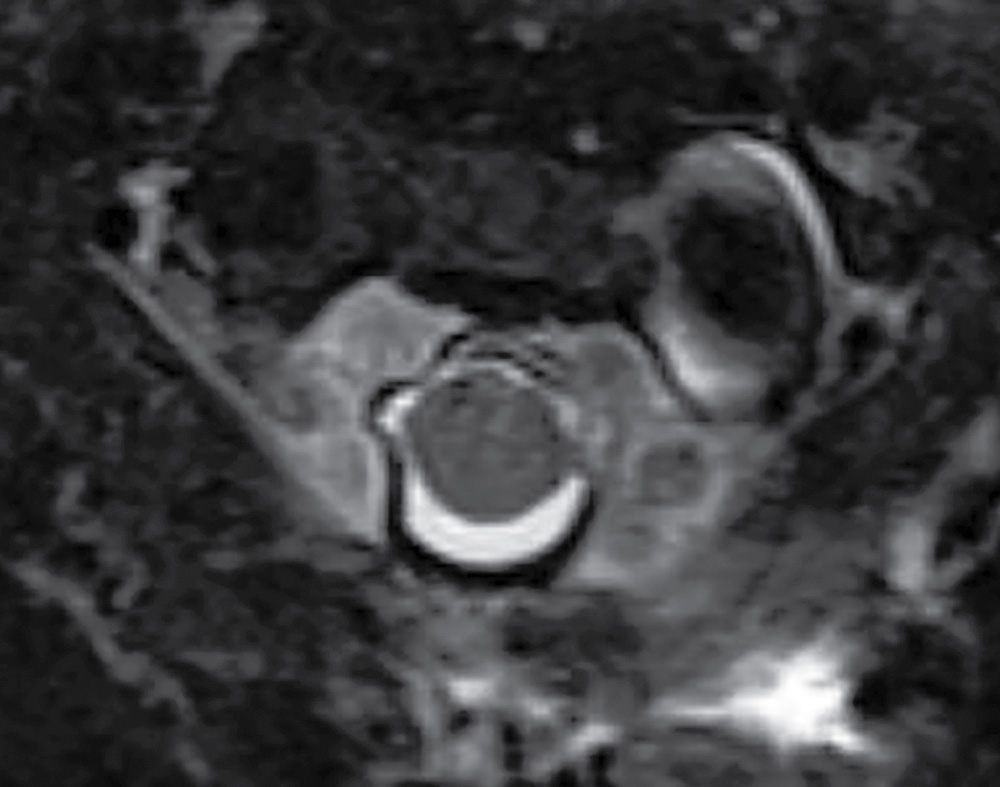
FIG. 17.20 An axial fat-saturated T2 image of the upper cervical spine obtained after a left retromastoid craniotomy reveals striking engorgement of the epidural venous plexus at the C1 to C2 level related to differences in intracranial pressure. This finding should not be misinterpreted as an epidural hematoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree