FIG. 22.1 Bones constituting the skull base. Graphical representation of bones of the skull base includes the unpaired midline bones, ethmoid, sphenoid, and occipital (red, teal, and blue, respectively), and the paired frontal temporal and parietal bones (purple, green, and orange, respectively).
(Courtesy of Amirsys, Inc.)
The floor of the anterior cranial fossa is formed anteriorly by the orbital plate of the paired frontal bones and the unpaired midline ethmoid bone and posteriorly by the lesser wings of the sphenoid, planum sphenoidale, and anterior clinoid process. The frontal bones and posterior wall of the frontal sinuses form the anterior and lateral boundaries of the anterior fossa. The superior relationships of the anterior cranial fossa are to the frontal lobes and cranial nerve I (CN I). The inferior relationships of the anterior skull base are to the roof of the orbits, nasal vault, and ethmoid and sphenoid sinuses.
Many landmarks of the anterior cranial fossa are important for disease recognition and surgical planning. The crista galli is a superiorly directed triangular midline process of the anterior ethmoid bone, which serves as an attachment to the anterior falx. The foramen cecum, anterior to the crista galli, is a blind-ending structure whose patency leads to developmental anomalies. The foramina of the cribriform plate (roof of the nasal vault) transmit afferent fibers from the olfactory bulbs to the nasal cavity. Important surgical landmarks of the anterior cranial fossa include the fovea ethmoidalis (roof of the ethmoid air cells), lateral to the cribriform foramina, and the anterior ethmoid foramina, which transmits the anterior ethmoid artery and vein, anterior to the cribriform foramina.
The central skull base is the floor of the middle cranial fossa and is created by the basisphenoid, the greater wing of the sphenoid, and the paired temporal bones anterior to the petrous ridge. The anterior margin is the planum sphenoidale (tuberculum sellae) and lesser wing of the sphenoid. The posterior margin of the central skull base is the dorsum sella and petrous ridges. The superior relationships of the central skull base include the pituitary gland, the cavernous sinus and its contents, the trigeminal (Meckel) cave, and the temporal lobes. Inferior to the central skull base is the deep spaces of the suprahyoid neck including the pharyngeal mucosal surface (or space), parapharyngeal space, masticator space, carotid space, and parotid space.
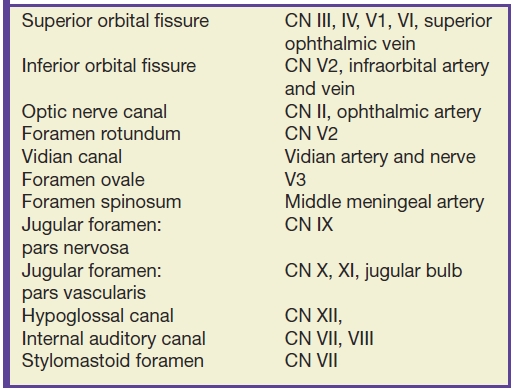
Many important cranial nerve foramina and the midline sella turcica are contained within the central skull base (Fig. 22.2). The sella turcica is a depression in the sphenoid body containing the pituitary gland. The optic canal, which transmits the optic nerve (CN II), is formed by the lesser wing of the sphenoid bone. The superior orbital fissure is lateral to the optic canal, is formed by a cleft between the lesser and greater wings of the sphenoid bone, and transmits cranial nerves III, IV, V1, and VI and the superior ophthalmic vein. The inferior orbital fissure is inferior and lateral to the optic canal, is formed by a cleft between the greater wing of the sphenoid and maxillary bones, and transmits the infraorbital artery, vein, and nerve and a branch of V2. The carotid canal is formed by the greater wing of the sphenoid bone and the temporal bone and transmits the carotid artery and associated sympathetic plexus.
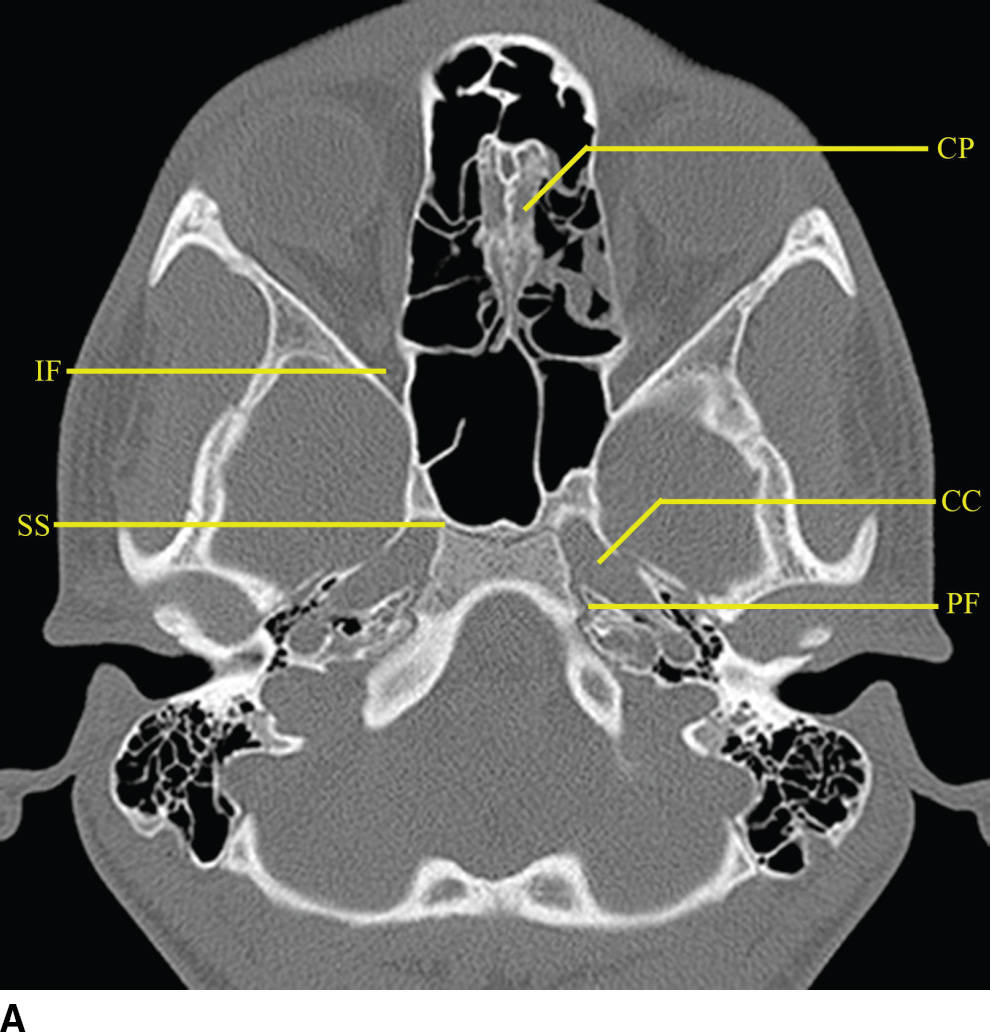
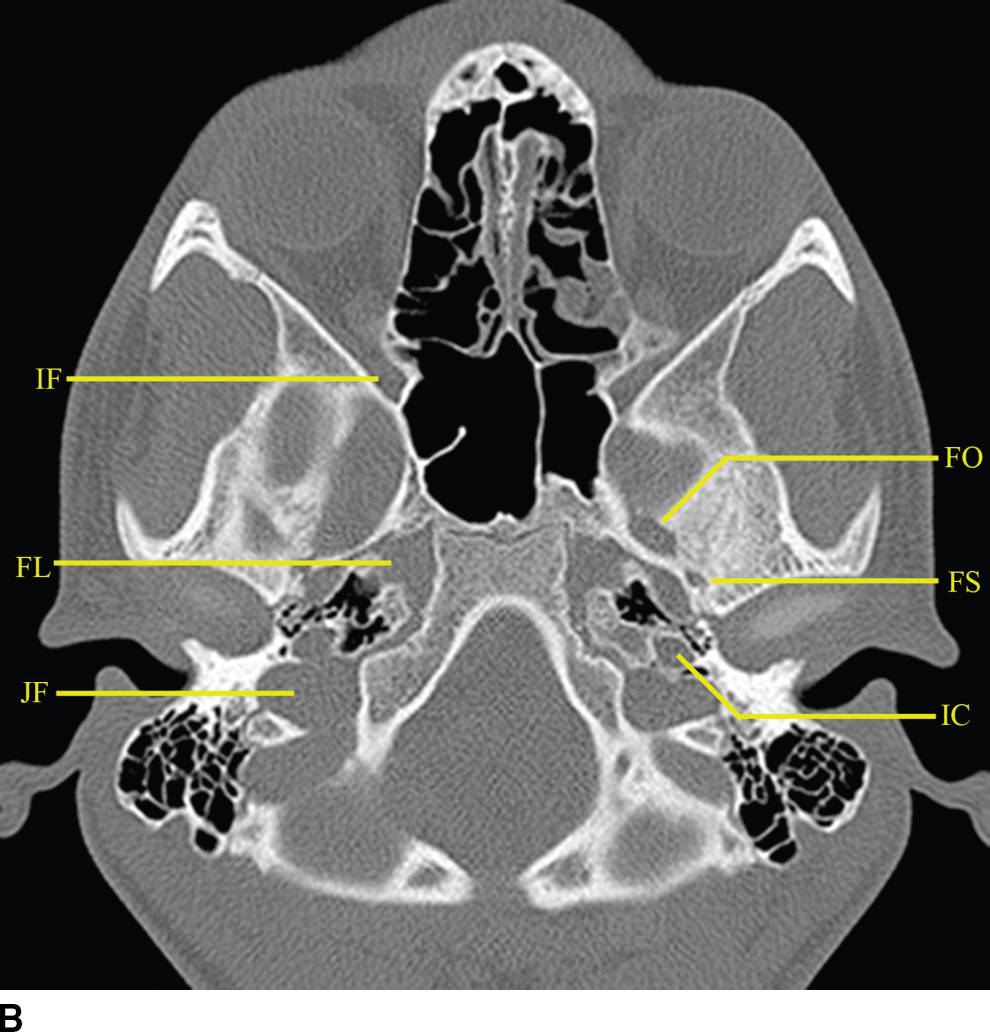
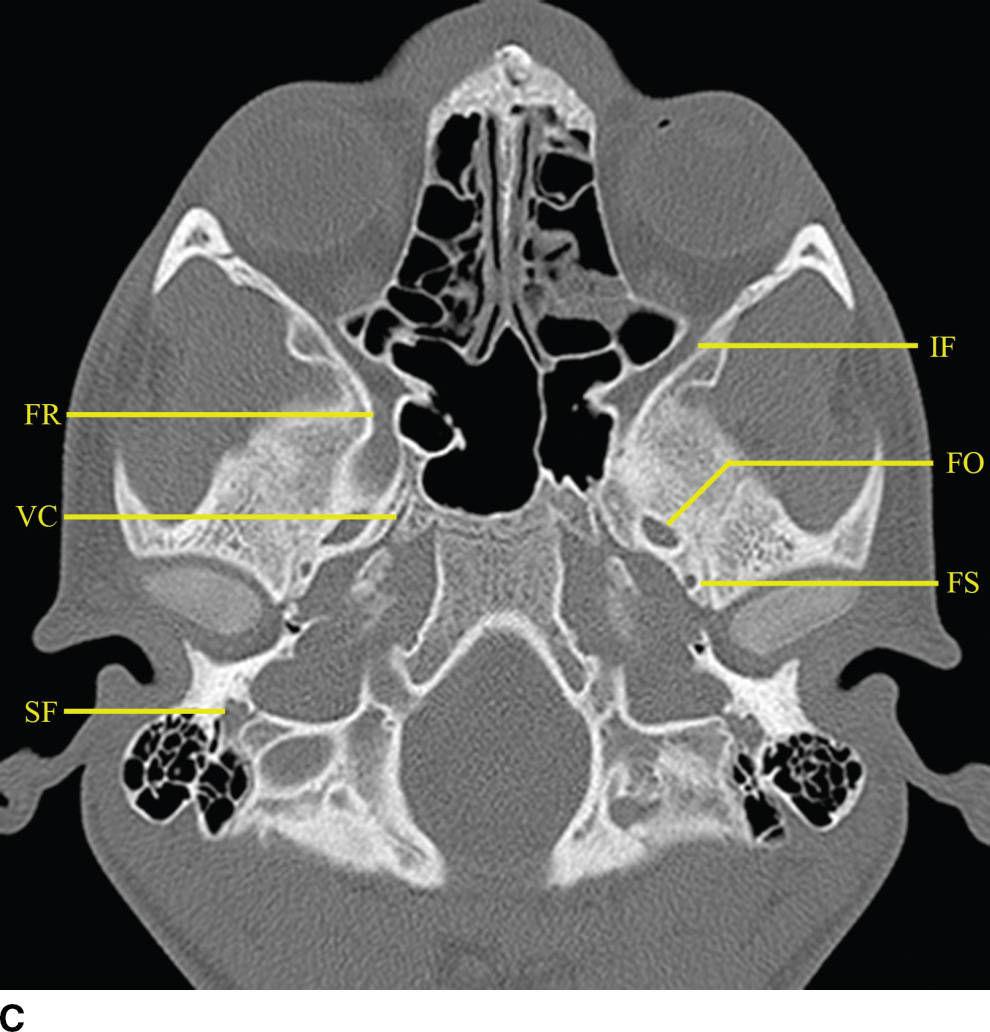
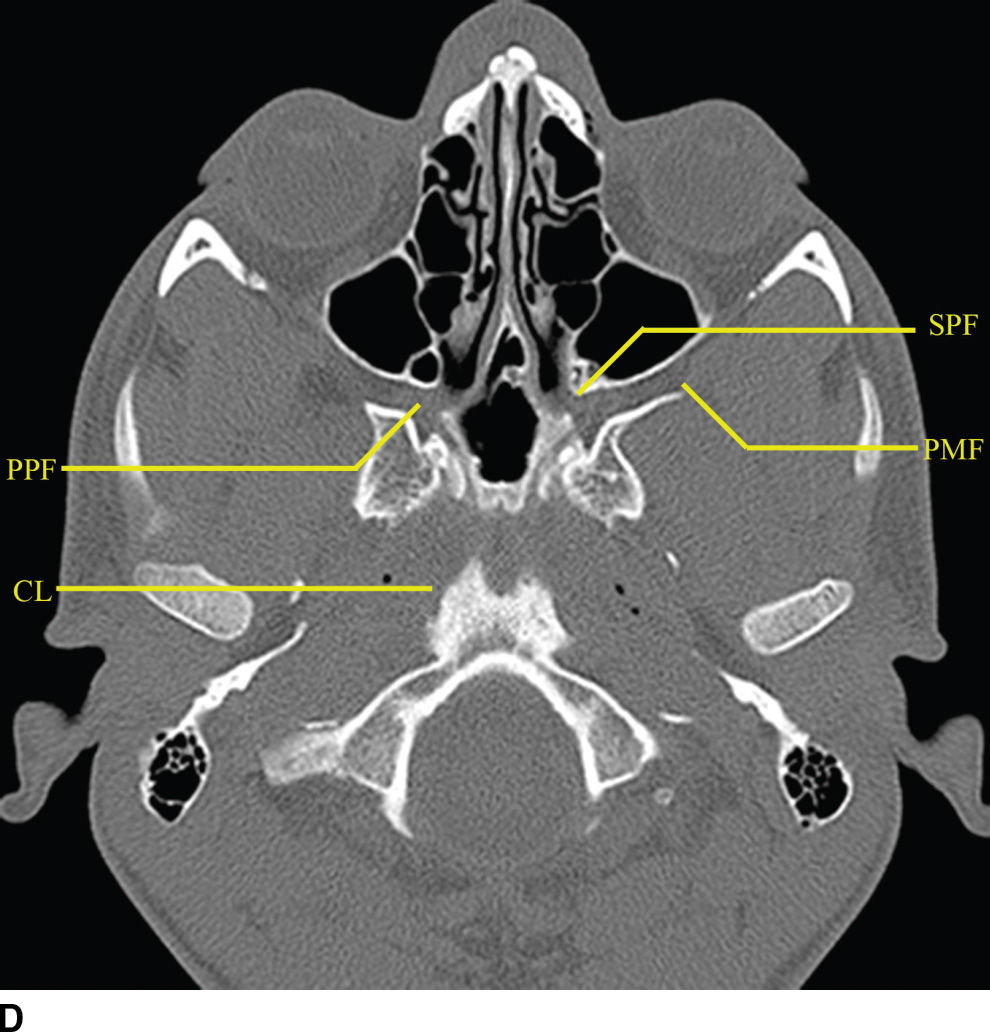
FIG. 22.2 Important foramina and structures of the skull base. A–D: Axial CT images in bone windows demonstrating important skull base structures discussed. Annotations are as follows: CP, cribriform plate; CC, horizontal portion of the petrous internal carotid canal; PF, petroclival fissure; IF, inferior orbital fissure; SS, sphenooccipital synchondrosis; FO, foramen ovale; FS, foramen spinosum; IC, vertical portion of the petrous ICA; FL, foramen lacerum; FR, foramen rotundum; VC, vidian canal; SF, stylomastoid foramen; SPF, sphenopalatine foramen; PMF, pterygomaxillary fissure; PPF, pterygopalatine fossa; CL, clivus.
The foramina entirely formed by the sphenoid bone include the rotundum, ovale, and spinosum. The vidian canal is also within the sphenoid bone. The foramen rotundum is a short round canal in the coronal plane that connects the middle cranial fossa to the pterygopalatine fossa and transmits V2. The foramen ovale and spinosum create a “high heel footprint” impression on the central skull base on axial CT imaging and transmit V3 (ovale) and the middle meningeal artery (spinosum), respectively. The vidian canal transmits the vidian nerve and is seen on axial imaging as a long, thin curvilinear canal and on coronal imaging as a round foramen inferior and medial to foramen rotundum. Foramina and their contents are summarized in Table 22.1.
Table 22.1 Central Skull Base Foramina and Contents
The pterygopalatine fossa is an important region of the deep face in understanding nerve pathways as well as pathways of the spread of tumor and/or infection (Fig. 22.2). This space is defined anteriorly by the posterior wall of the maxillary sinus, posteriorly by the base of the pterygoid process, and medially by the vertical portion of the palatine bone. The pterygopalatine fossa communicates with the middle cranial fossa through the foramen rotundum and the vidian canal, the nasal cavity through the sphenopalatine foramen, the infrazygomatic masticator space of the suprahyoid neck through the pterygomaxillary fissure, the oral cavity through the palatine canal, and the orbit through the infraorbital fissure and orbital apex.
The relationship of the central skull base with the clivus and occipital bones is important in understanding disease processes that extend into the central skull base region. The clivus is a midline bone, which lies posterior to the sphenoid sinus and nasopharynx and inferior to the sella, separated from the sphenoid by the sphenooccipital synchondrosis. Laterally, the petrooccipital fissure separates the clivus from the petrous portion of the temporal bone.
Pathology
Pathologic lesions of the skull base may arise intrinsically from the bones of the skull base (Table 22.2); from the endocranial meningeal surfaces; exocranially from mucosal surfaces of the nasal cavity, sinuses, and olfactory tissue; or from embryologic remnants and synchondroses of the skull base proper (3–8). Subsections of this chapter are organized by lesions intrinsic to the bones of the skull base, lesions most commonly found involving the anterior skull base, and lesions most commonly found involving the central skull base. Additionally, lesions that arise from neural tissue such as the pituitary gland and cranial nerves and from vascular structures and the cavernous sinus can also affect the skull base. These lesions are, however, primarily discussed separately in chapters related to brain neoplasms, cranial nerve pathology, and intracranial vascular diseases and are mentioned briefly in relation to their effect on the skull base proper.
Table 22.2 Differential Diagnosis of Intrinsic Skull Base Lesions

Intrinsic skull base lesions
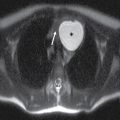
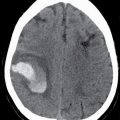
Metastatic disease is the most common neoplastic process of the skull base. The appearance can be nonspecific and related to properties of the primary neoplasm. Lesions can present as either lytic or sclerotic foci on CT and be hypo- or hypervascular on postcontrast cross-sectional imaging (9). Common malignancies include breast, lung, and prostate cancers; however, many other malignancies can be found. A clinical history of primary neoplasm is most helpful making the diagnosis. The most common locations are the sphenoid bone, clivus, and petrous apex due to the higher marrow content but are usually multiple and can be found anywhere (Fig. 22.3). It is important to evaluate for any associated soft tissue mass or extraosseous extension.
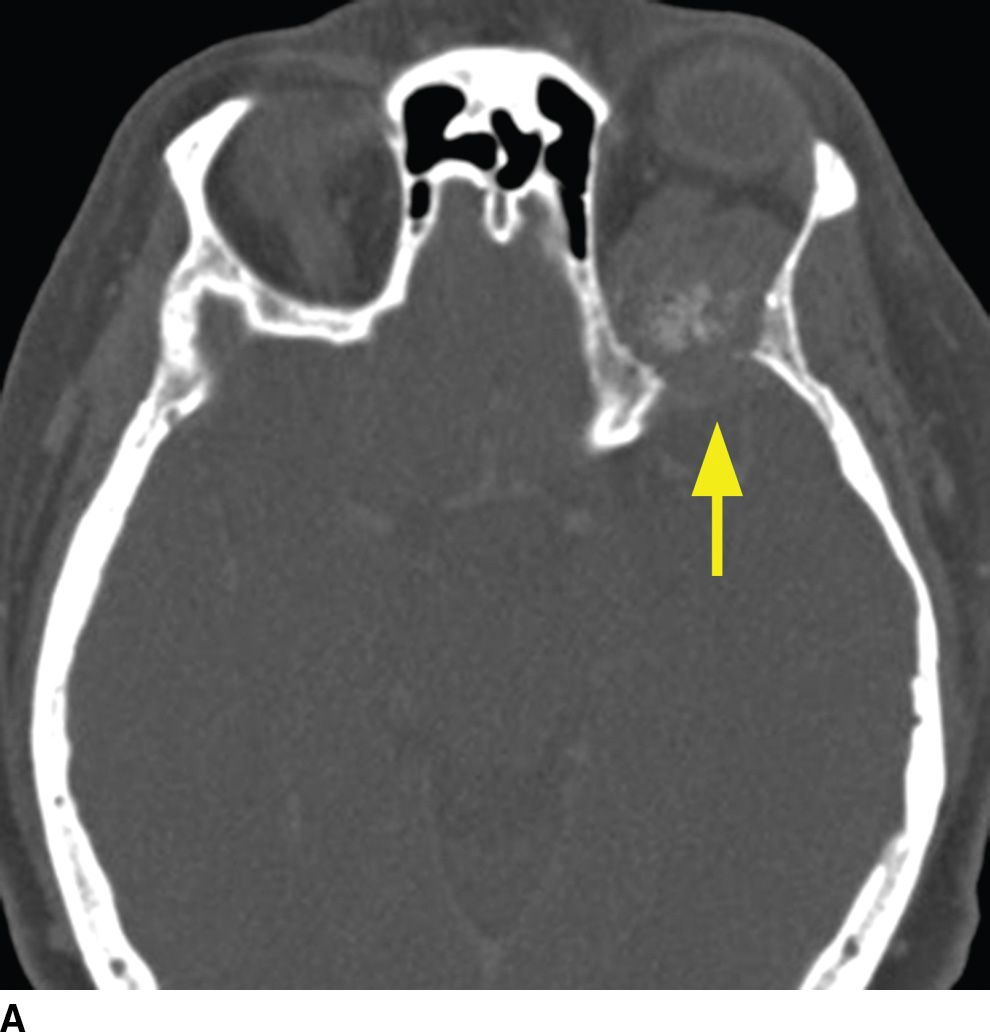
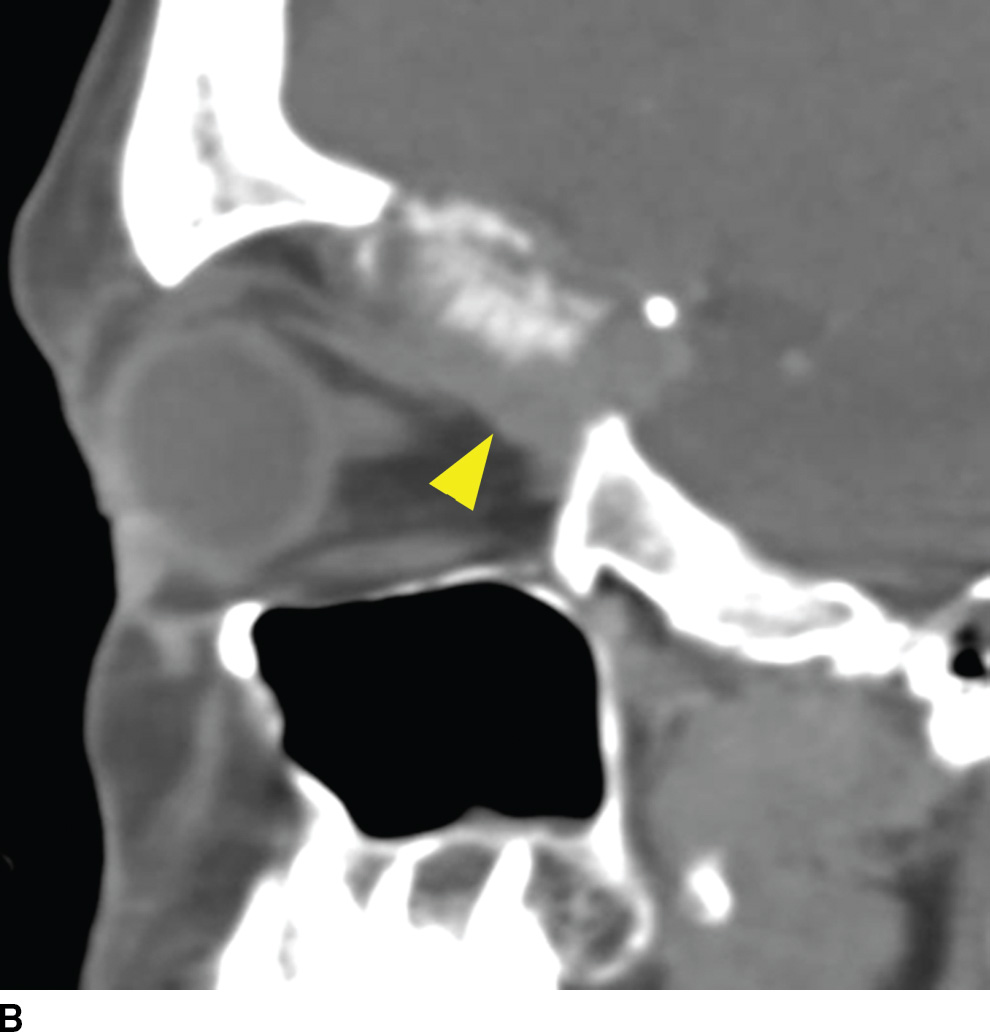
FIG. 22.3 Skull base metastasis. A: Axial contrast-enhanced CT in bone windows showing metastatic adenocarcinoma of an unknown primary to the floor of the anterior cranial fossa (arrow). B: The same lesion in the sagittal plane. Note the degree of extraosseous extent into the orbit and anterior cranial fossa (arrowhead).
Multiple myeloma, a malignancy of monoclonal plasma cells, can also present as multiple lytic lesions of the skull base on CT and is in the differential of metastatic disease (10). Key features are nonsclerotic margins of the lytic lesions with typically homogenous postcontrast enhancement (Fig. 22.4). Within the spectrum of monoclonal plasma cell lesions is a solitary plasmacytoma of the skull base, which presents as a solitary mass with identical imaging characteristics of the multiple myeloma lesions. The skull base plasmacytoma, however, is larger and typically has a significant extraosseous soft tissue component, which can appear hyperdense on noncontrast CT relative to the brain due to high cellularity.
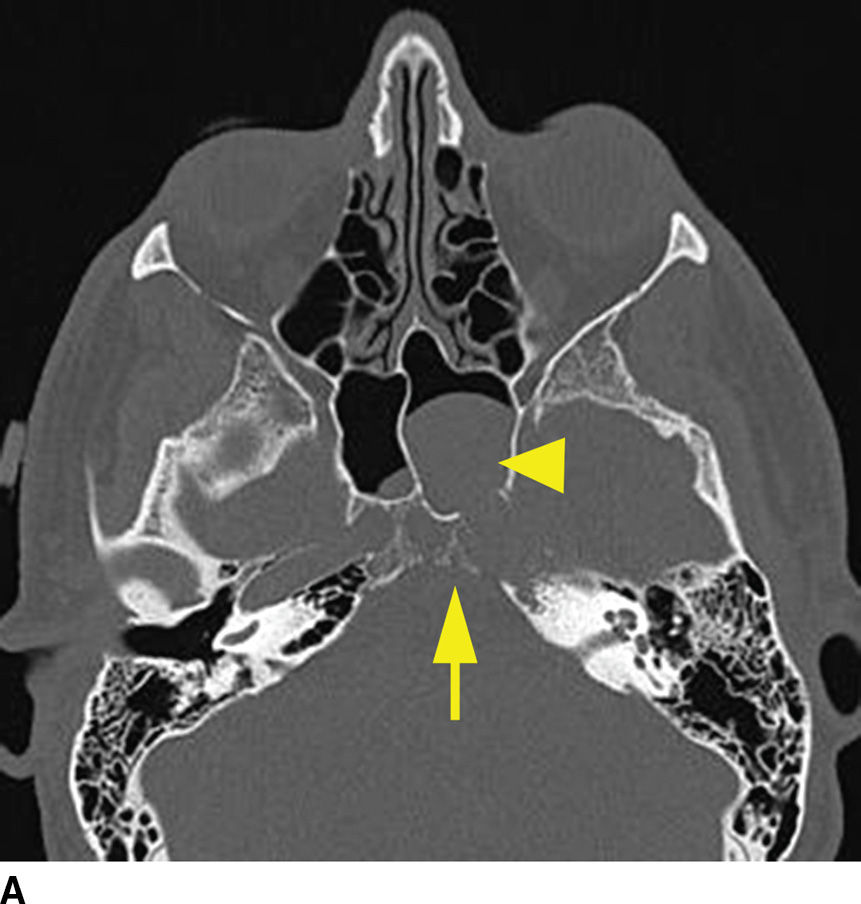
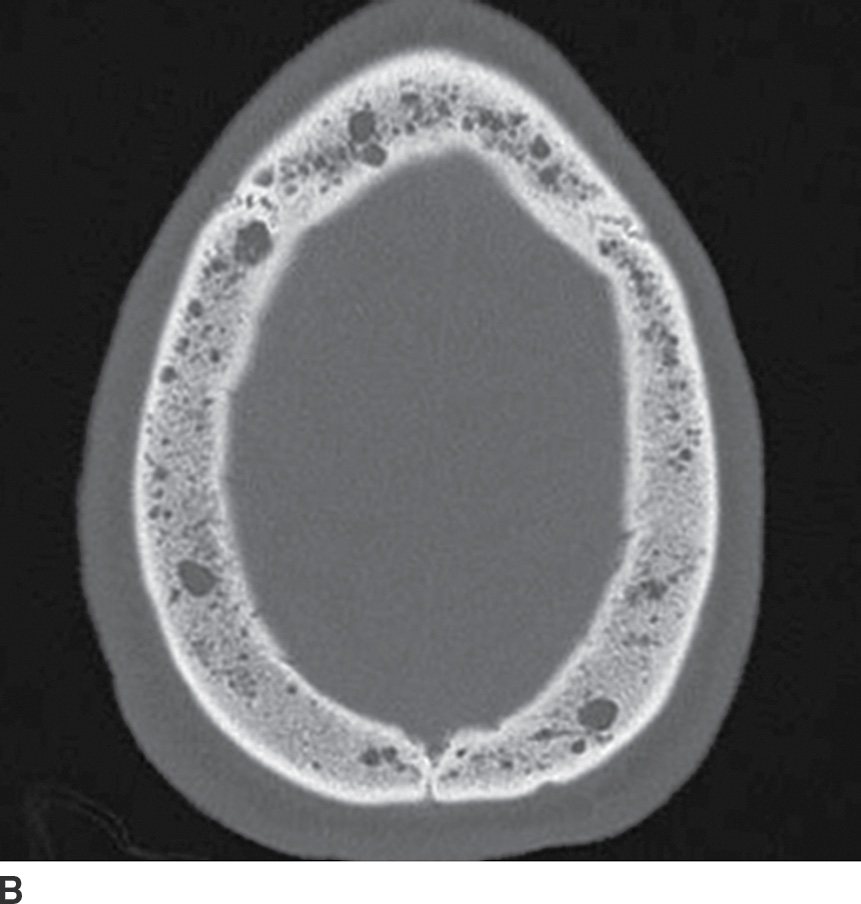
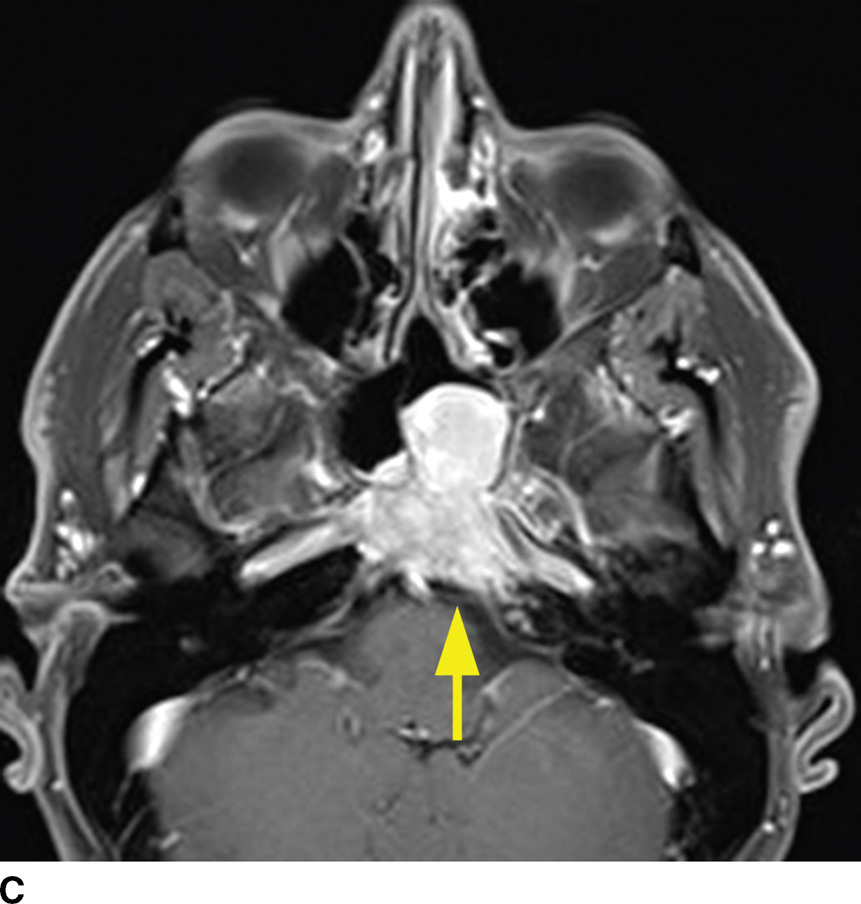
FIG. 22.4 Multiple myeloma. A: Noncontrast CT image demonstrating a permeative lesion of the central skull base (arrow) with an associated soft tissue component breaking through into the left sphenoid sinus (arrowhead). B: Same noncontrast CT demonstrating numerous additional lytic myeloma lesions of the calvarium. C: T1 postgadolinium fat-saturated MR image demonstrates homogeneous enhancement of the myeloma lesion (arrow).
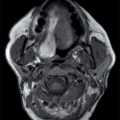
Additional lesions that can originate in the skull base and present as a lytic destructive soft tissue mass include Langerhans cell histiocytosis (LCH) and non-Hodgkin lymphoma (NHL). LCH, focal proliferation of histiocytes, can have a variety of appearances and is classically described as lytic calvarial lesions with beveled edges in the pediatric population. When LCH involves the skull base, it can present as a geographic lesion with a significant soft tissue component and heterogeneous enhancement (11). Because this lesion often has intracranial/intradural involvement, particularly in the sellar region, contrast-enhanced MR evaluation is indicated. NHL can also originate within the bone of the skull base or dural surface and also has a variety of appearances (Fig. 22.5) including extension from a primary origin in the sinonasal cavity, as detailed below. When presenting as a skull base mass, differentiating features include high density on noncontrast CT and hypointensity on T2-weighted images due to high nuclear to cytoplasmic ratio and homogeneous enhancement (12).
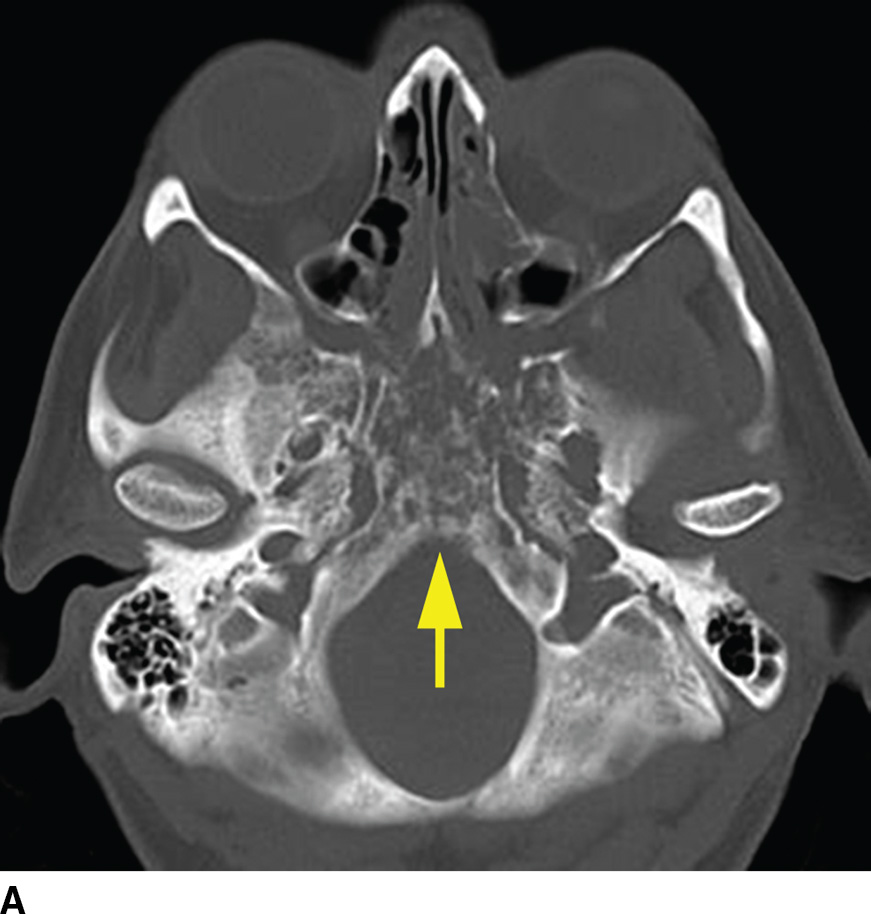
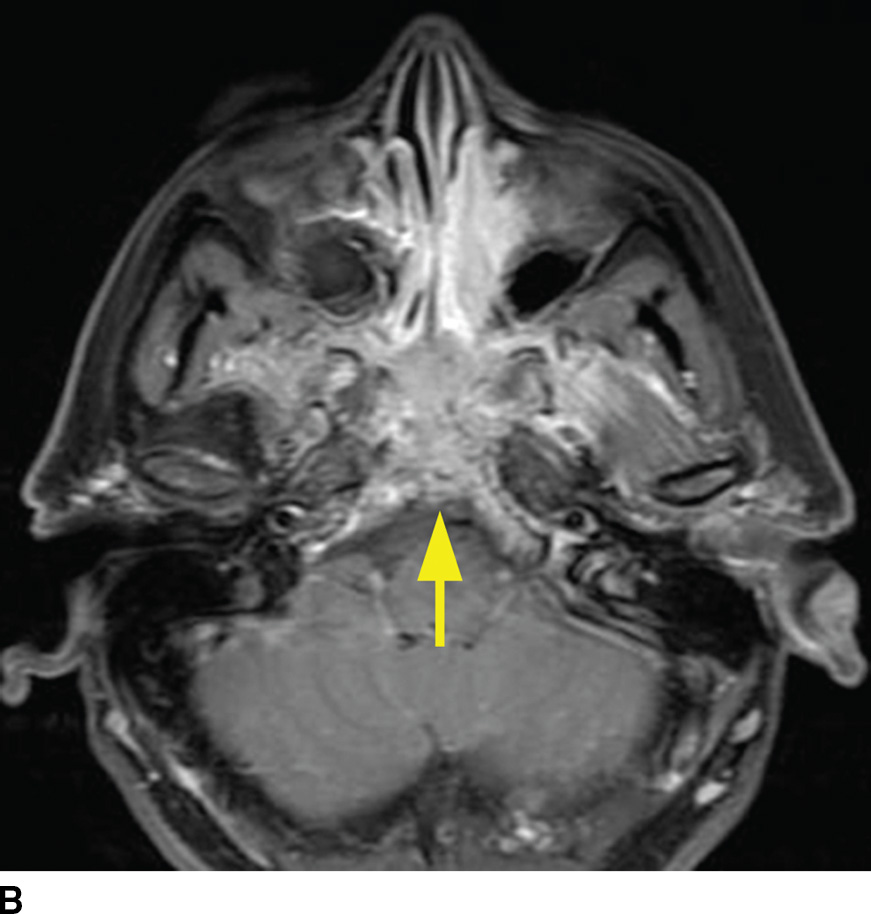
FIG. 22.5 Non-Hodgkin lymphoma. A: Noncontrast CT demonstrates one imaging presentation of NHL as a nonspecific permeative lesion of the central skull base (arrow). B: MR image at the same level, T1 postgadolinium with fat saturation, demonstrates enhancement of the permeative lesion (arrow).
Many fibro-osseous lesions affect the craniofacial complex including a variety of bone dysplasias, neoplasms, and metabolic diseases (13). The most commonly encountered benign fibro-osseous lesions intrinsic to the skull base include fibrous dysplasia and Paget disease (osteitis deformans). Many key features differentiate the two disease processes, often best delineated by CT imaging (Table 22.3). The MR appearance of these lesions can be confusing, and both can be mistaken for more aggressive primary and secondary disease processes of bone.
Table 22.3 Fibrous Dysplasia Versus Paget Disease
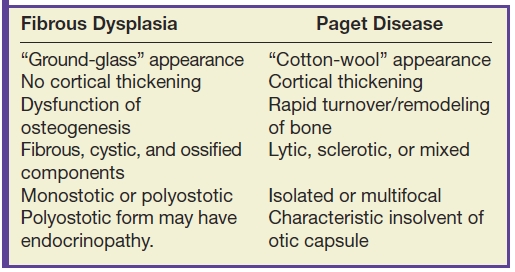
Fibrous dysplasia is a relatively common fibro-osseous lesion of the skull base often involving the sphenoid and occipital bones (14). This genetic alteration in osteogenesis can present in either a monostotic or a polyostotic form. The monostotic form is more common. The polyostotic form, however, is more commonly associated with skull base involvement and can be associated with other abnormalities including endocrinopathies in the McCune-Albright syndrome variant. CT characteristics are classically described as the “ground-glass” appearance of myxofibrous tissue and immature woven bone, although the imaging appearance can be variable based on the amounts of fibrous, cystic, and ossified components. Boney expansion is demonstrated, which can narrow important foramina. Sparing of the cortex is often demonstrated, which is a distinguishing feature from Paget disease. The MR appearance can be concerning for malignancy, showing fatty marrow replacement with low to intermediate T1 signal, elevated T2 signal, and variable, often impressive enhancement (Fig. 22.6). In the absence of clinical history, CT may be needed to exclude this potential pitfall (15).
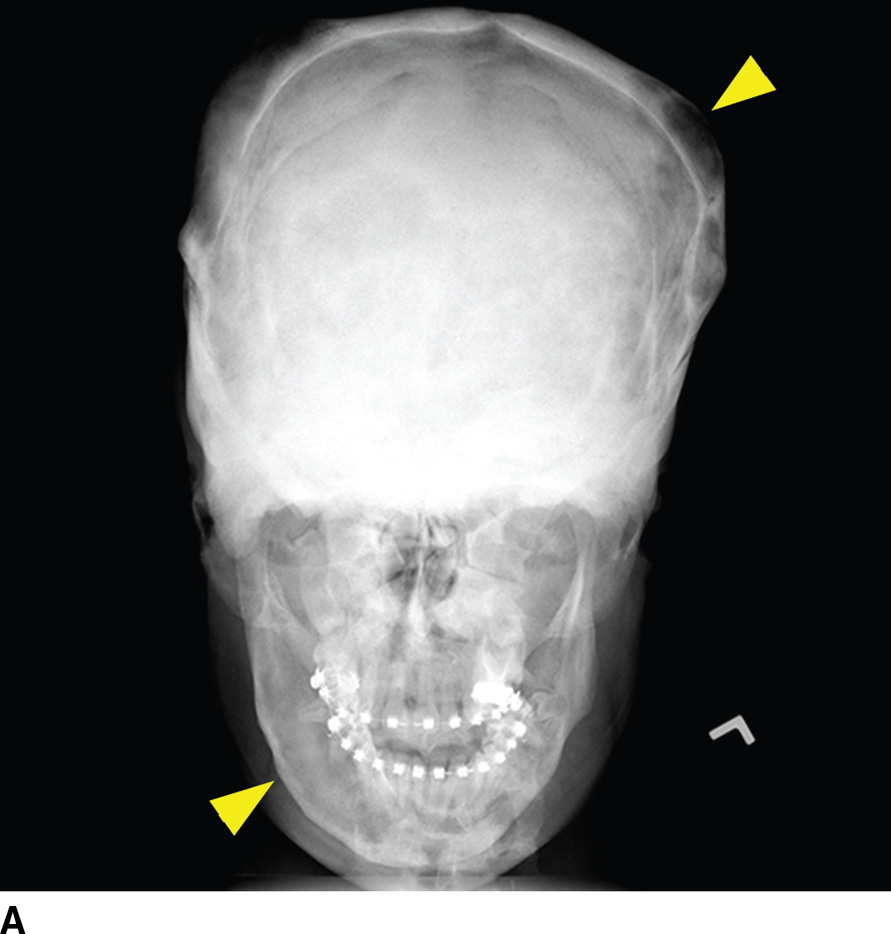
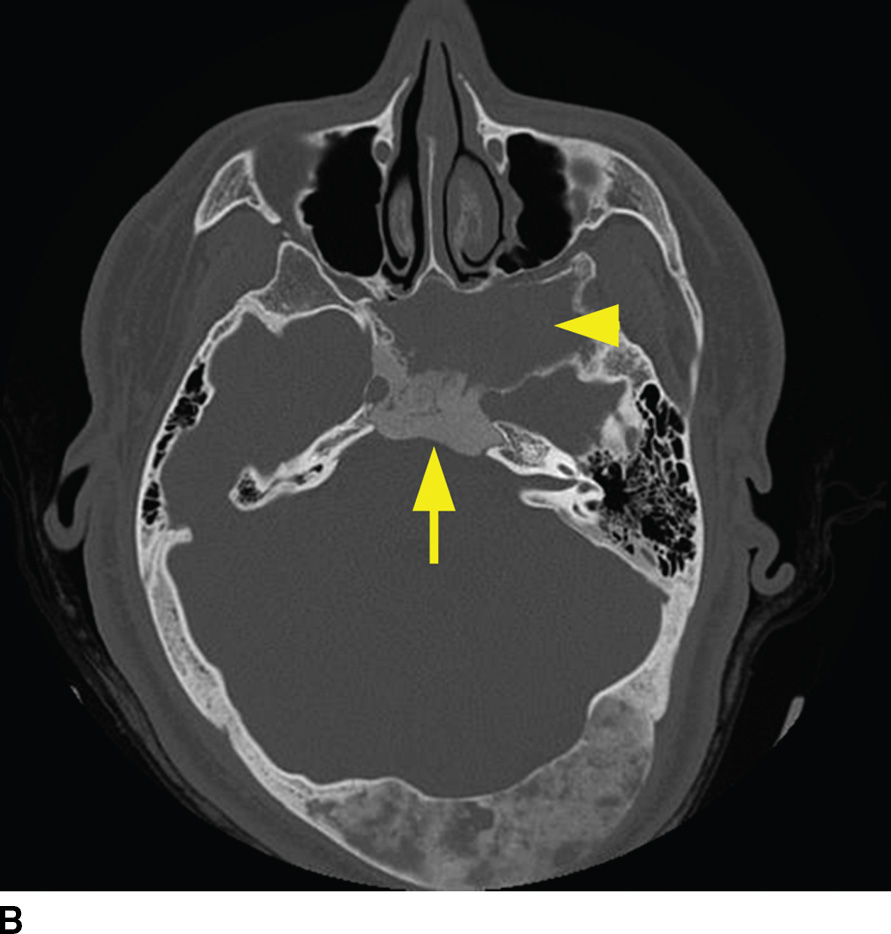
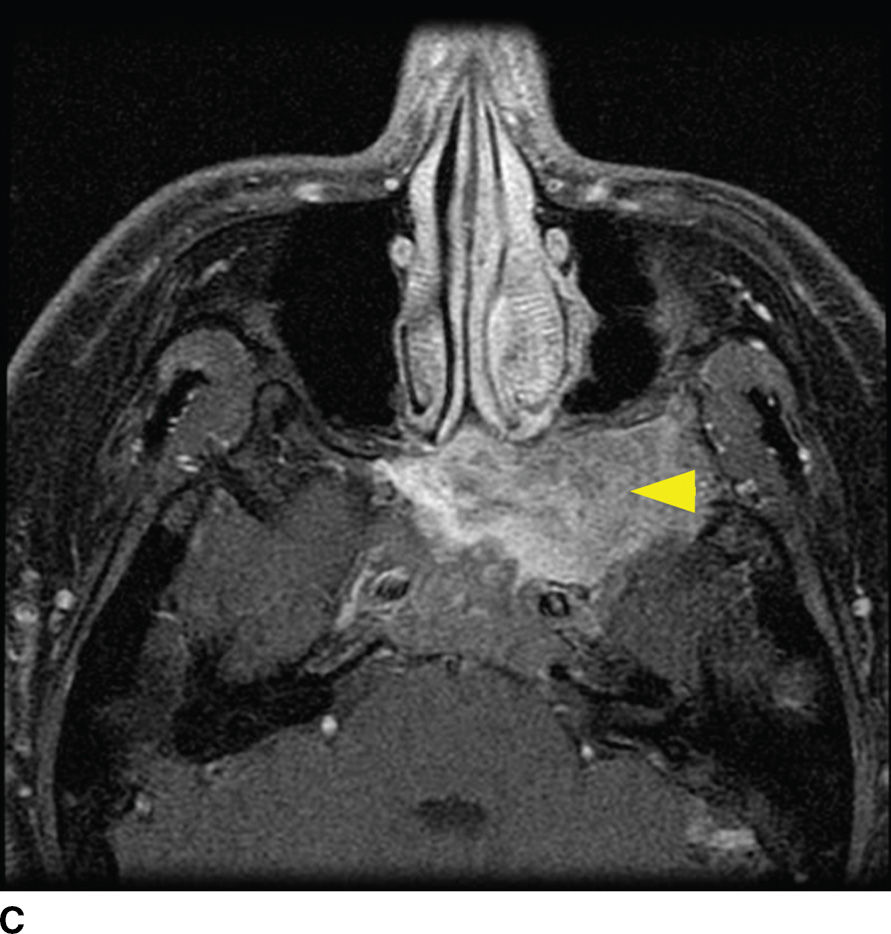
FIG. 22.6 Fibrous dysplasia. A: Skull radiograph demonstrating polyostotic fibrous dysplasia with expansile lesions of the calvarium and mandible (arrowheads). B: CT image of the central skull base demonstrates a heterogeneous lesion of the skull base with ground-glass ossified components (arrow) and lytic fibrous and cystic components (arrowhead). C: MRI can have a confusing appearance concerning for a more aggressive process. Note the heterogeneous enhancement and expansile mass–like appearance on this fat-saturated T1 postgadolinium image (arrowhead).
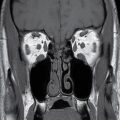
Paget disease is a pathologic process characterized by rapid turnover and remodeling and expansion of bone showing lytic, sclerotic, and mixed phases radiographically (16). This disorder presents most commonly in the fifth and sixth decades as opposed to fibrous dysplasia, which is most commonly a disease of children and young adults. While it too can be isolated or multifocal, it is commonly found in the craniofacial complex resulting in a variety of symptoms including skull deformity, cranial neuropathies from compression, sensorineural or conductive hearing loss from either directly affecting the ossicles or compression of the cochlear nerve, and basilar invagination. Involvement of the otic capsule is a distinctive feature of Paget disease. The CT appearance is classically described as “cotton-wool,” coarsened trabeculae, and cortical thickening/expansion of bone (Fig. 22.7). The MR appearance demonstrates low to intermediate T1 signal, heterogeneous T2 signal, and heterogeneous enhancement. Regions of new bone formation can demonstrate avid enhancement.
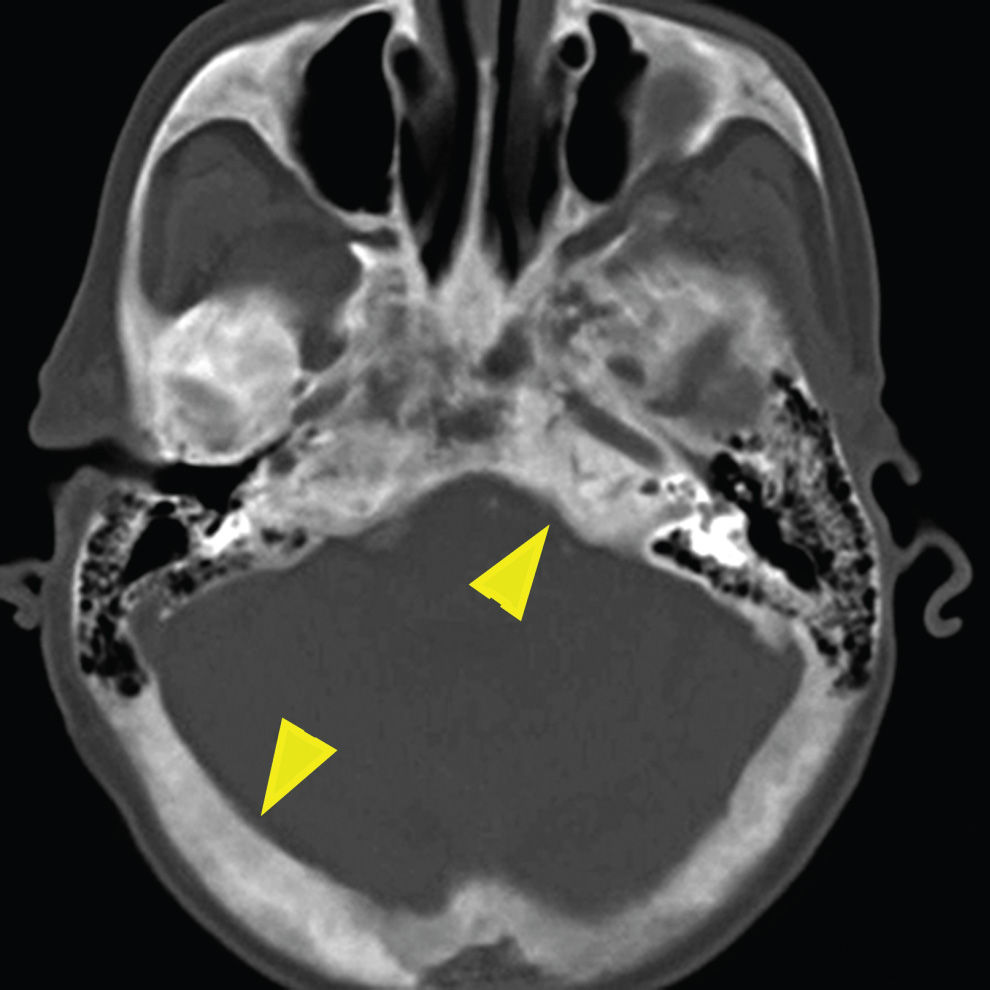
FIG. 22.7 Paget disease. Noncontrast CT image of the skull base demonstrates the characteristic “cotton-wool” appearance of the bones with expansion of bone with both cortical and trabecular thickening (arrowheads).
(Courtesy of Amirsys, Inc.)
Anterior skull base, sinonasal, and orbital region pathology
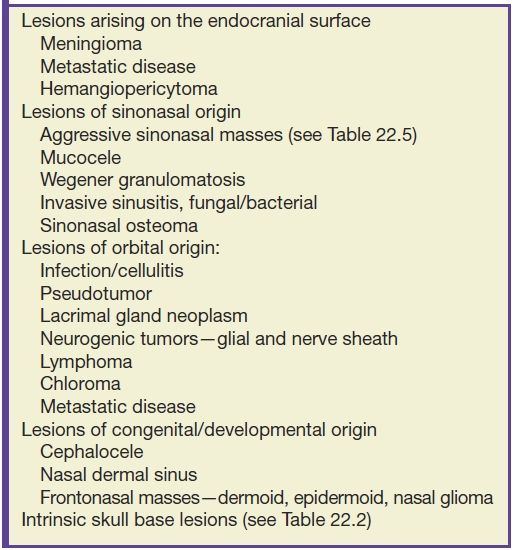
The differential diagnosis for an anterior skull base lesion is quite broad (Table 22.4). The discussion of commonly encountered lesions of the anterior skull base will be broken down into five major topics of discussion: tumors of intracranial origin, tumors of sinonasal origin, tumors originating from the orbit, congenital abnormalities, infectious/inflammatory, and other conditions. Major imaging characteristics of each entity on CT and MRI will then be reviewed. These disease processes that are discussed are not necessarily only found in the anterior skull base and, in many cases, are frequently seen to a lesser degree in the central skull.
Table 22.4 Differential Diagnosis of Anterior Skull Base Lesions
The most common lesion arising from the endocranial surface of the anterior skull base is the meningioma. The olfactory grove is the most common location of a meningioma of the anterior skull base, followed by the tuberculum sella (17). These lesions may become quite large and have various effects on bone, including hyperostosis, permeative destructive changes, or erosion. Meningioma lesions at the olfactory grove can penetrate the cribriform plate and enter the nasal cavity (Fig. 22.8). The CT imaging typically demonstrates a mass that is hyperdense to the brain, may contain calcifications, and has the above described bony changes. The MR imaging demonstrates an isointense lesion to the brain on most sequences. The majority of these lesions demonstrate avid postcontrast enhancement and are often associated with a broad dural attachment and dural tail. Other lesions of the endocranial surface are much less common but may be difficult to distinguish from a meningioma, including dural-based metastases and the rare hemangiopericytoma, which typically shows a more aggressive growth pattern.
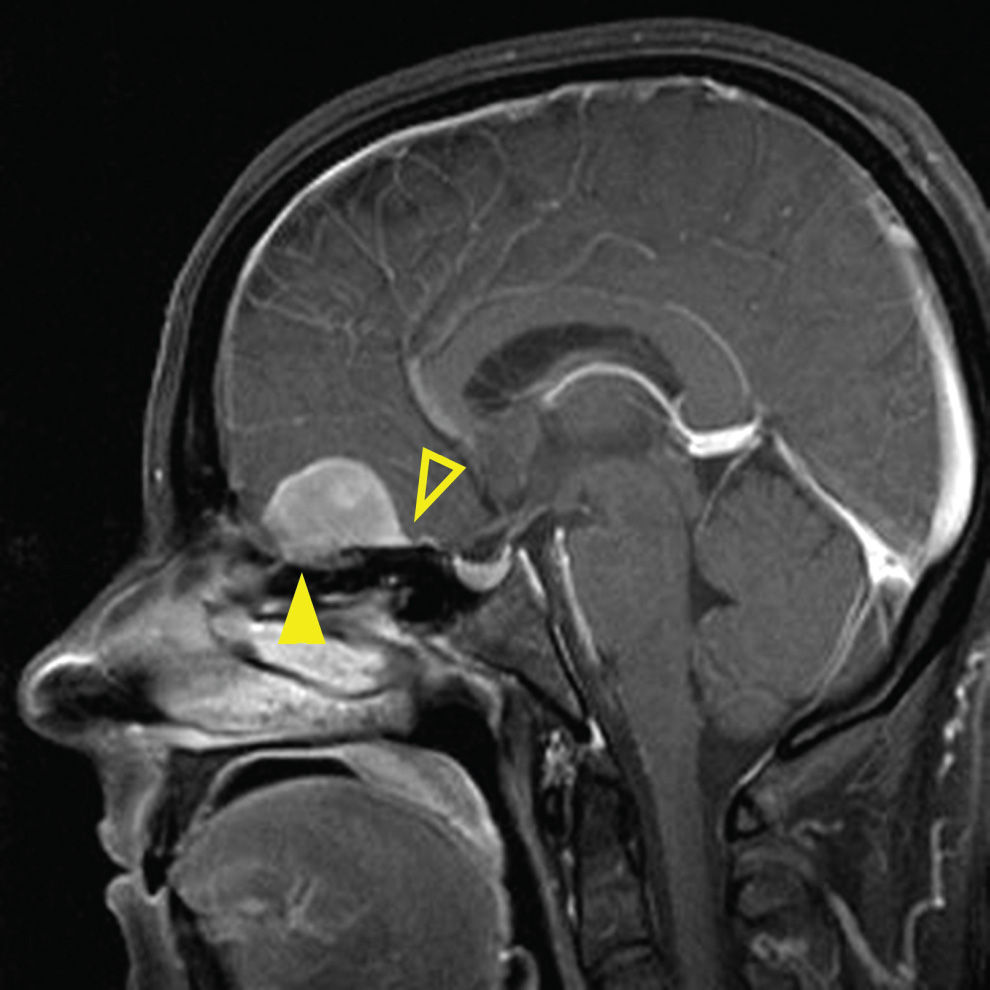
FIG. 22.8 Olfactory groove meningioma. MR image, sagittal T1 postcontrast with fat saturation, demonstrates an avidly enhancing dural-based lesion along the olfactory groove with upward mass effect on the inferior frontal lobe. Dural tail is noted distinctive of meningioma (open arrowhead). Note the extension through the cribriform plate into ethmoid sinuses (arrowhead).
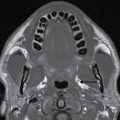
Tumors of sinonasal origin share many similar imaging characteristics and often present as an aggressive, ill-defined enhancing mass spreading beyond the confines of the nasal cavity (18–21) (Fig. 22.9). Understanding the complex anatomy of the face and its relationship to the anterior and central skull base is crucial in the role of imaging. Making the diagnosis is not often the dilemma as these lesions are often clinically accessible for inspection and tissue biopsy. Direct extension of disease is often seen through the lamina papyracea into the orbit, through the posterior wall of the maxillary sinus, or sphenopalatine foramen to the pterygopalatine fossa, through the cribriform plate to the anterior cranial fossa, or to the middle cranial fossa through the orbital apex, foramen rotundum, ovale, or the vidian canal. Additionally, many of these lesions extend along these same pathways primarily by way of perineural tumor spread, discussed in the central skull base section. These important anatomic regions deserve particular attention on all preoperative and follow-up imaging of sinonasal masses and are best characterized by contrast-enhanced MRI.
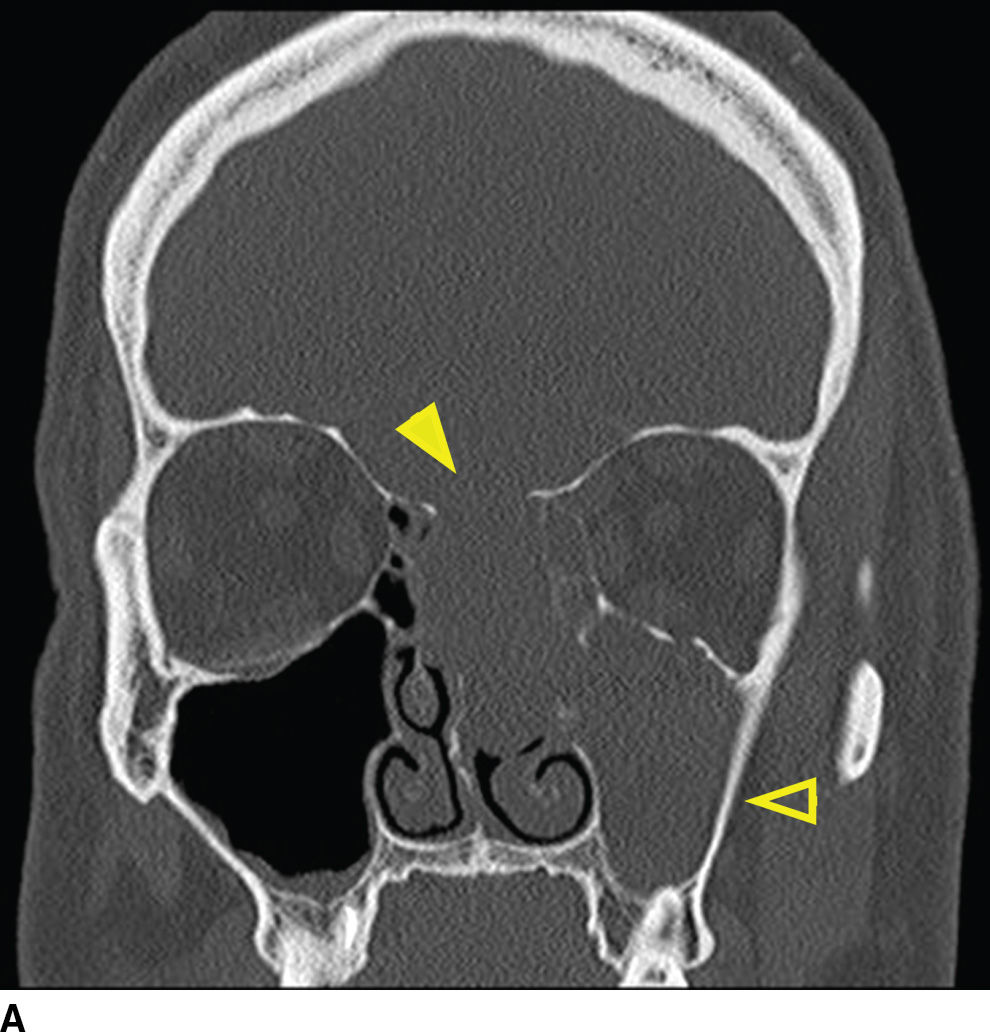
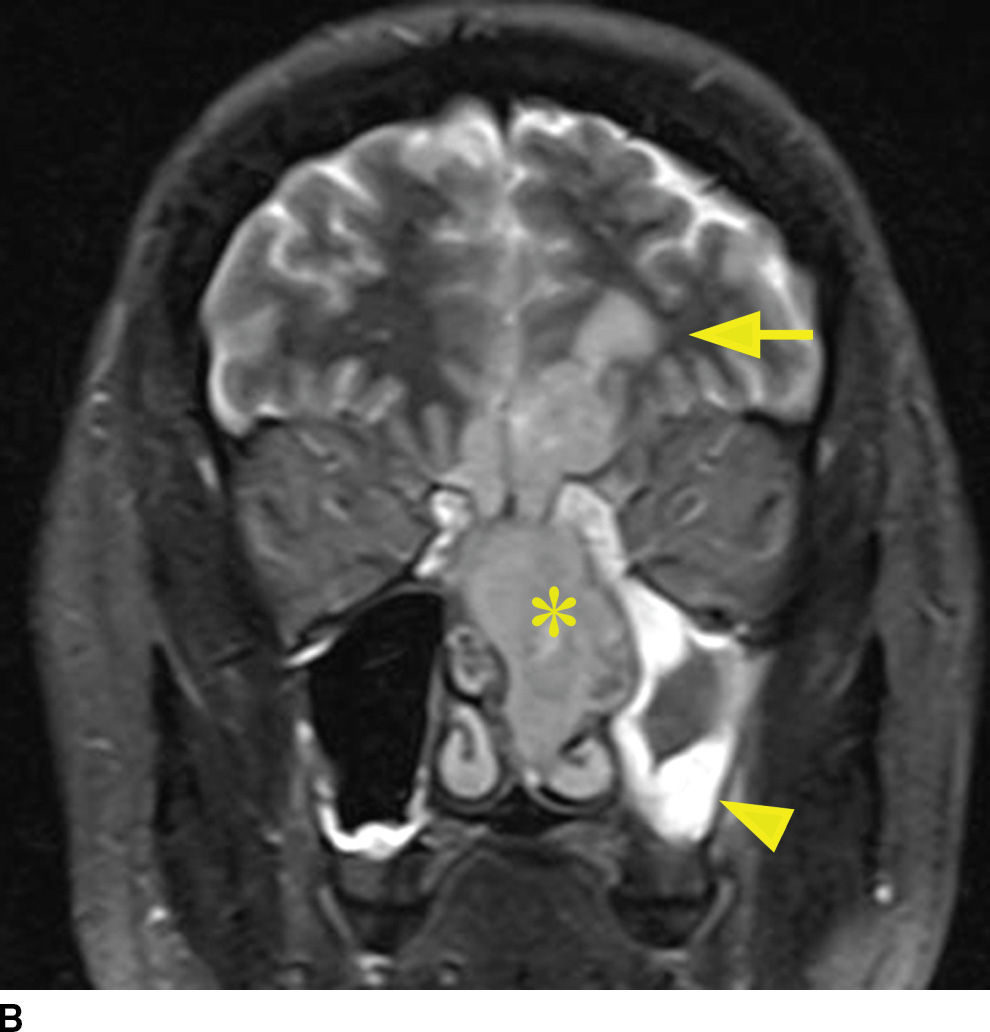
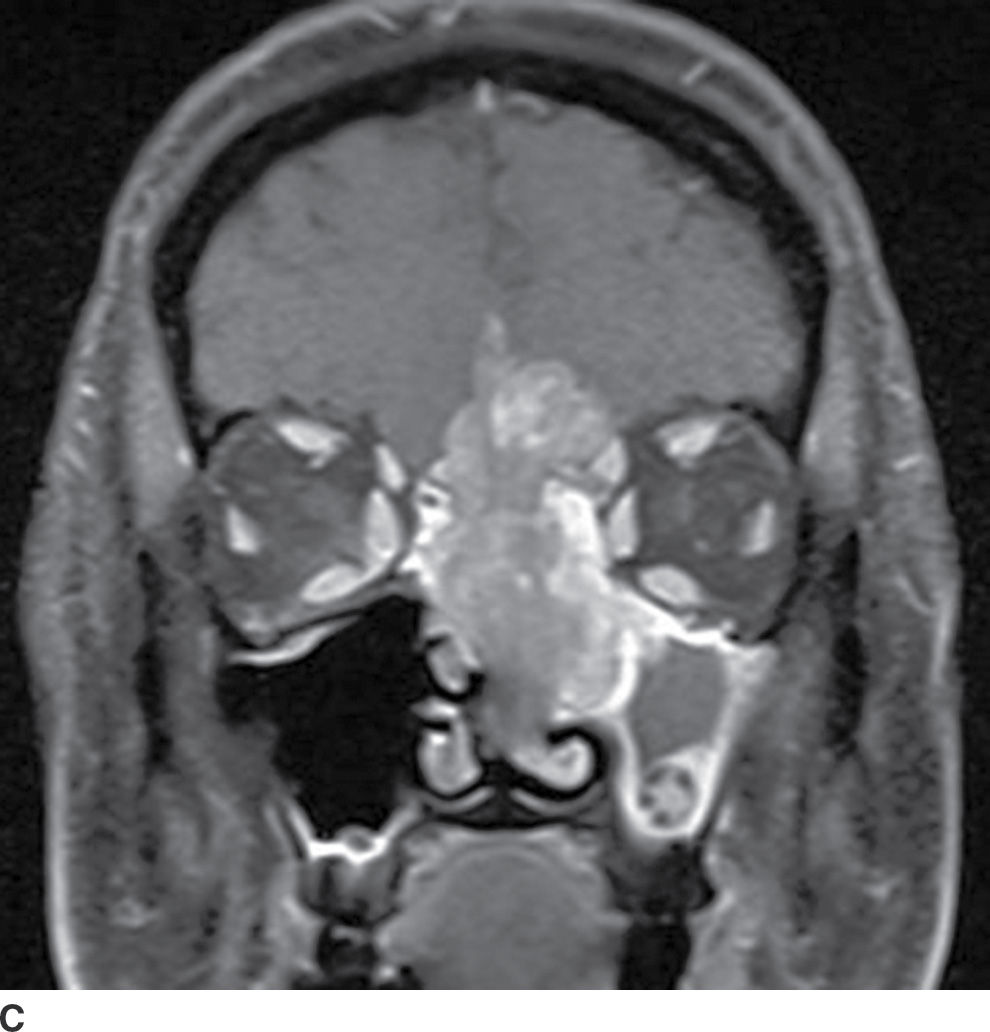
FIG. 22.9 Aggressive sinonasal mass with skull base invasion. A: Coronal noncontrast CT in bone windows demonstrates an aggressive sinonasal mass with osseous destruction of structures in the sinonasal cavity and extension through the skull base (arrowhead). Note an obstructed left maxillary sinus (open arrowhead). B: Coronal T2 with fat suppression demonstrates heterogeneous T2 signal within the mass (asterisk). Note the intracranial extension with reactive changes of the brain (arrow). Also note the thickened T2 bright mucosa of the obstructed left maxillary sinus (arrowhead). C: Heterogeneous enhancement on coronal T1 image with fat suppression. These imaging findings in (A–C) are nonspecific and include a large differential of aggressive sinonasal masses. In this case, the lesion was a sinonasal undifferentiated carcinoma (SNUC).
The majority of sinonasal tumors arise from elements of the sinonasal mucosal epithelium. Sinonasal mucosal lesions include in decreasing frequency: squamous cell carcinoma, intestinal-type adenocarcinoma, minor salivary gland tumors, and undifferentiated carcinomas. Additional, less common lesions of sinonasal origin include esthesioneuroblastoma, melanoma, and non-Hodgkin lymphoma (Table 22.5). In the pediatric population, mesenchymal tumors such as rhabdomyosarcoma are also found within the sinonasal region.
Table 22.5 Differential Diagnosis of Aggressive Sinonasal Masses with Skull Base Invasion
Squamous cell carcinoma is the most common sinonasal malignancy. These lesions originate most commonly in the antrum of the maxillary sinus. When originating in the second most common location, the ethmoid sinuses, there is a higher propensity of direct extension through the cribriform plate into the anterior cranial fossa (22). Regardless of its primary location, this is an aggressive lesion that has the propensity to invade directly into adjacent structures as well as to enter the anterior and middle cranial fossa by way of perineural tumor spread. Aggressive bone destruction is usually demonstrated on CT with squamous cell carcinoma cases. The MR characteristics are fairly nonspecific with intermediate signal on T1 and T2. Enhancement is intermediate, somewhat to a lesser degree than other sinonasal masses. Contrast-enhanced MR is key for characterization of perineural tumor spread (23,24). Secondary findings that are common include obstruction of the egress of the paranasal sinuses, resulting in trapped secretions, and obstruction of the eustachian tubes with resulting mastoid effusions.
Intestinal-type adenocarcinoma and minor salivary gland tumors are additional aggressive-appearing sinonasal lesions that can invade the skull base. Intestinal-type adenocarcinoma is frequently found in the nasal cavity and ethmoid sinuses (25). Minor salivary gland tumors can be found in the ethmoid sinuses; however, most commonly arise at the hard palate. Their imaging characteristics are also nonspecific and difficult to distinguish from squamous cell carcinoma. T2 prolongation can be an occasionally seen distinguishing feature. This is unfortunately not reliable as these lesions can also be isointense on T2. Minor salivary gland tumors can have a less aggressive appearance on CT. Adenoid cystic subtype has the propensity to spread perineurally and present with cranial neuropathy.
Although uncommon, sinonasal undifferentiated carcinoma (SNUC) has similar imaging characteristics to squamous cell carcinoma and should be included in the differential diagnosis of an aggressive sinonasal mass that invades the skull base (26) (Fig. 22.9). This lesion is typically large at diagnosis, tends to show rapid growth, and has a poor prognosis. Again, most imaging features are nonspecific; however, the presence of lymphadenopathy and distant metastasis can be a characteristic feature.
Esthesioneuroblastoma is a tumor of neuroectodermal origin, arising from the olfactory mucosa within the superior recess of the nasal cavity. These can present as a high unilateral nasal mass with remodeling of the skull base or as a large dumbbell-shaped mass with a narrow waist at the point of erosion through the cribriform plate. Large, higher stage lesions, which have invaded the anterior cranial fossa, are common due to late presentation. Avid postcontrast enhancement is typical with esthesioneuroblastomas, and retropharyngeal lymph nodes can be seen. Many of the imaging features are nonspecific and similar to squamous cell carcinoma. There is, however, a distinguishing feature of this lesion, which can clinch an imaging diagnosis. That is the presence of intracranial cysts, which form at the periphery of the tumor at its interface with the brain (27) (Fig. 22.10). Uncommonly, these lesions can also contain calcification, which can be a distinguishing feature. Meningeal spread can be seen in advanced lesions, as well as orbital invasion through the lamina papyracea.
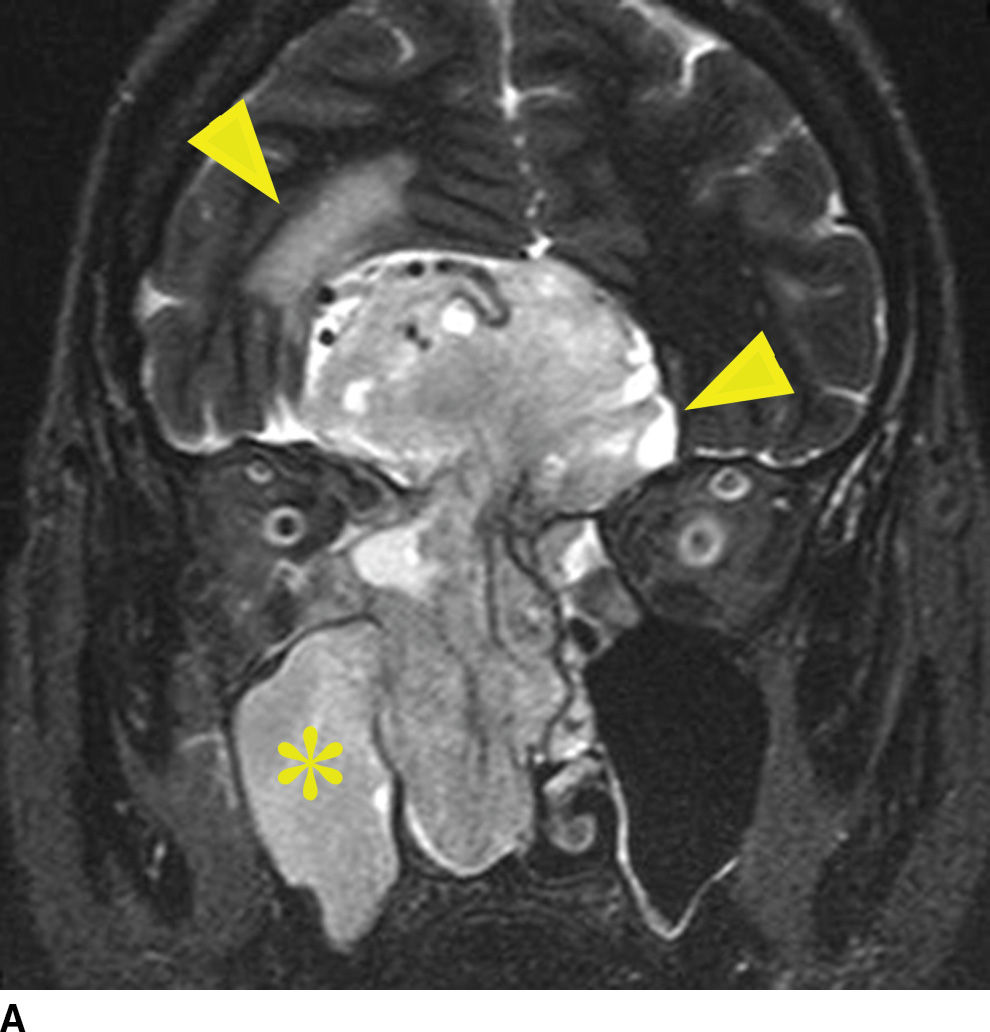
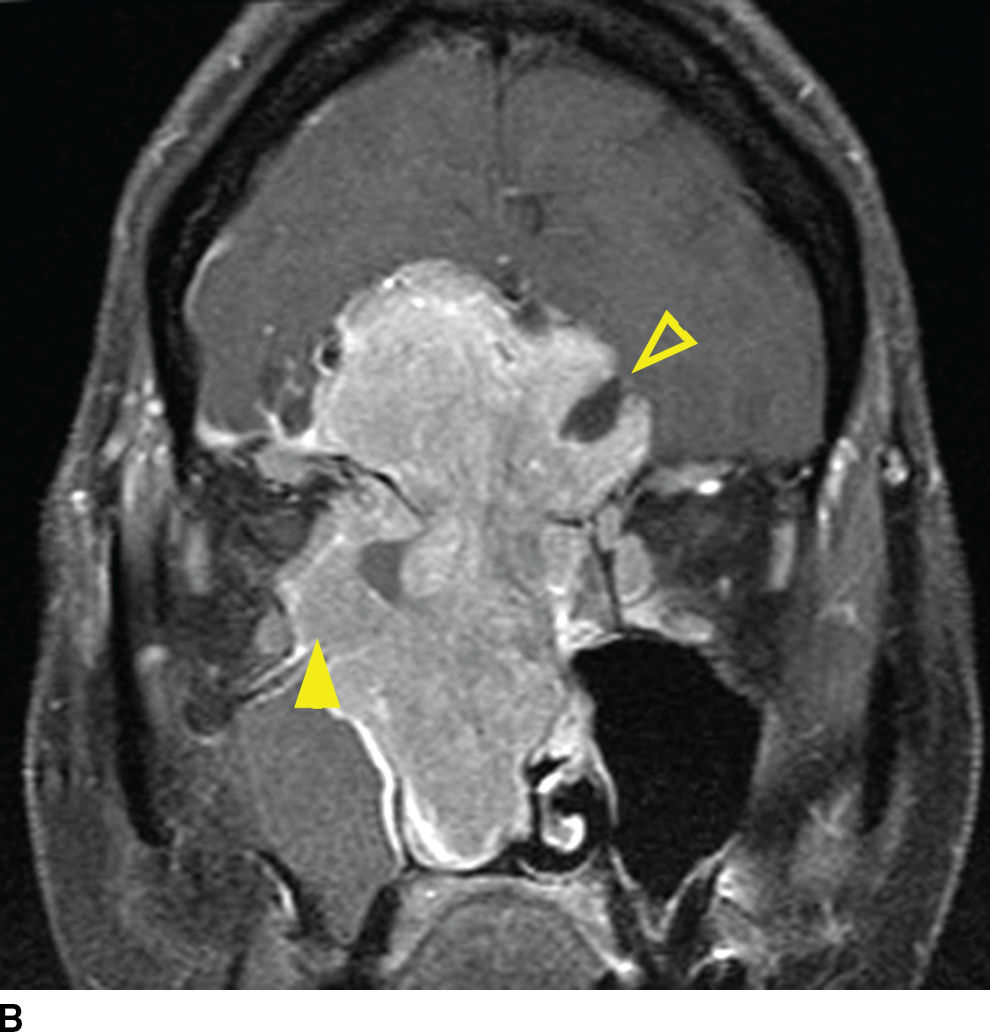
FIG. 22.10 Esthesioneuroblastoma. A: Coronal T2 fat-suppressed MR image again demonstrates mostly nonspecific findings of an aggressive sinonasal mass. Note the T2 hyperintense cysts that form at the tumor brain interface (arrowhead), which is a distinguishing characteristic. Note the reactive changes in the brain (top arrowhead) and right maxillary sinus obstruction (asterisk). B: Coronal T1 postgadolinium with fat saturation demonstrates the T1 hypointense nonenhancing cysts adjacent to the enhancing lesion (open arrowhead). Note the orbital invasion (arrowhead).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree