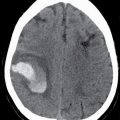
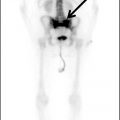
Ellen G. Hoeffner, MD, and Laurie A. Loevner, MD
LEARNING OBJECTIVES
1. Review the imaging findings of common acute infectious and inflammatory conditions of the neck and learn the fundamentals of acute neck trauma.
2. Understand the vital role of imaging in accurately diagnosing neck infections, precisely delineating the anatomic extent of disease, facilitating early detection of potentially life-threatening complications, and guiding therapy.
3. Recognize that computed tomography is the primary imaging modality for working up head and neck infections. Magnetic resonance imaging is reserved for assessing intracranial extension or osteomyelitis and monitoring response to therapy. Ultrasound is preferred for assessment of lymph nodes, salivary glands, and neck abscesses and plays an important role in imaging of the pediatric population.
Early diagnosis and management of neck infectious and inflammatory conditions is a common challenge in the acute setting. Although the clinical diagnosis is obvious in many cases, some infections may be misleading in their severity and extent due to the limitations of physical examination and complexity of the anatomy involved. Delay in appropriate management increases the risk of life-threatening complications, because of compromise of vital structures like the airway, cervical vessels, optic nerves, intracranial space, and spinal canal (1). Imaging plays a central role in delineating the anatomic extent of the disease process, identifying the infection source, and detecting complications. The utility of imaging to differentiate between a solid phlegmonous mass and an abscess cannot be overemphasized, the latter being an indication for drainage. This chapter briefly describes and pictorially illustrates the pertinent anatomy of the fascial layers and spaces of the neck and imaging manifestations of common infectious and inflammatory conditions of the head and neck, with a brief review of acute neck trauma.
Normal Anatomy
The superficial cervical fascia underlies the skin and is composed of adipose tissue, superficial neurovascular structures, lymphatics, and the platysma muscle (Fig. 21.1). Infection in the superficial space usually manifests as cellulitis, lymphadenitis, or abscess. The deep cervical fascia is divided into three layers (superficial, middle, and deep) that enclose the contents of the head and neck and give rise to the deep spaces of the neck (Fig. 21.2). The parotid, masticator (including the infratemporal fossa), submandibular, and the prestyloid parapharyngeal space (PPS) are exclusively suprahyoid in location. The anterior visceral space is exclusively infrahyoid in location. The prevertebral space, retropharyngeal space (RPS) and poststyloid PPS (carotid space) traverse the neck from the skull base down to the mediastinum. The relevant anatomy of each of these spaces is discussed along with their respective conditions.
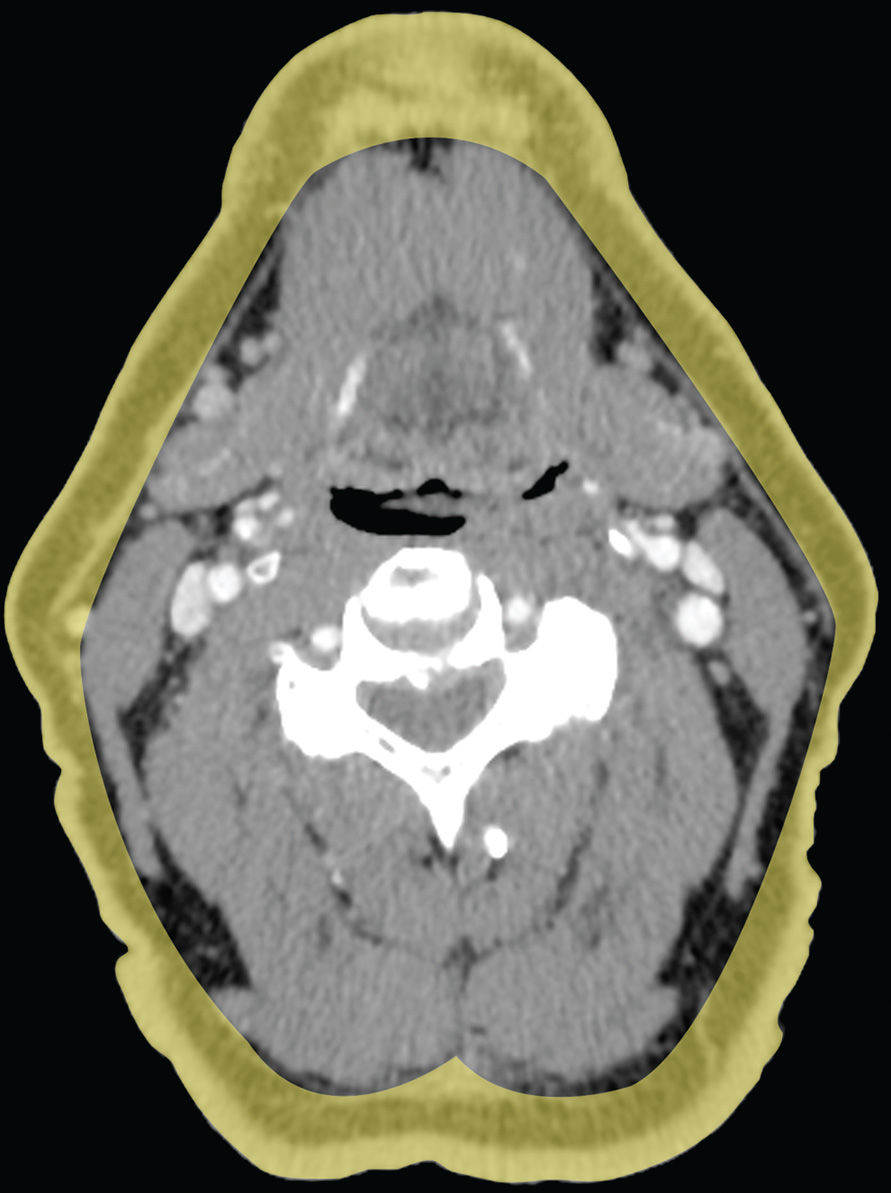
FIG. 21.1 Superficial cervical fascia. The superficial cervical fascia is shaded in yellow.
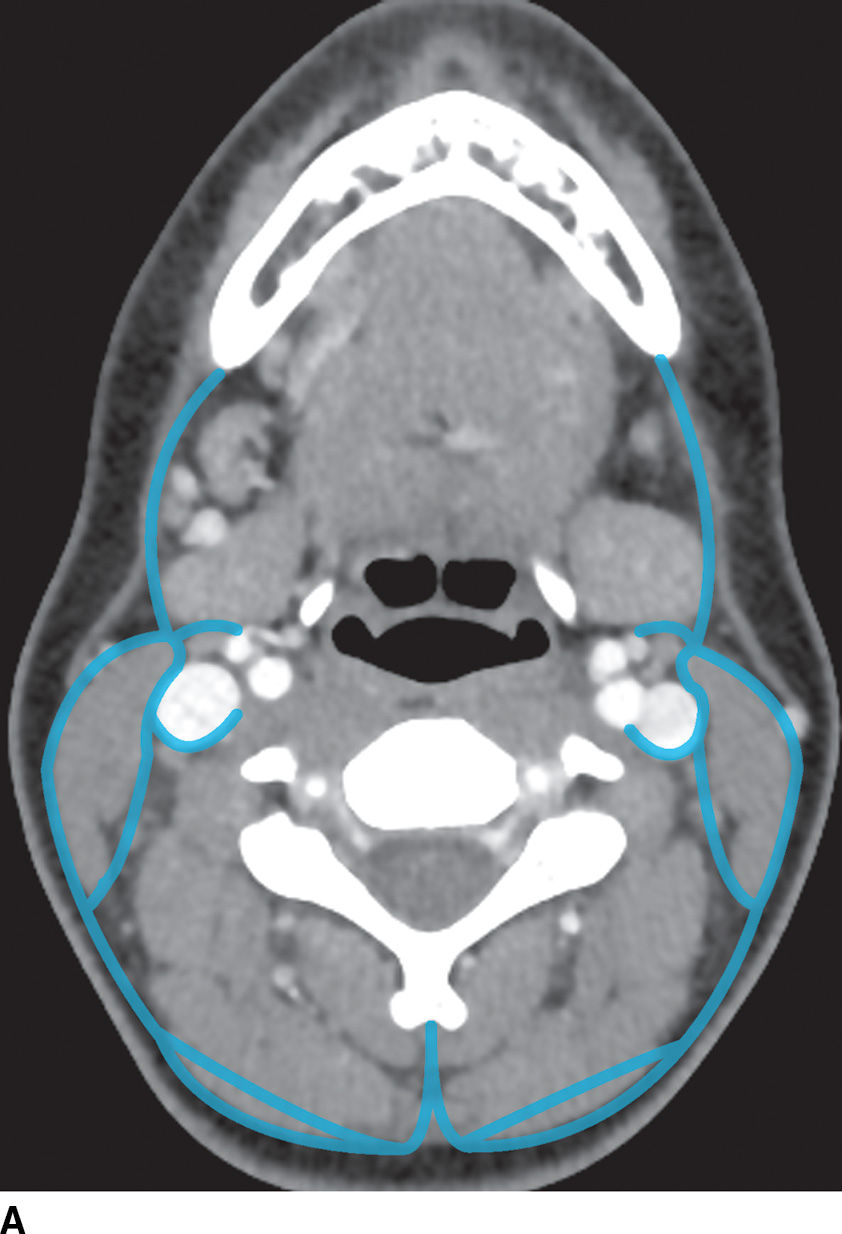
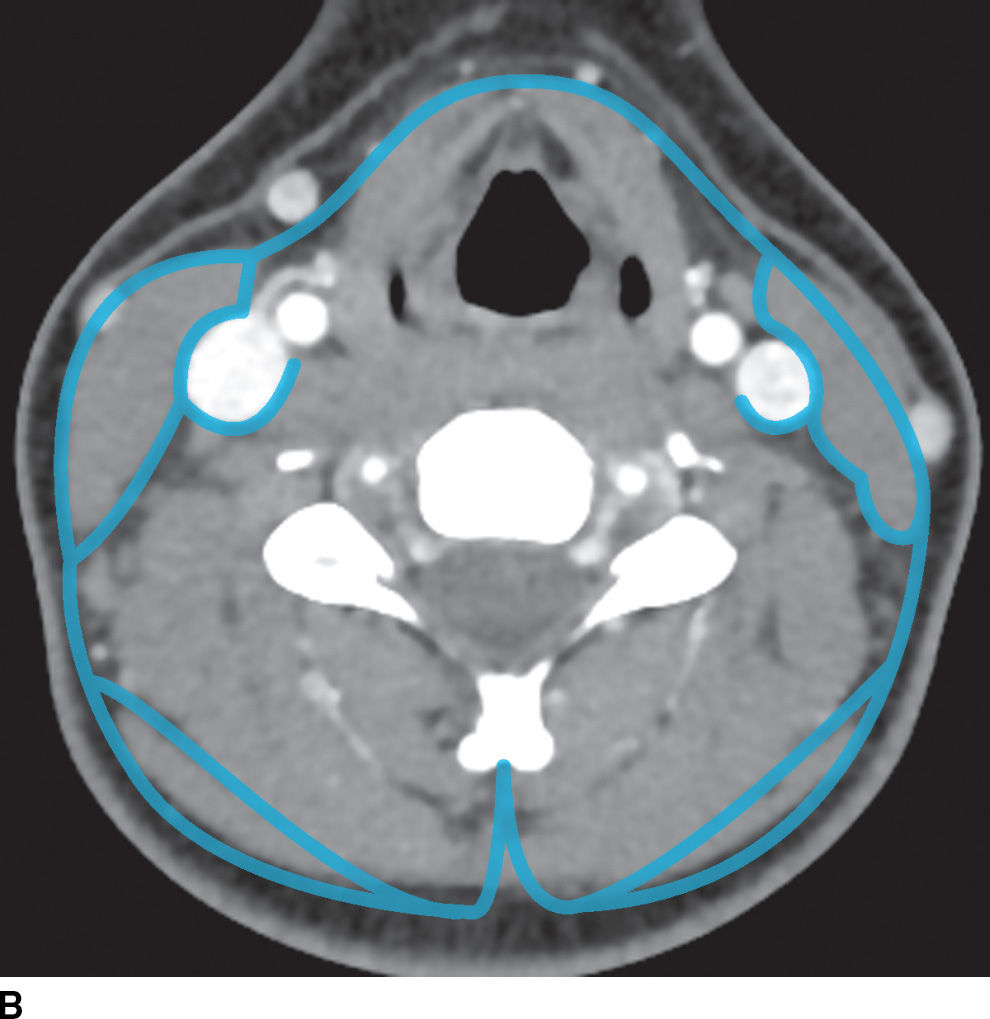
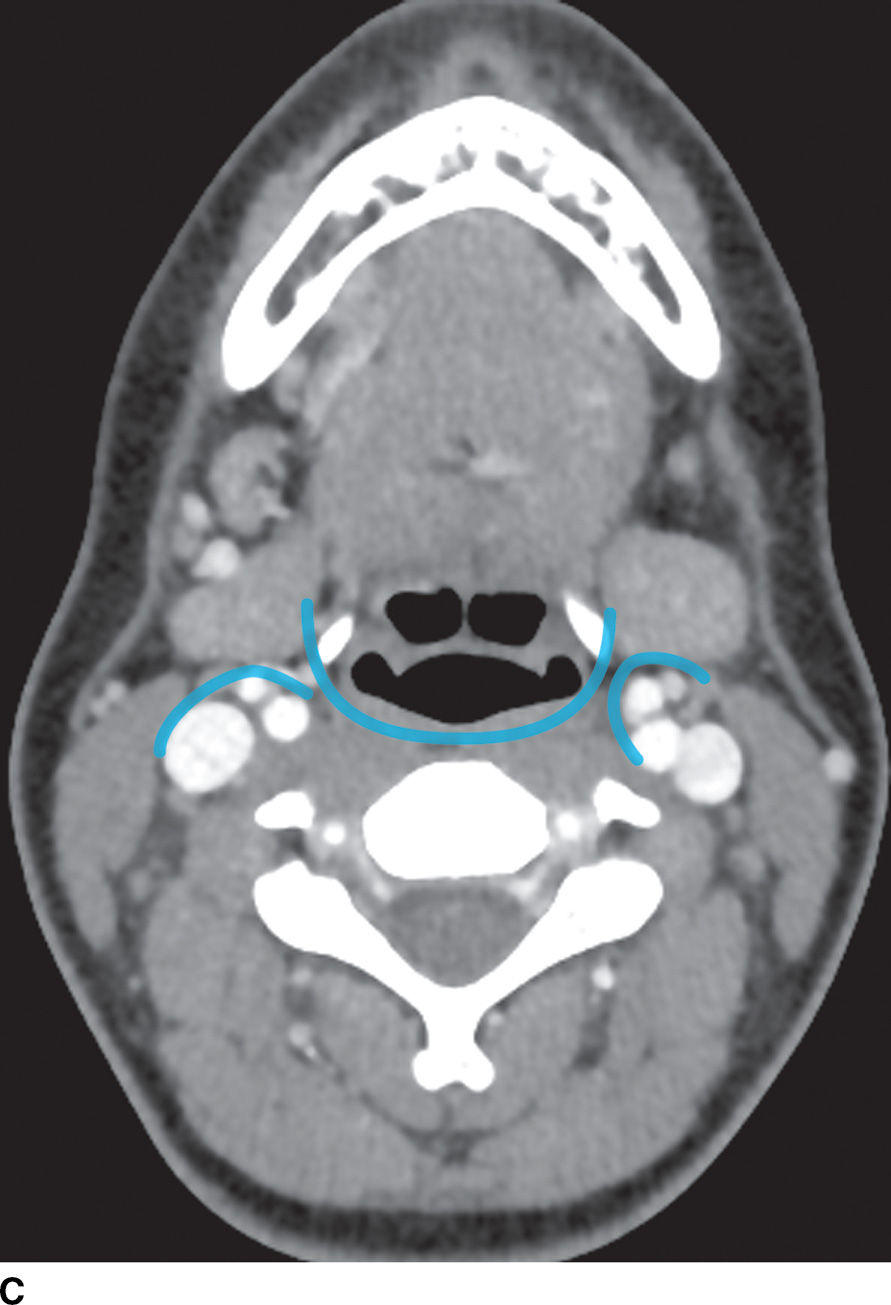
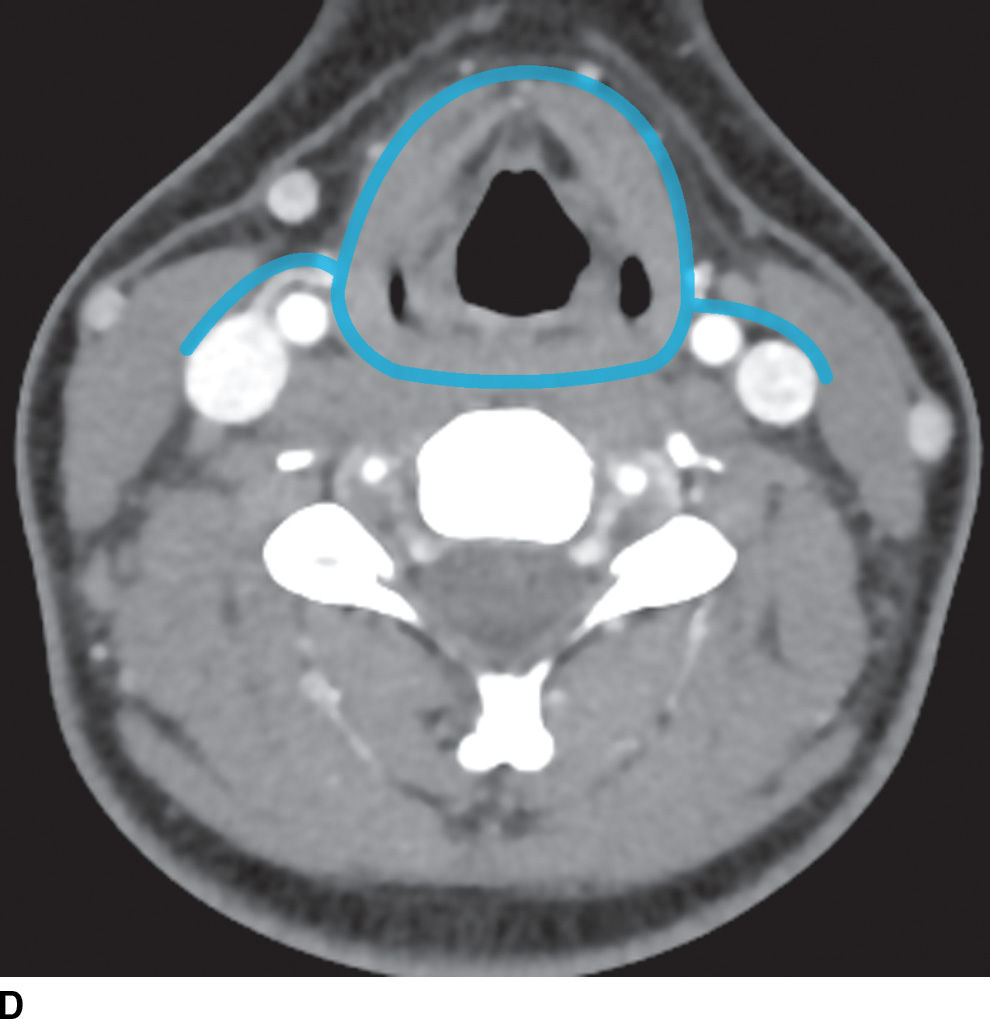
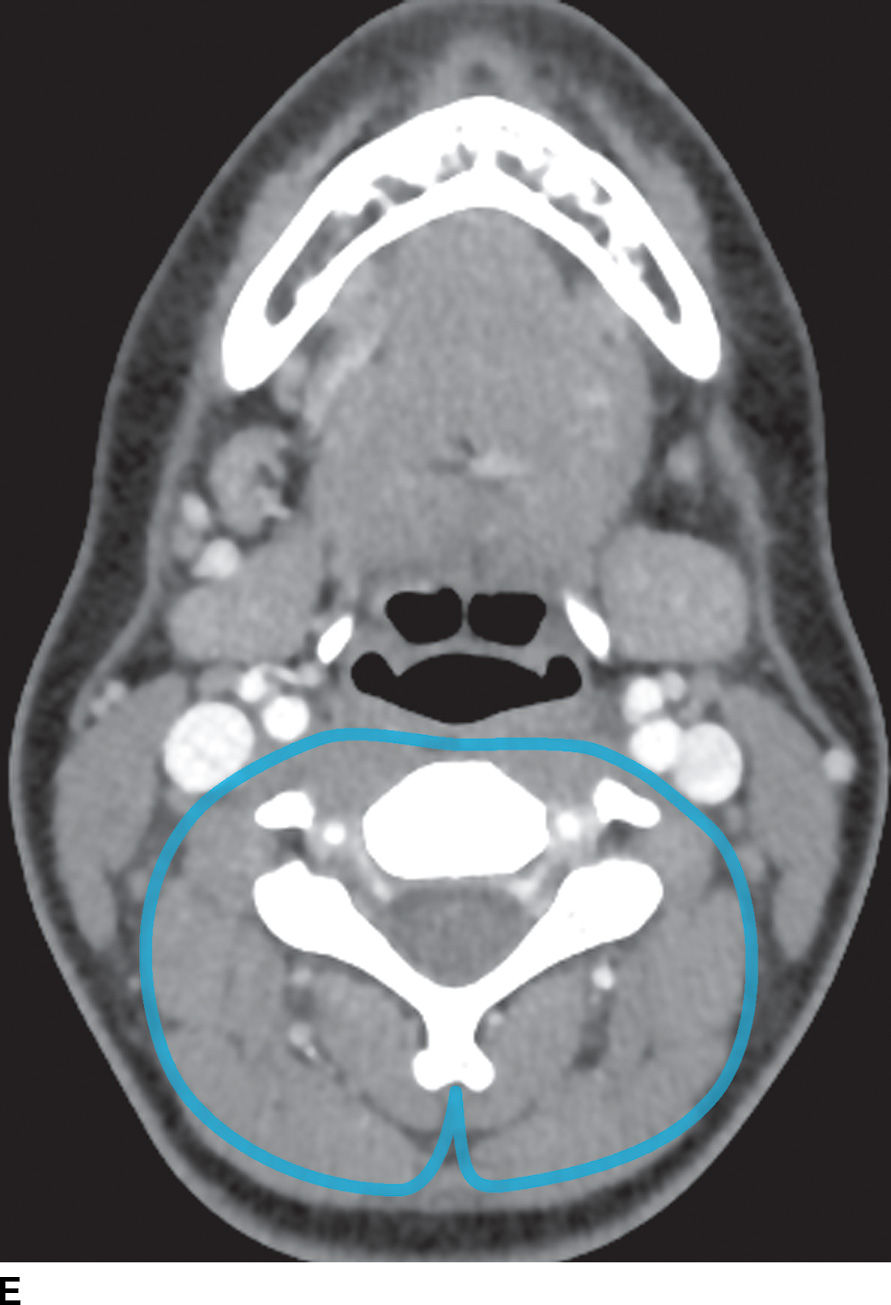
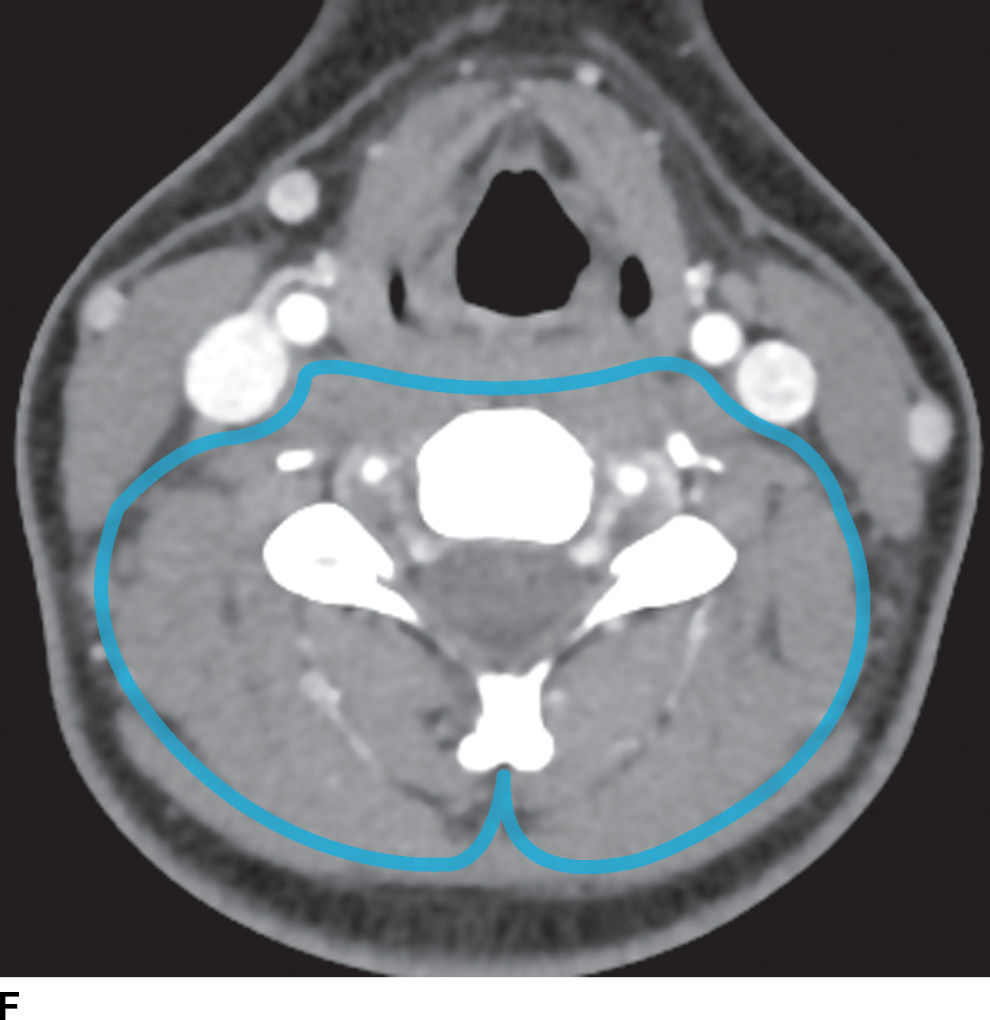
FIG. 21.2 Deep cervical fascia. A: The superficial layer of the deep cervical fascia in the suprahyoid neck is outlined in blue. B: The superficial layer of the deep cervical fascia in the suprahyoid neck is outlined in blue. C: The middle layer of the deep cervical fascia in the suprahyoid neck is outlined in blue. D: The middle layer of the deep cervical fascia in the infrahyoid neck is outlined in blue. E: The deep layer of the deep cervical fascia in the suprahyoid neck is outlined in blue. F: The deep layer of the deep cervical fascia in the infrahyoid neck is outlined in blue.
Acute Infectious Conditions
Oral cavity
Odontogenic infection
Odontogenic infections are the most common source of infections in the head and neck among adults (2,3), with the most common organisms being S. viridans, Prevotella, Staphylococcus, and Peptostreptococcus (4). Inflammatory disease of the teeth can be divided into periodontal infection that originates as gingivitis, resulting in radiolucency along the side of the tooth, or endodontal infection that originates as dental caries, resulting in bone resorption at the tooth apex (2).
The most common path of spread of maxillary odontogenic infection originating in the incisors, canines, premolars, and first molars is to the buccal space, whereas infection originating in the second maxillary molar tends to spread to the masticator space. Infection of the third maxillary molar tends to spread to the parapharyngeal space (5).
The submandibular space extends from the floor of the oral cavity to the attachment of the superficial layer of the deep fascia to the mandible to the hyoid bone (Fig. 21.3). It is divided by the mylohyoid muscle into sublingual (supramylohyoid) and submandibular (inframylohyoid) compartments. The mylohyoid muscle originates from the mylohyoid line of the mandible; the posterior fibers insert to the body of the hyoid, whereas the middle and anterior fibers are inserted into a median fibrous raphe. The free posterior border of the mylohyoid allows communication of infection across the sublingual and submandibular spaces.
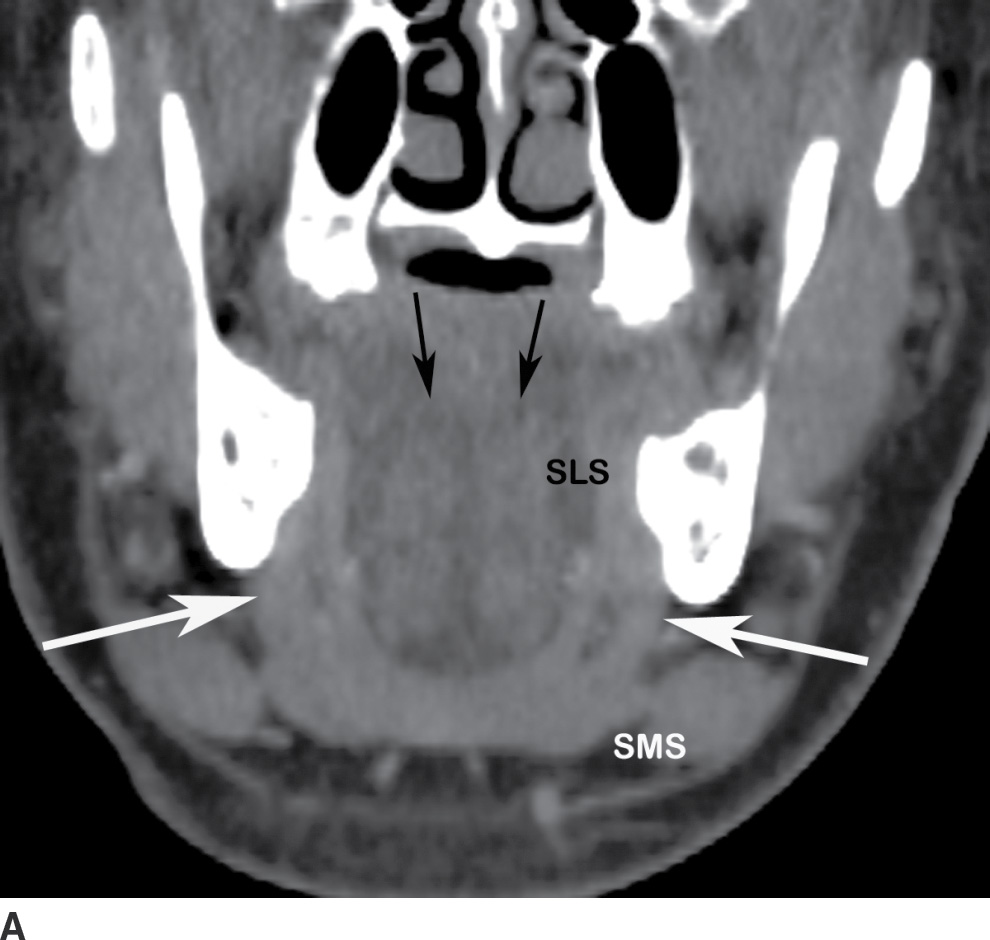
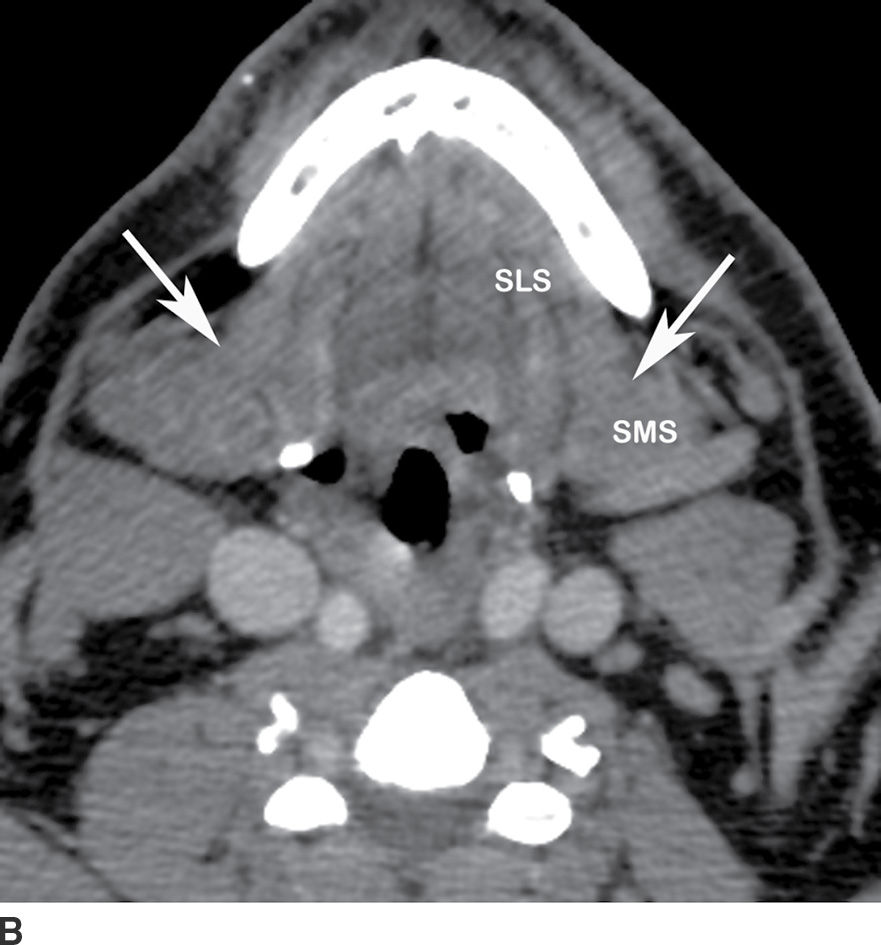
FIG. 21.3 Submandibular space. A: On coronal reformatted postcontrast CT image, the mylohyoid muscle (white arrows) is seen as a sling forming the floor of the mouth. The sublingual compartment (SLS) is superior and medial to and the submandibular compartment (SMS) inferior to the mylohyoid muscle. The genioglossus and geniohyoid muscles (black arrows) are also seen. B: The free posterior border of the mylohyoid muscle (arrows) is seen on this axial postcontrast CT image, allowing communication between the SLS and SMS of the submandibular space.
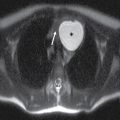
Odontogenic infection originating in the mandibular incisors, canines, or first premolars spreads into the sublingual space. Because the second and third mandibular molar tooth roots extend below the mylohyoid muscle insertion, odontogenic infection originating from these teeth tends to spread into the submandibular space (2). Complications of odontogenic infections include oroantral fistula, which occurs secondary to resorption of the tooth root, or odontogenic sinusitis, which refers to secondary infection of the paranasal sinuses originating from dental infection (2) (Fig. 21.4). The maxillary sinus is the main sinus involved because of its proximity to the posterior maxillary teeth. Unusual complications include osteomyelitis and rarely intraorbital extension.
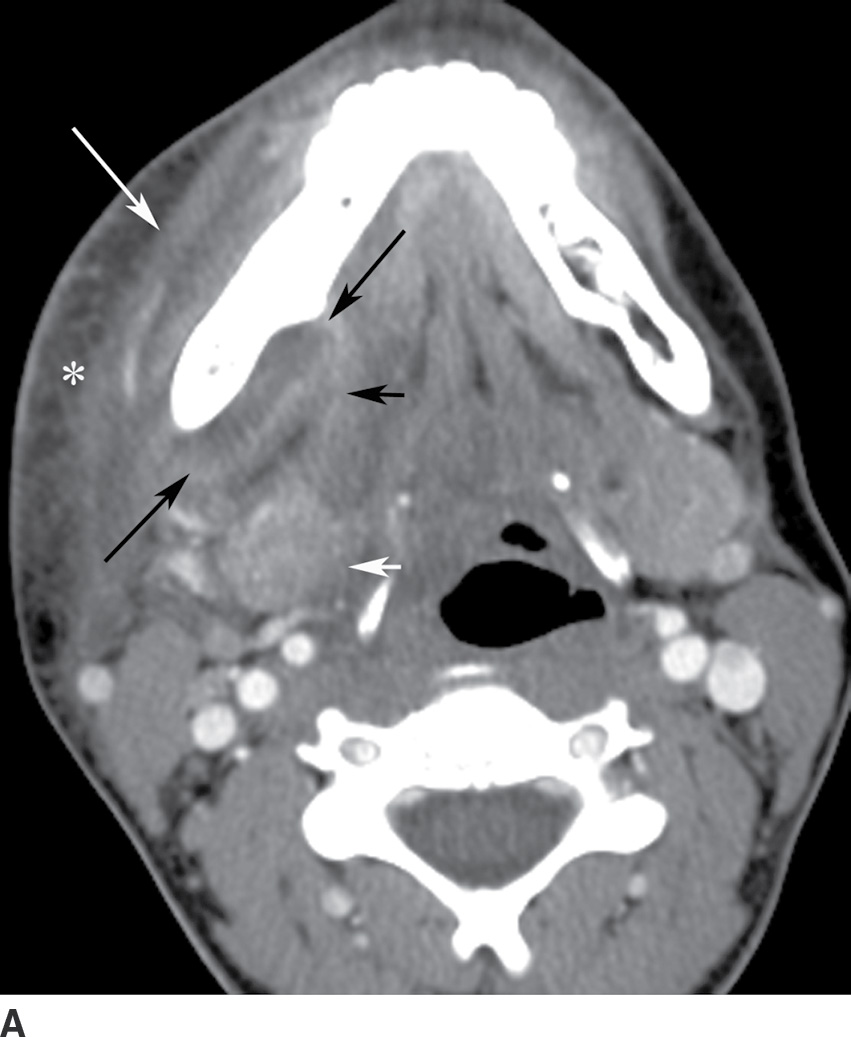
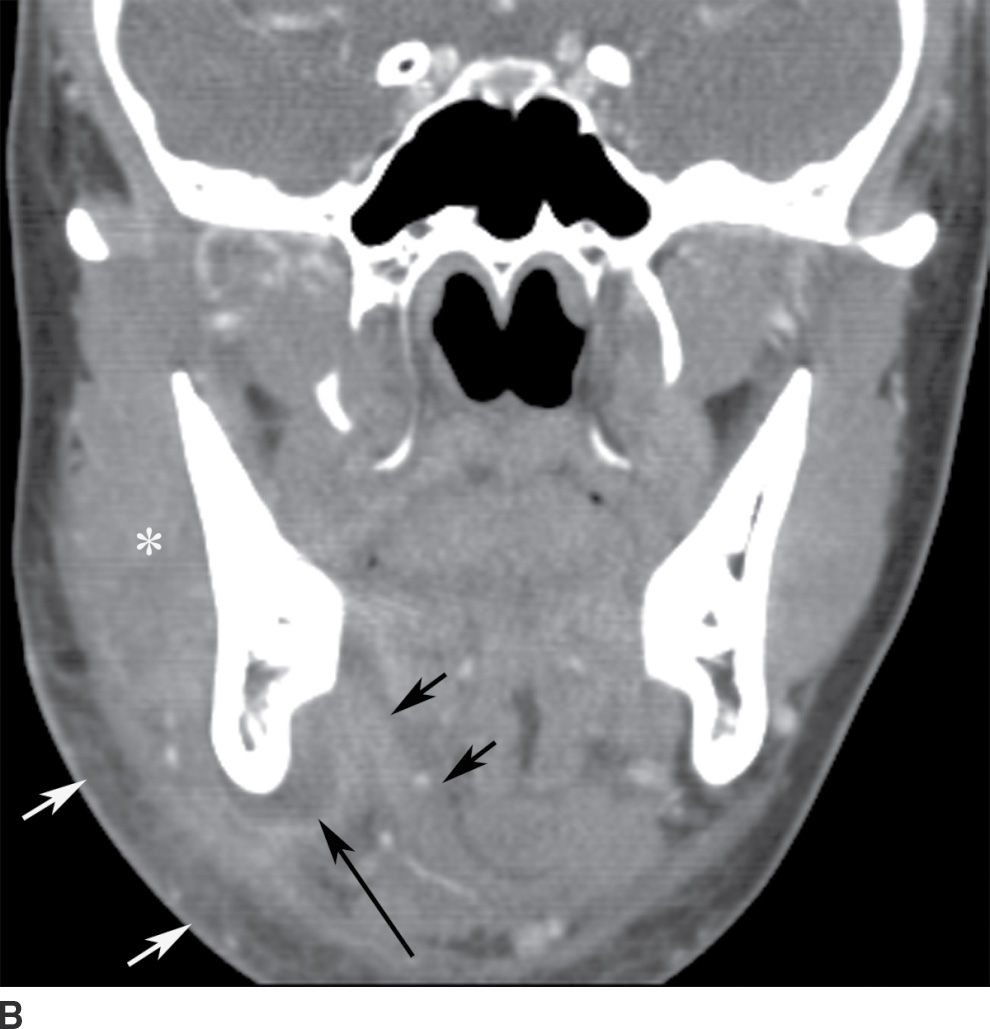
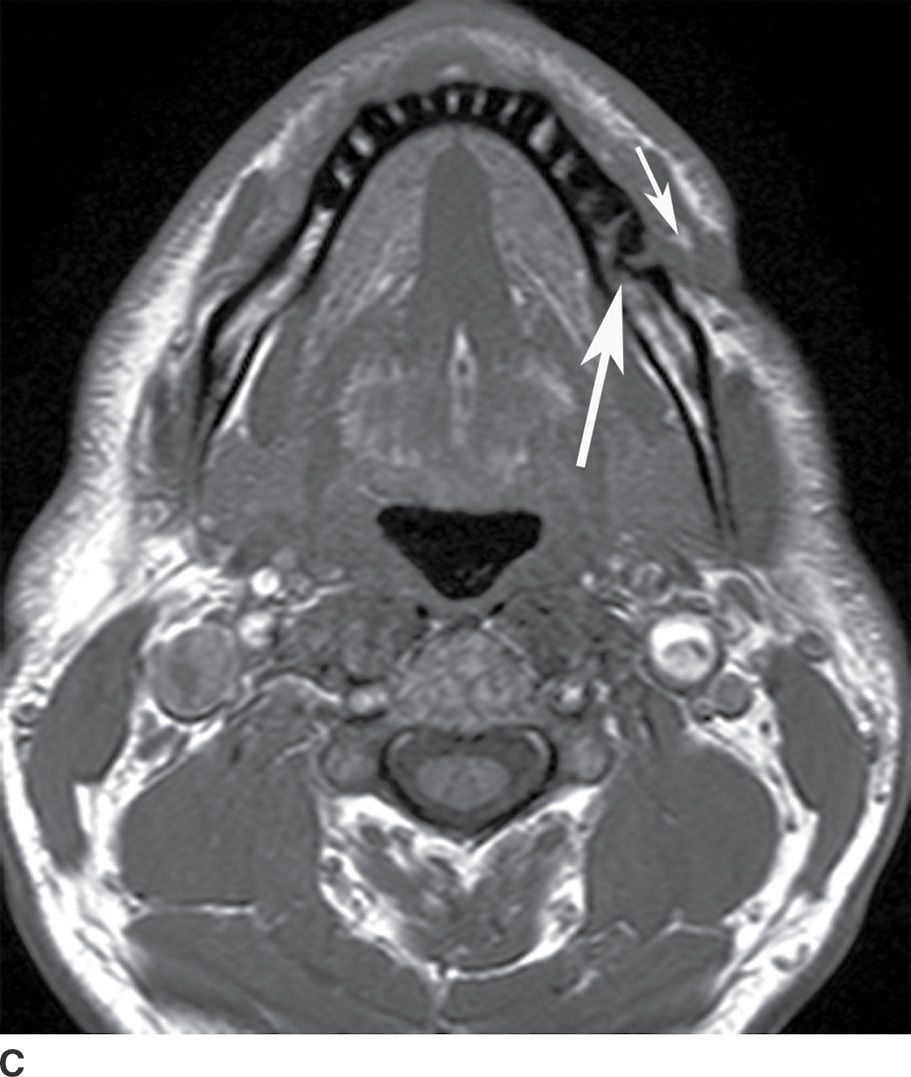
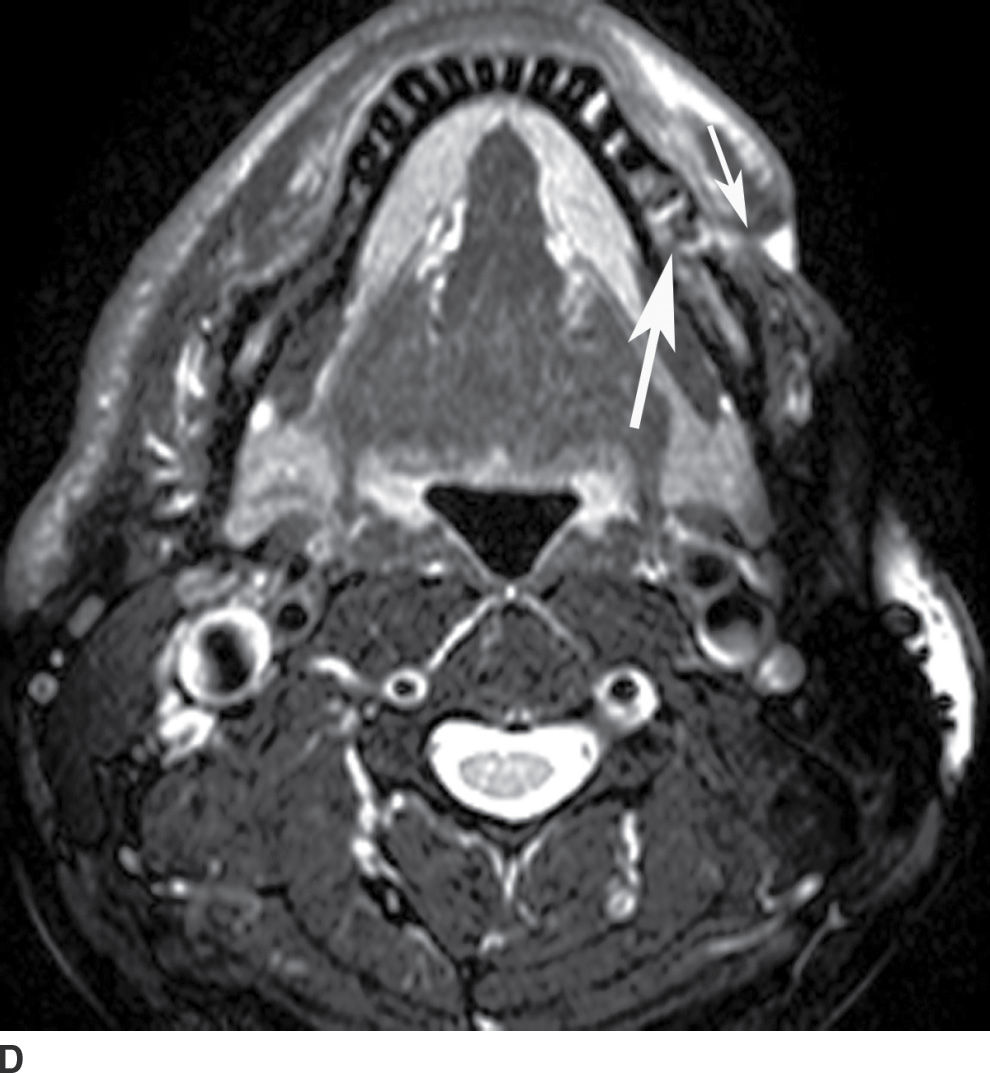
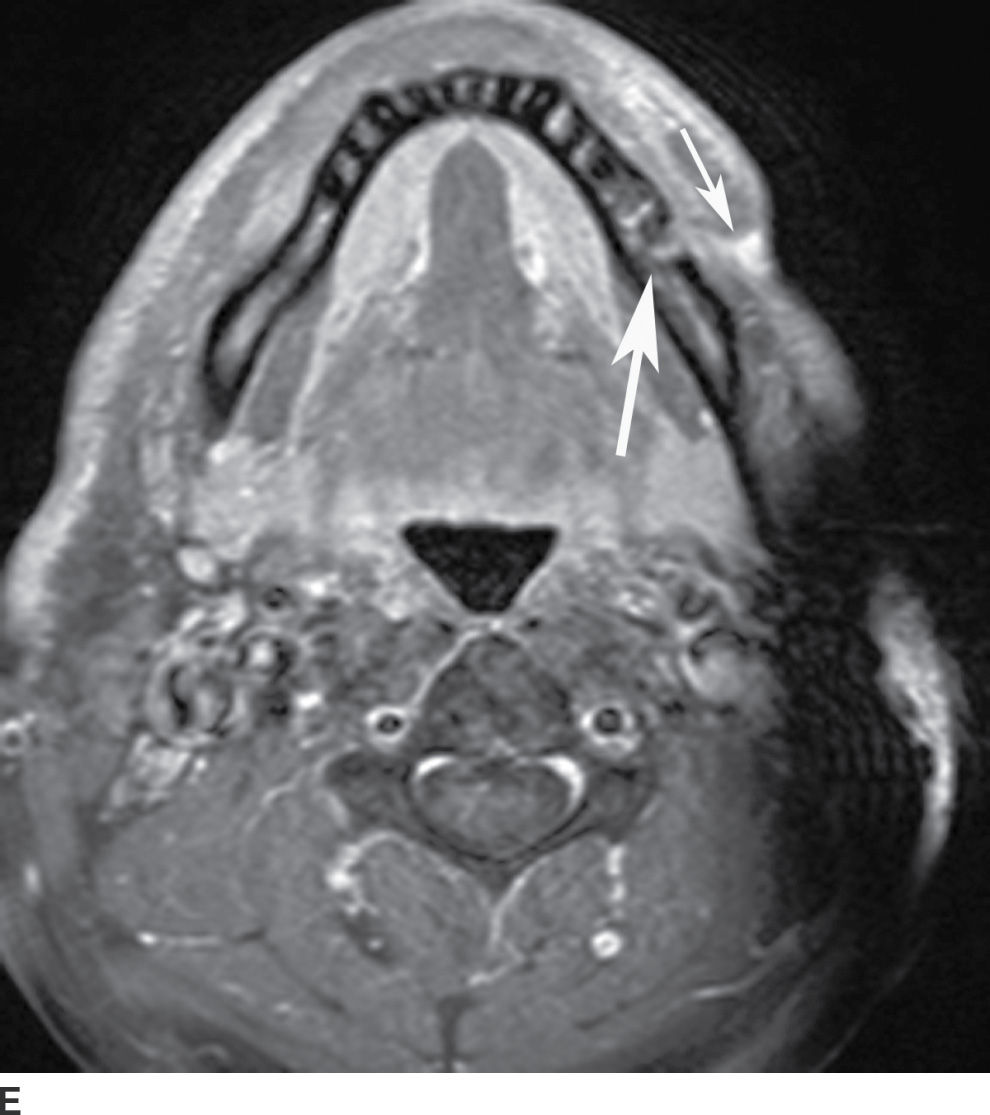
FIG. 21.4 Odontogenic infection. A: Axial postcontrast CT image shows a low-attenuation, rim-enhancing mass (long black arrows) along the lingual surface of the mandible, inferior to the displaced mylohyoid muscle (short black arrow). There is thickening of the platysma muscle (white arrow) and stranding of the subcutaneous fat (asterisk). B: Coronal reformatted postcontrast CT image shows the rim-enhancing mass (long black arrow) along the lingual surface of the mandible, inferior to the displaced mylohyoid muscle (short black arrows). There is stranding of the subcutaneous fat (white arrows) and swelling of the right masseter muscle (asterisk). Findings are related to a submandibular space abscess with cellulitis. C: Axial T1-weighted MRI image from a different patient shows bone destruction around the left first molar (long arrow) with associated intermediate signal soft tissue fullness extending to skin surface (short arrow). D: Corresponding axial T2-weighted image shows abnormal high signal around the left first molar (long arrow) extending to the skin surface (short arrow). E: Postcontrast, fat-saturated axial T1-weighted image demonstrates enhancement around the left first molar (long arrow) extending to the skin surface (short arrow). Findings compatible with osteomyelitis and draining sinus tract in a patient with history of chronic left mandibular area infection draining tract.
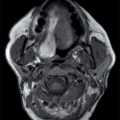
Ludwig angina
Ludwig angina is an aggressive, potentially life-threatening cellulitis of bilateral supra and inframylohyoid compartments, named after a German physician, Wilhelm Friedrich von Ludwig, who first described this condition in 1836. The most common source of infection is odontogenic, in approximately 80% of cases, usually an infection of the third mandibular molar tooth or pericoronitis (an infection of the gums surrounding the partially erupted lower third molar tooth), with other sources of infection including submandibular sialadenitis, lymphadenitis, and trauma. Infection initially may be limited to either the sublingual or submandibular space. However, infection can communicate across the posterior border of the mylohyoid and across the midline if it is not adequately treated (6). Patients present with pain, swelling, dysphagia, fever, tongue elevation, drooling, and an inability to swallow. At physical examination, the submandibular tissues are firm and hard, and crepitation may be present. As soft tissue swelling displaces the tongue into the pharyngeal airway, the patient may experience difficulty breathing or stridor, with a potential risk of direct spread to the parapharyngeal space or the RPSs, and subsequent rapidly progressive airway obstruction.
Although Ludwig angina is a cellulitis with diffuse infiltration, subsequent abscess formation is not uncommon. Imaging is performed to assess airway patency and, for the presence of gas-forming organisms, an underlying dental infection, or a drainable abscess (6). CT may demonstrate either a diffusely infiltrative process or a more discrete low-attenuation area reflecting abscess (Fig. 21.5). Prompt and aggressive treatment with antibiotics, airway management, and surgical decompression, if required, are critical (7).
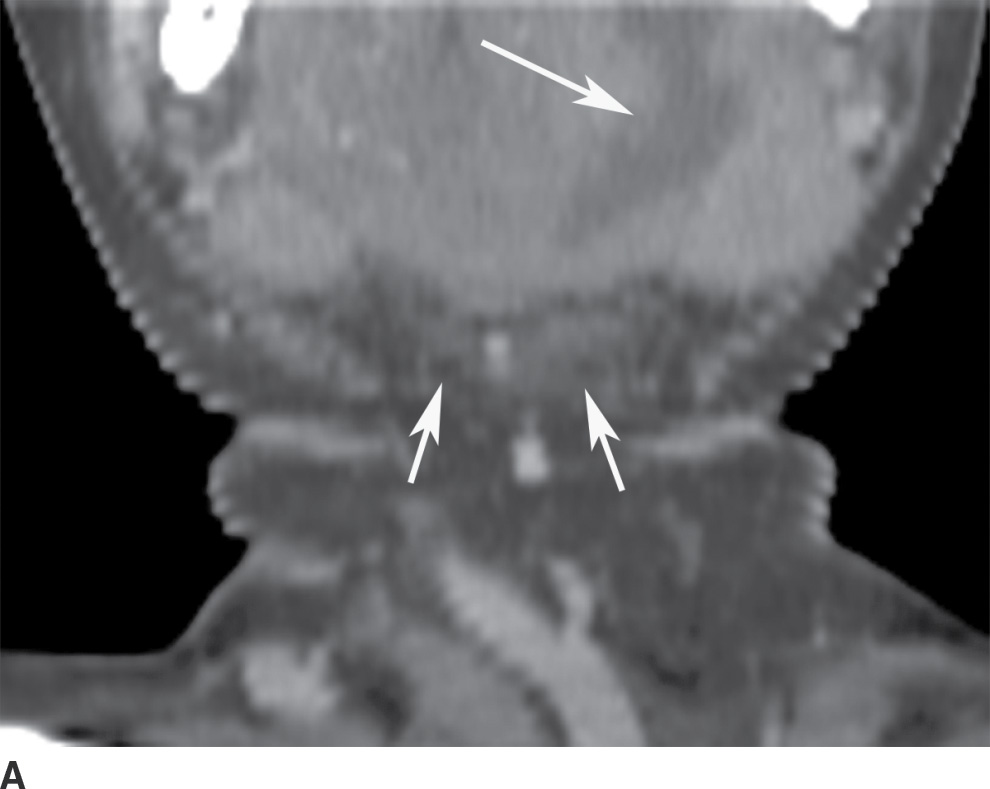
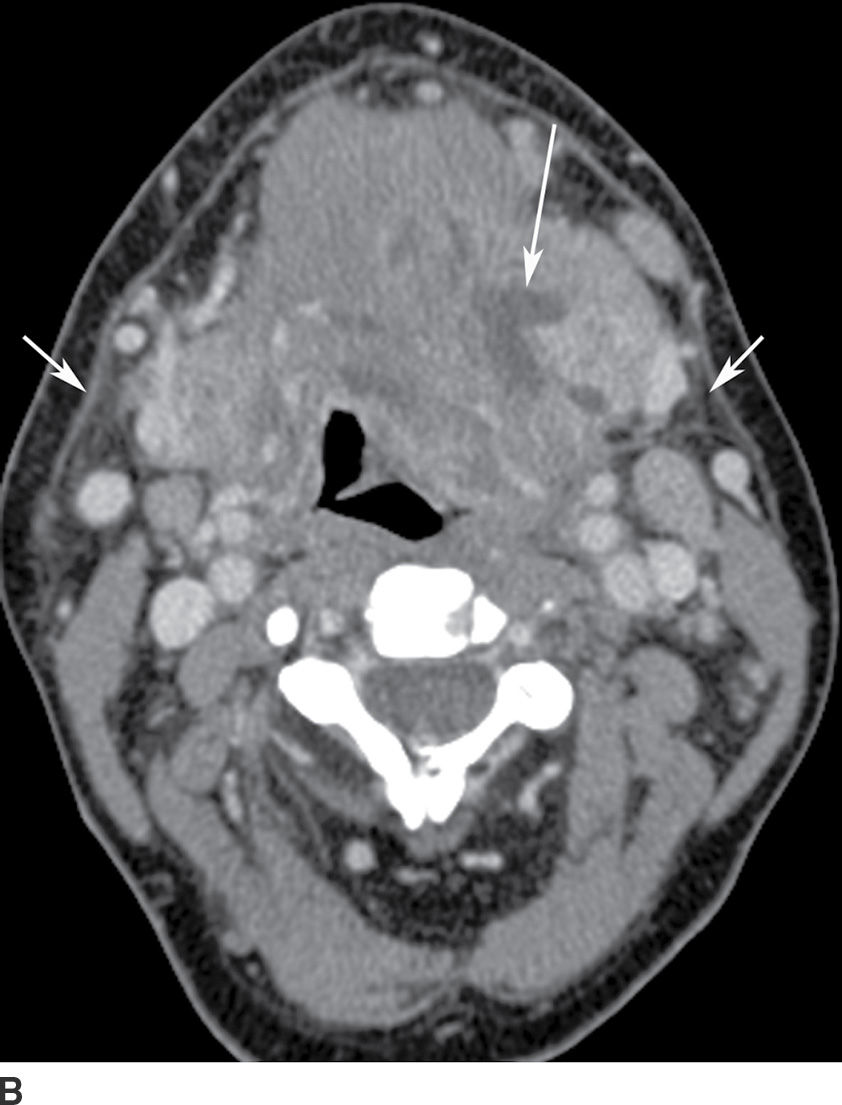
FIG. 21.5 Ludwig angina. A: Coronal reformatted postcontrast CT image shows a low-attenuation, rim-enhancing mass in the left sublingual space (long arrow). There is stranding in the fat of the submandibular space (short arrows). B: Axial postcontrast CT image shows the rim-enhancing sublingual space mass (long arrow) and soft tissue stranding in the bilateral submandibular spaces (short arrows). Findings compatible with Ludwig angina with abscess formation.
Oropharynx
Tonsillitis and peritonsillar abscess
Tonsillitis and peritonsillar abscess are the most commonly encountered acute neck infections among adolescents and young adults, with peritonsillar abscess accounting for one-third of all soft tissue abscesses of the head and neck (8,9). Symptoms include severe unilateral sore throat, fever, tender cervical lymphadenopathy, dysphagia, pharyngotonsillar exudates, otalgia, and trismus. The most common pathogens are β-hemolytic Streptococcus, Staphylococcus aureus, pneumococcus, and Haemophilus influenzae. Acute tonsillitis may suppurate and internally cavitate to create an intratonsillar abscess; however, a true tonsillar abscess is rare. Infection that penetrates the fibrous tonsillar capsule and the peritonsillar space—a potential space between the tonsillar capsule and the superior constrictor muscle—is more common. The infection may then extend into the adjacent parapharyngeal, masticator, or submandibular spaces. The resulting peritonsillar cellulitis resolves over several days when antibiotics are administered; however, if it goes untreated, a peritonsillar abscess develops, typically along the superior tonsillar pole.
Imaging is not routinely performed if the diagnosis is clinically apparent (10). However, contrast-enhanced CT is used if the diagnosis is uncertain, a full clinical examination is difficult because of severe trismus, a deep neck space infection or complication is suspected, or the patient does not respond to therapy. CT findings of peritonsillar cellulitis include tonsillar enlargement and linear, striated enhancement of the palatine tonsils and posterior pharyngeal soft tissues. Medial apposition of the enlarged tonsils results in a “kissing tonsils” appearance (Fig. 21.6A). Central liquefaction with surrounding rimlike enhancement is diagnostic of peritonsillar abscess (Fig. 21.6B). Peritonsillar cellulitis is treated with antibiotics, whereas abscess requires needle aspiration or surgical drainage (9,11).
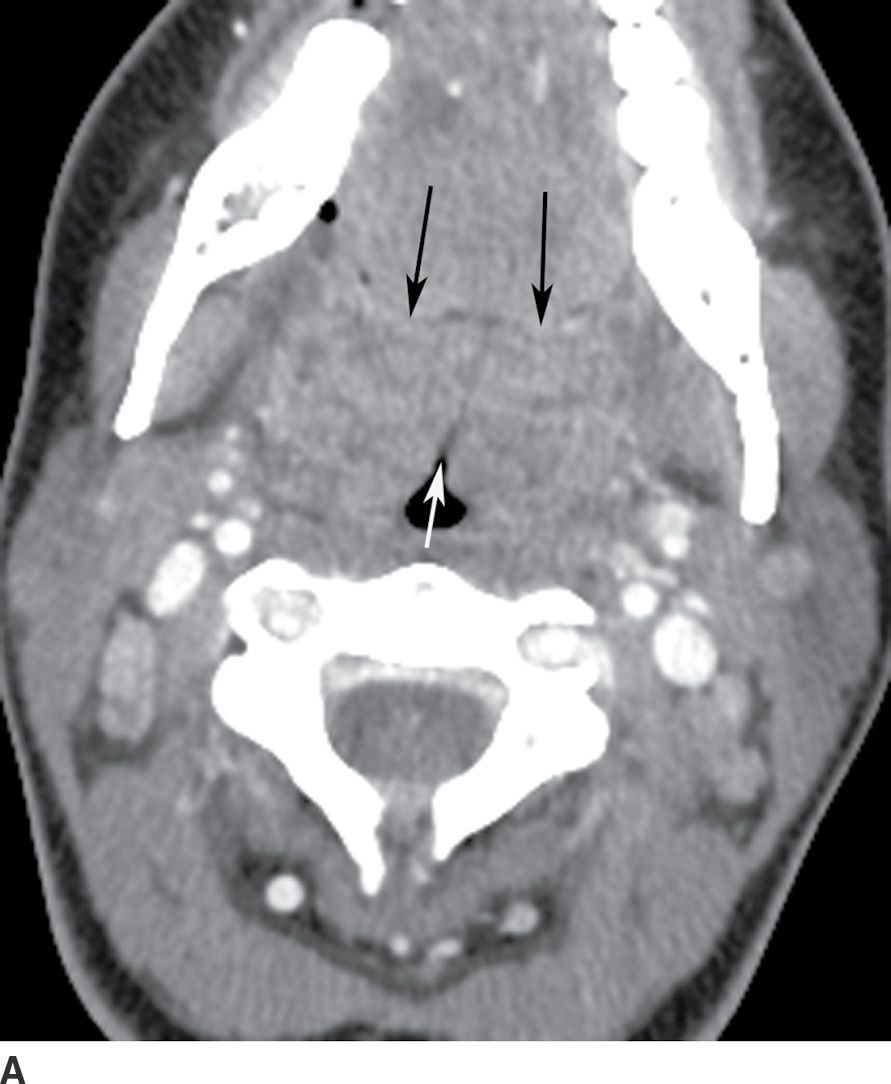
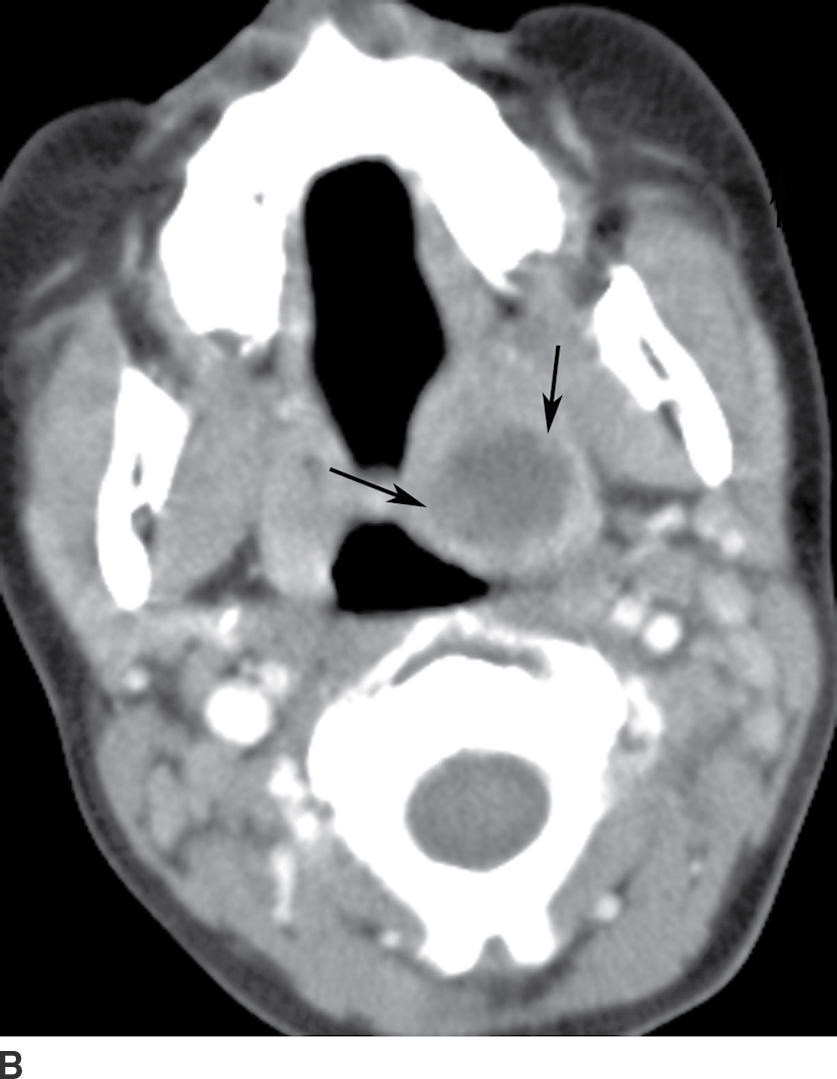
FIG. 21.6 A: Tonsillitis. Axial postcontrast CT image shows enlargement of bilateral palatine tonsils with linear striated enhancement (black arrows). Enlarged tonsils are apposed in the midline (white arrow) giving a “kissing tonsil” appearance. B: Peritonsillar abscess. Axial postcontrast CT image shows a low-attenuation, rim-enhancing mass in the left palatine tonsil, compatible with a peritonsillar abscess, with purulent material found at tonsillectomy.
Retropharynx
The RPS extends from the skull base into the mediastinum up to the T2 thoracic vertebral level. It is bordered anteriorly by the visceral fascia (middle layer of the deep cervical fascia) and posteriorly by the alar fascia (a division of the deep layer of deep cervical fascia), which separates it from the danger space. Immediately posterior to the alar fascia is the danger space (Fig. 21.7A), which extends inferiorly into the posterior mediastinum. It is not possible to differentiate the RPS from the danger space on imaging, and danger space infection can be inferred only if the infection spreads below the T4 level.
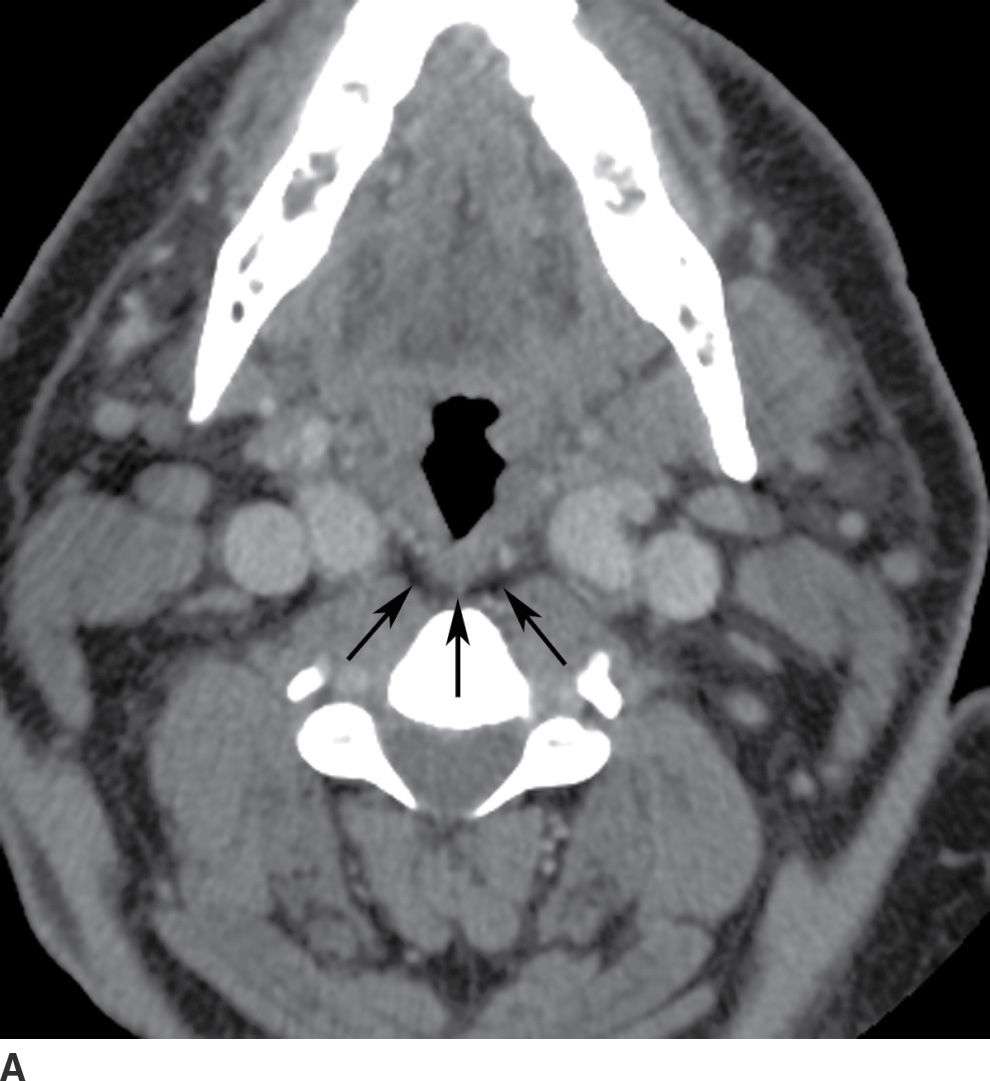
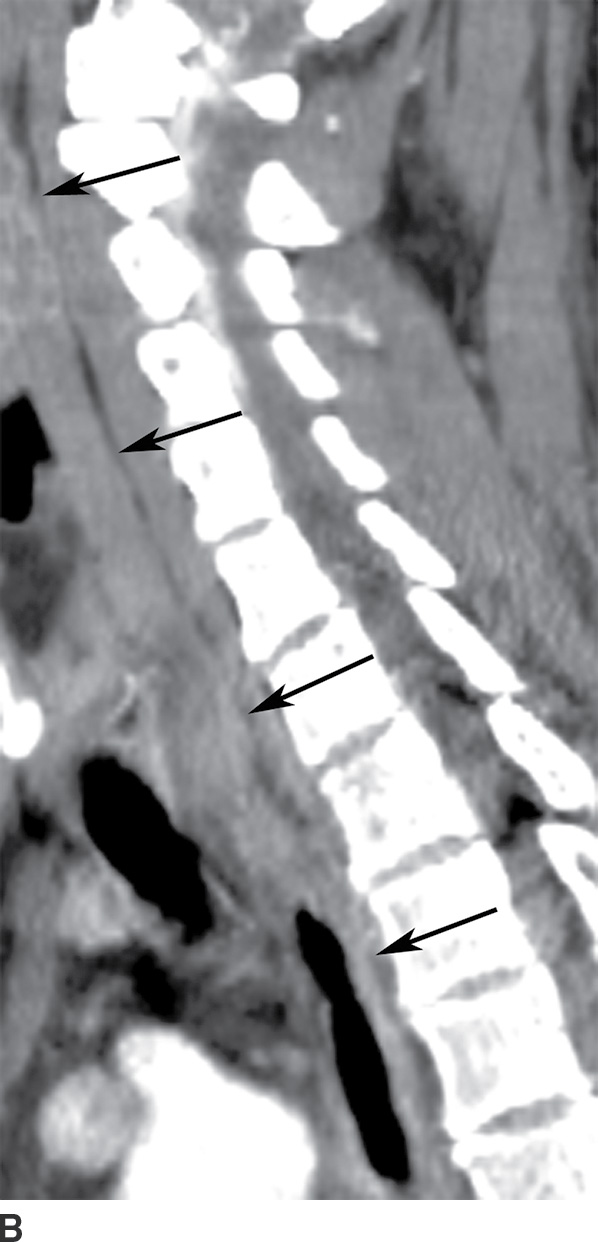
FIG. 21.7 Retropharyngeal space. A: On axial postcontrast CT image, the retropharyngeal space (RPS) is seen as a thin strip of fat density (arrows) posterior to the pharynx and anterior to the prevertebral muscles. B: On sagittal reformatted postcontrast CT image, the retropharyngeal fat stripe (arrows) can be seen in continuity throughout the neck from the skull base to the upper mediastinum. The RPS cannot be distinguished from the danger space. Danger space infection is inferred only if the infection spreads below the T4 level.
On imaging, the RPS appears as a thin stripe of fat density posterior to the pharynx (Fig. 21.7B). The contents of the RPS include fibrofatty tissue, blood vessels, and lymph nodes. Retropharyngeal lymph nodes are classified into medial and lateral groups. Lateral retropharyngeal lymph nodes are located just medial to the carotid sheath and anterolateral to the longus colli and longus capitis muscles, whereas medial retropharyngeal lymph nodes are located near the midline overlying the spine, immediately anterior to the prevertebral musculature. The prevertebral space lies posterior to the danger space, separated by prevertebral fascia, and extends down to the coccyx.
Suppurative retropharyngeal adenopathy
The RPS lymph nodes are prominent in young children but involute in late childhood. This explains the prevalence of retropharyngeal abscesses in the pediatric age group, which develop secondary to the suppuration of these lymph nodes following upper respiratory tract infections. Infections of the RPS can also originate from the parapharyngeal space, or may be posttraumatic, particularly in adults. On imaging, retropharyngeal suppurative adenitis is heralded by the appearance of enlarged paramedian retropharyngeal lymph nodes that contain low-attenuation centers (Fig. 21.8) (8).
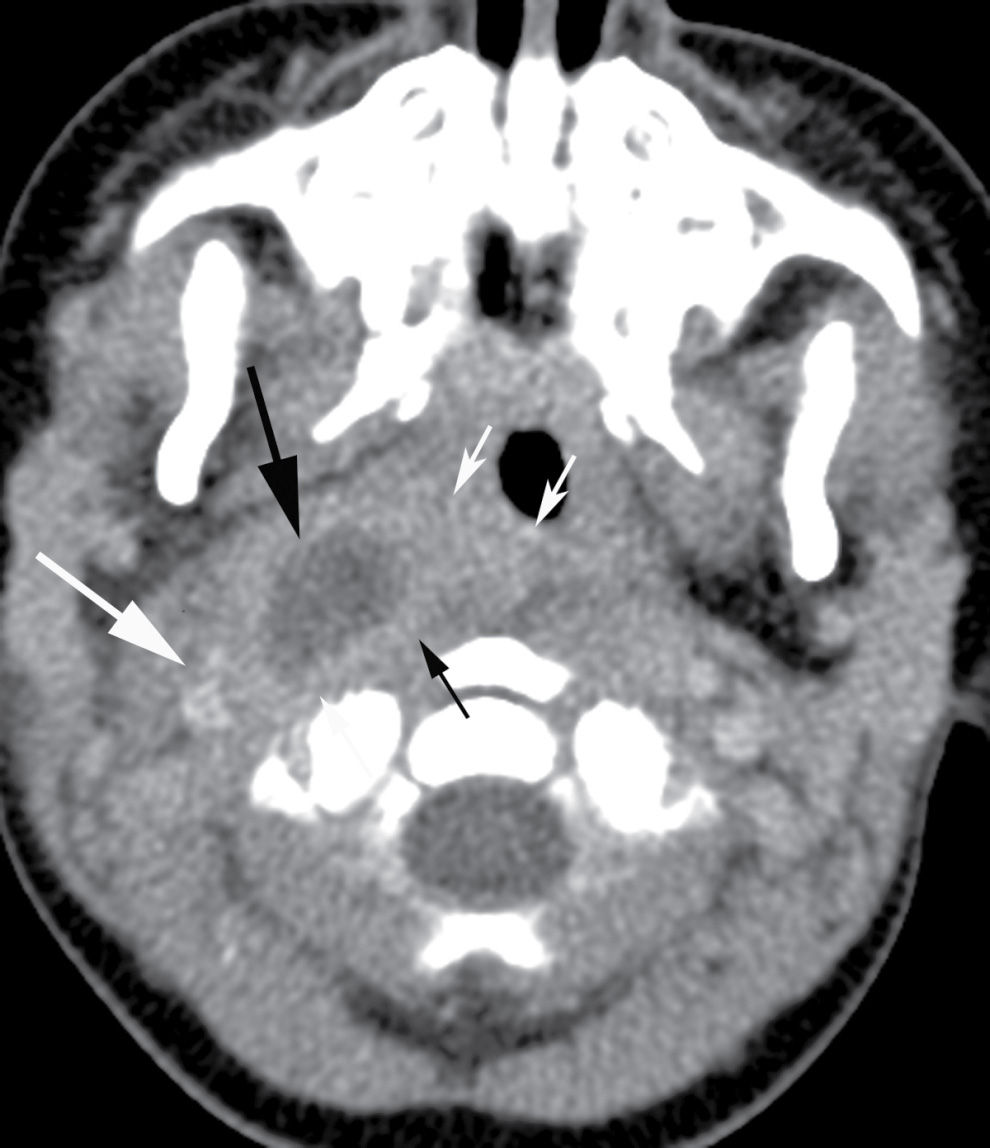
FIG. 21.8 Suppurative retropharyngeal adenopathy. A mass with a low-attenuation center (long black arrow) is seen medial to carotid sheath structures (long white arrow), anterolateral to distorted prevertebral muscles (short black arrows), and lateral to the constrictor ring (short white arrows) on an axial postcontrast CT image, compatible with a suppurative lateral retropharyngeal lymph node.
Retropharyngeal effusion/abscess
Unlike peritonsillar abscess, which is medial to the constrictor ring, parapharyngeal and retropharyngeal abscesses are lateral. The most common source of retropharyngeal abscess in children is suppurative adenitis (12), which is difficult to differentiate from frank abscess. A retropharyngeal abscess commonly results from a rupture of a suppurated retropharyngeal node. On imaging, abscesses appear as low-attenuation rim-enhancing collections with mass effect and flattening of the prevertebral muscles. They tend to displace the carotid sheath laterally and the parapharyngeal space anterolaterally. Life-threatening complications, such as airway obstruction and aspiration, may occur following rupture of an abscess into the airway. Treatment frequently requires surgical intervention for abscess drainage and intravenous antibiotics. Aggressive airway management is necessary in these patients to avoid airway obstruction and aspiration of pus.
MRI may help to delineate retropharyngeal abscess better and differentiate it from cellulitis. Symmetric and smooth expansion of the RPS by fluid density without an enhancing rim (termed retropharyngeal edema or nonabscess fluid) may be seen in early infection, internal jugular vein thrombus, postradiation states, and prevertebral calcific tendonitis.
The differentiation of RPS edema from RPS abscess on imaging is important, as the former does not require surgical drainage. RPS edema lacks an enhancing rim and is almost always confined to the level of the oropharynx (13) (Table 21.1; Fig. 21.9). RPS abscesses may extend posteriorly to involve the danger and prevertebral spaces as well as cause osteomyelitis of the spine.
Table 21.1 Fluid in the Retropharyngeal Space: RPS Nonabscess Fluid (Benign Edema) Versus RPS Abscess
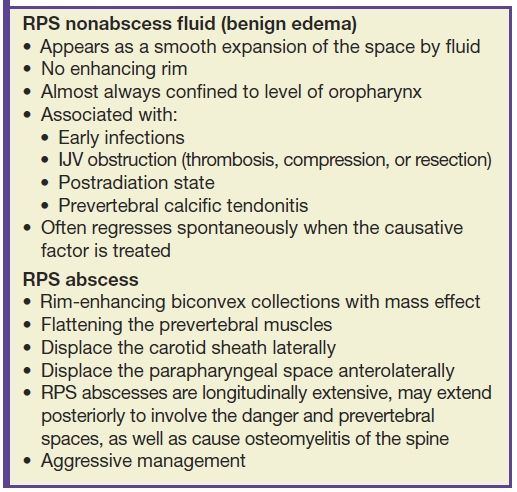
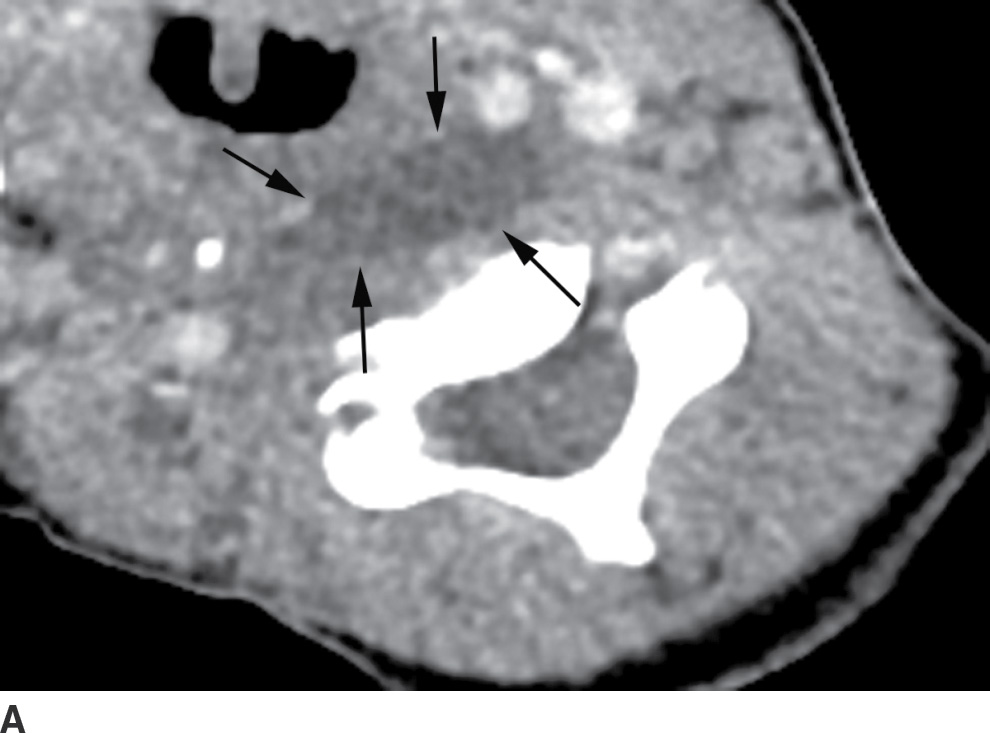
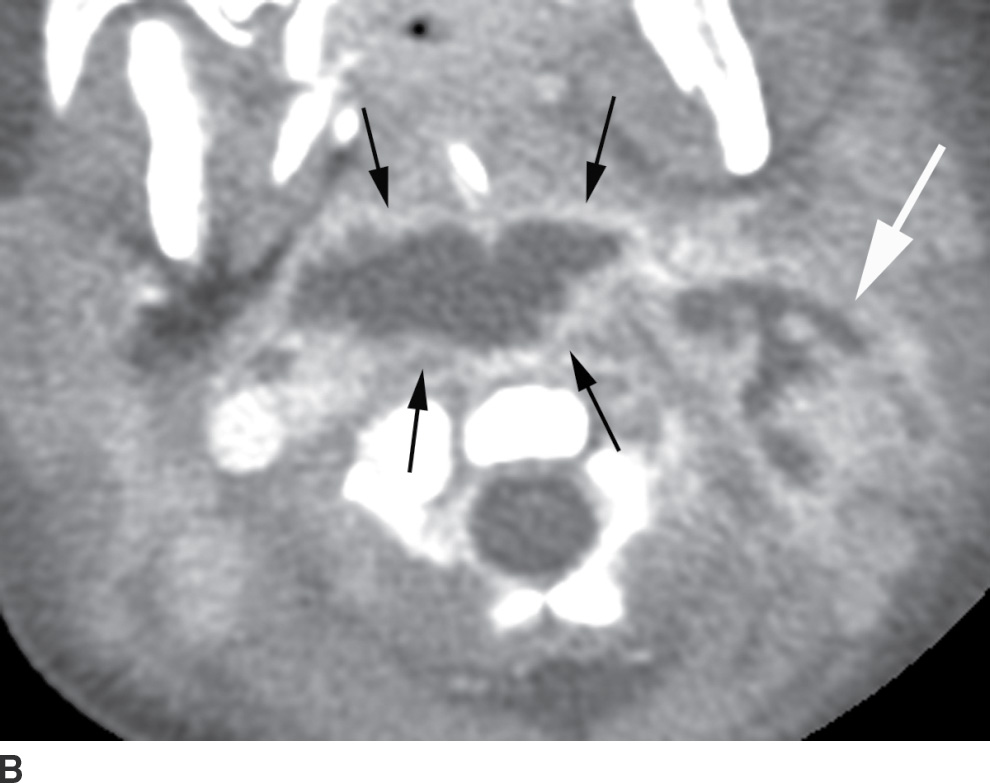
FIG. 21.9 Retropharyngeal effusion and abscess. A: A low-attenuation collection without enhancement (arrows) is present in the retropharyngeal space (RPS) on this axial postcontrast CT image, consistent with an effusion. B: An axial postcontrast CT images demonstrates a rim-enhancing, biconvex collection in the RPS (black arrows) compatible with an abscess. There is spread to the left carotid space (white arrow). Cultures grew methicillin-resistant Staphylococcus aureus (MRSA).
Descending necrotizing mediastinitis (danger space)
This is a rare but emergent complication of periodontal infection with a very high mortality rate of up to 40% (8), primarily due to delayed diagnosis, a result of nonspecific, subtle symptoms and clinical findings. Infection most commonly spreads from the oral cavity or oropharynx to the mediastinum by way of the RPS (the “danger” space), but it also may spread by way of the carotid space.
Contrast-enhanced CT allows the most accurate and rapid detection of descending necrotizing mediastinitis, often depicting both the source of infection and the route of spread. CT findings include mediastinal fluid collections, stranding of mediastinal fat, gas locules, and pericardial or pleural effusions (Fig. 21.10). Aggressive treatment is essential and includes airway management; broad-spectrum antibiotics; and early mediastinal exploration, debridement, and drainage (14).
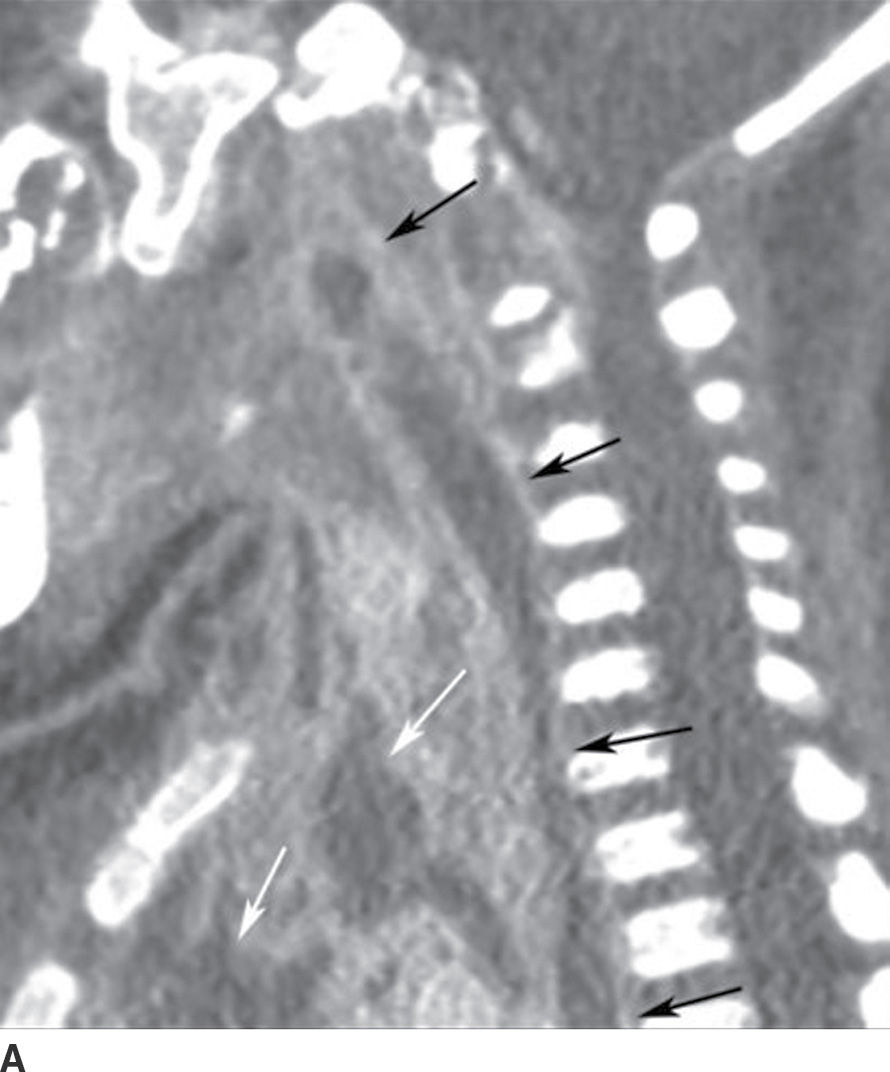
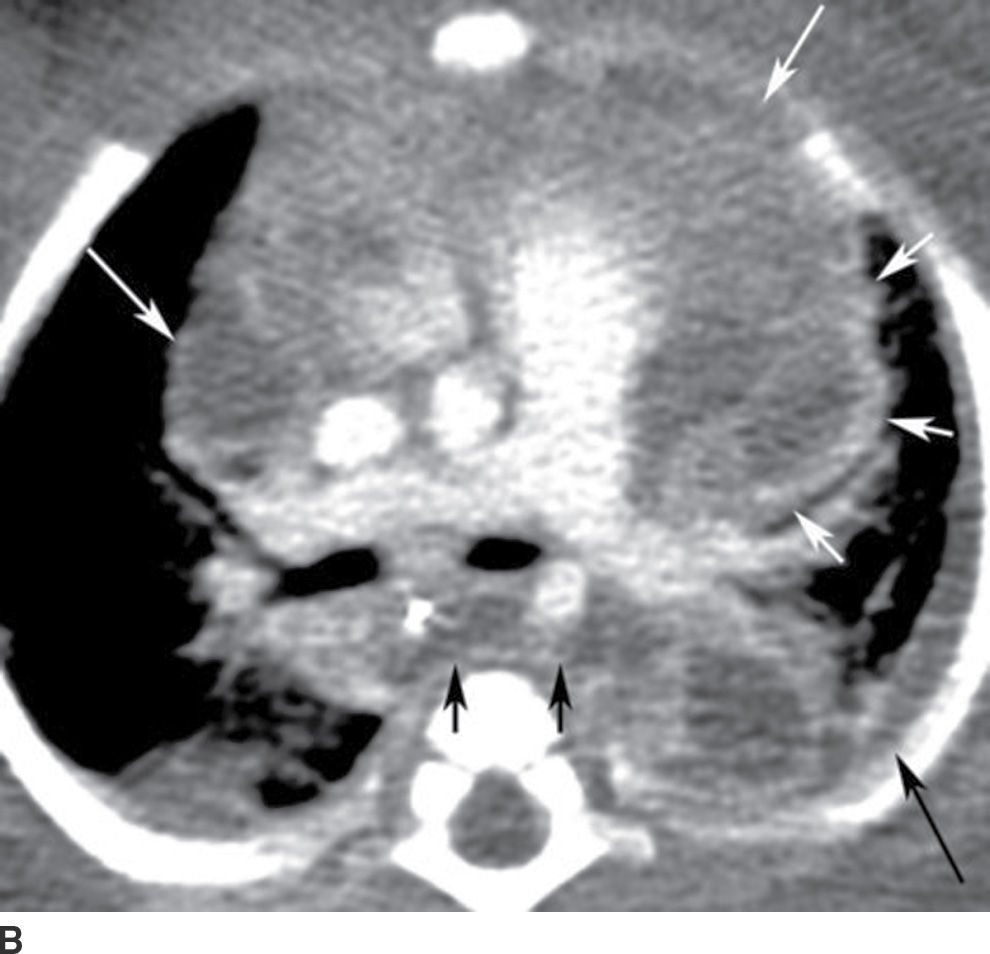
FIG. 21.10 Descending necrotizing mediastinitis. A: Sagittal reformatted postcontrast CT image shows enhancing collection in the retropharyngeal space (RPS) with extension inferiorly into the posterior mediastinum (black arrows), consistent with abscess. Similar collections are seen in the anterior and middle mediastinum (white arrows). B: Axial postcontrast CT image obtained at the level of T5 vertebra shows rim-enhancing collection, consistent with abscess in the posterior mediastinum (short black arrows), implicating involvement and spread via the danger space. Low attenuation is also seen in anterior and middle mediastinum (long white arrows) with some enhancement (short white arrows). Overall findings suggest abscess and phlegmon, which were confirmed at surgery. There is also a left pleural effusion (long black arrow).
Laryngeal/airway abnormalities
Epiglottitis
Acute epiglottitis is a cellulitis of the epiglottis, aryepiglottic folds, and adjacent tissues. It is one of the most serious emergent conditions of the pediatric head and neck, between the ages of 1 and 5 years, because of its association with life-threatening airway obstruction (2). Fortunately, acute epiglottitis has become rare in the United States due to routine vaccination for Haemophilus influenzae type b (Hib), which was the most common causative pathogen (15). Other causative organisms are Streptococcus and Staphylococcus in immunocompetent hosts and Pseudomonas and Candida in immunocompromised hosts.
Fever, sore throat, drooling, posturing with neck extension, and respiratory distress are common clinical symptoms (16). It is normally diagnosed on the basis of clinical findings. Imaging is reserved only for select cases where the diagnosis is unclear and should not interfere with bronchoscopy or maintenance of airway patency. If the patient’s condition permits, lateral neck radiography is the preferred modality, which shows thickening of the epiglottis (the “thumb sign”) and aryepiglottic folds (Fig. 21.11). CT is neither necessary nor recommended in the acute setting; however, CT images obtained in patients with unsuspected epiglottitis reveal a thickened epiglottis and narrowing of the airway as well as extension of the inflammatory process into the adjacent deep spaces of the neck (16). Besides epiglottitis, other causes of epiglottic and aryepiglottic fold thickening include ingestion of a caustic substance or foreign body, angioedema, hemorrhage, epiglottic cyst, and postirradiation edema and fibrosis. Endoscopy is performed emergently to confirm the diagnosis. Treatment includes securing an airway and initiating intravenous antibiotics.
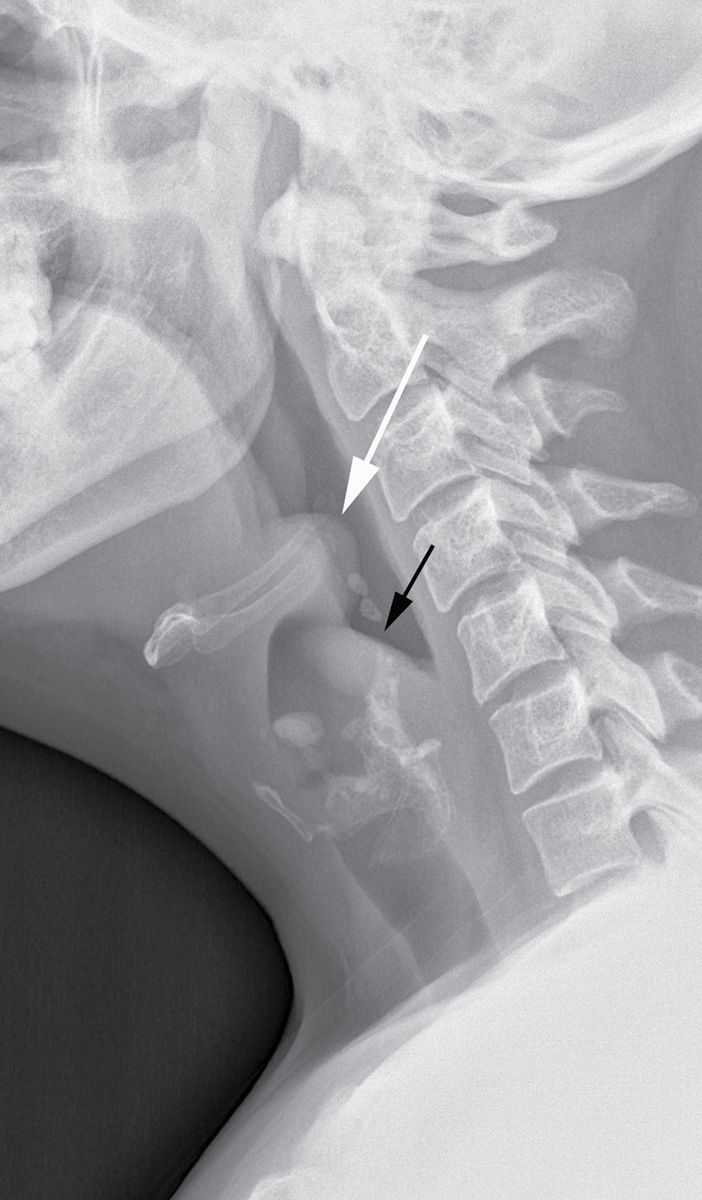
FIG. 21.11 Epiglottitis. The epiglottis (white arrow) and aryepiglottic folds (black arrow) are thickened on the lateral neck x-ray. On flexible nasal laryngoscopy, the epiglottis was significantly enlarged with exudate and erythema present.
Laryngotracheobronchitis
Laryngotracheobronchitis, also known as “subglottitis,” or “croup,” most commonly occurs between the ages of 6 months and 3 years. The most common causative pathogen is parainfluenza virus (16). The condition typically occurs during the fall and winter months, with nonspecific prodromal symptoms of viral infection, followed by fever, inspiratory stridor, hoarseness, and a classic “barking” cough. Imaging is performed to determine whether another cause of inspiratory stridor is present that may require emergent intervention, for example, epiglottitis and foreign body ingestion. Frontal radiographs demonstrate symmetric straightening of the normally convex appearance of the “shoulders” of the subglottic airway, a finding also referred to as the “steeple sign.” Lateral radiographs show narrowing of the subglottic airway with distention of the hypopharynx (Fig. 21.12). Differential considerations include subglottic stenosis (congenital or related to prior intubation), inflammatory causes such as Wegener granulomatosis, and developmental lesions such as subglottic hemangioma. It is often managed on an outpatient basis with oral and inhaled corticosteroids, with inpatient management, and with intubation, reserved for severe cases (16).
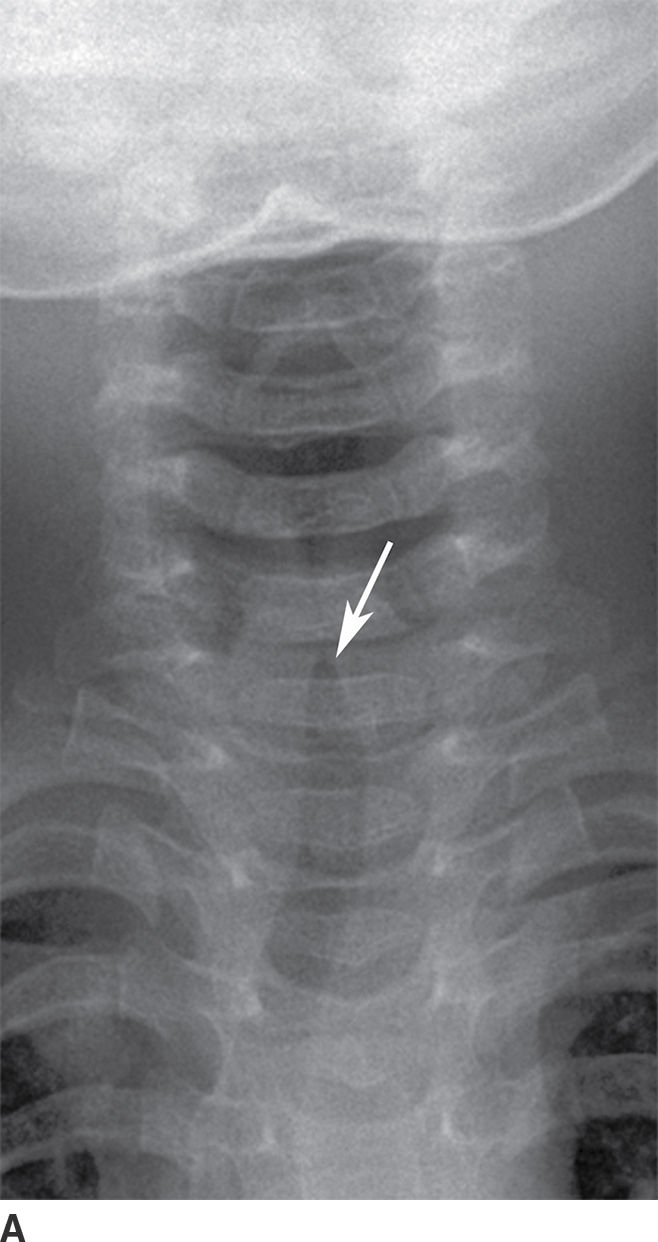
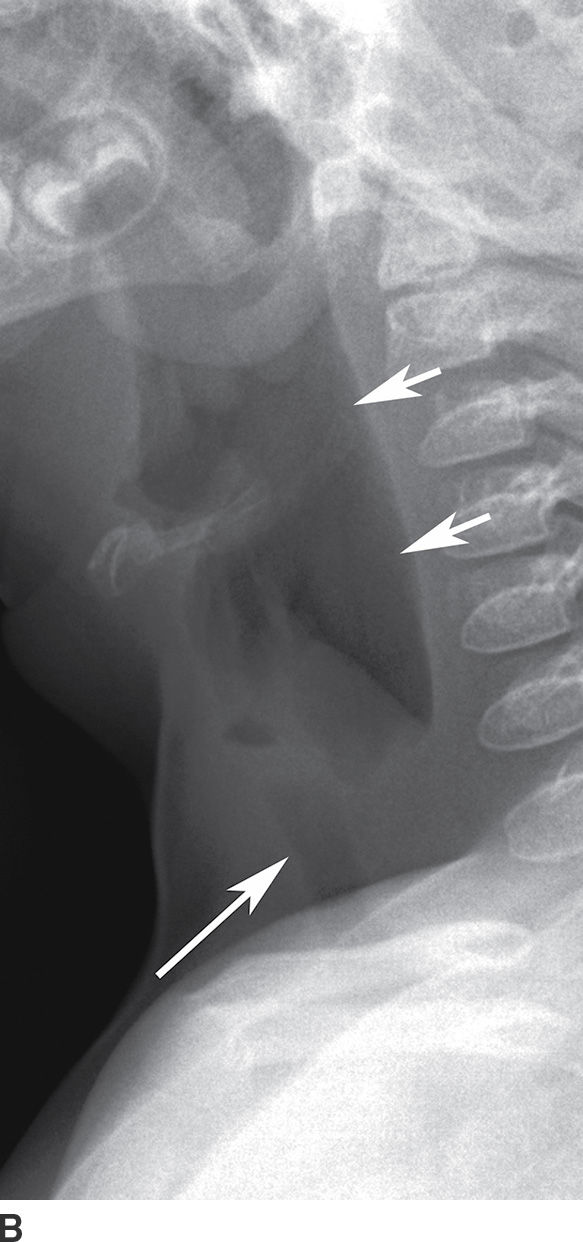
FIG. 21.12 Laryngotracheobronchitis. A: Frontal neck x-ray shows narrowing of subglottic airway with “steeple sign” (arrow). B: Lateral neck x-ray shows a distended hypopharynx (short arrows) proximal to the narrowed subglottis (long arrow).
Laryngopyocele
A laryngocele is an abnormal dilation of the anterior laryngeal ventricle, which extends superiorly between the false vocal cord and the inner aspect of the thyroid cartilage. Laryngopyoceles are rare infectious complications of laryngoceles. Laryngopyoceles may present with life-threatening airway obstruction, palpable neck mass, hoarseness, dyspnea, dysphagia, odynophagia, and fever (17). CT demonstrates a rim-enhancing fluid or mixed fluid–air density collection emanating from the laryngeal ventricle, with superolateral extension into the paraglottic fat. Management includes airway support, needle or surgical drainage, broad-spectrum antibiotics, and steroids.
Salivary glands
Sialolithiasis/sialadenitis/abscesses
Sialolithiasis refers to salivary gland stones or calculi and is the most common benign condition affecting the salivary glands. The submandibular (Wharton) duct is by far most commonly affected in about 80% to 90% of cases followed by the parotid (Stensen) duct in 10% to 20% of cases. They occur most often in the submandibular duct because of its larger diameter and ascending course, more mucinous, alkaline, and viscous secretions and the presence of salivary stasis (18,19). Most stones are radiopaque, but CT is more sensitive than radiography to demonstrate sialothiasis and its complications, including sialadenitis and abscesses.
Sialadenitis, or glandular inflammation of the salivary glands, is most commonly caused by sialolithiasis. Patients with acute sialadenitis present with painful swelling that is exacerbated by eating, a condition often referred to as salivary colic. The most common causative organism of sialadenitis is S. aureus. Calculus-induced sialadenitis or bacterial sialadenitis is typically unilateral, whereas viral parotiditis is bilateral in 75% of cases and may also involve the submandibular and sublingual glands. Viral parotiditis is a self-limiting condition, with peak prevalence in patients aged 5 to 9 years. The mumps virus (paramyxovirus) is the most common pathogen. Other pathogens include influenza virus, parainfluenza virus, coxsackievirus, cytomegalovirus, and adenovirus. Secondary sialadenitis often occurs as a result of ductal obstruction from squamous cell carcinoma in the floor of the mouth.
On contrast-enhanced CT, the affected gland is diffusely enlarged and enhancing, with ill-defined fuzzy margins; ductal dilatation may be seen secondary to an obstructive calculus or stenosis, with associated adjacent cellulitis and myositis (Fig. 21.13) (10). The salivary duct may be dilated and thick walled, and an obstructing sialolith can be visualized.
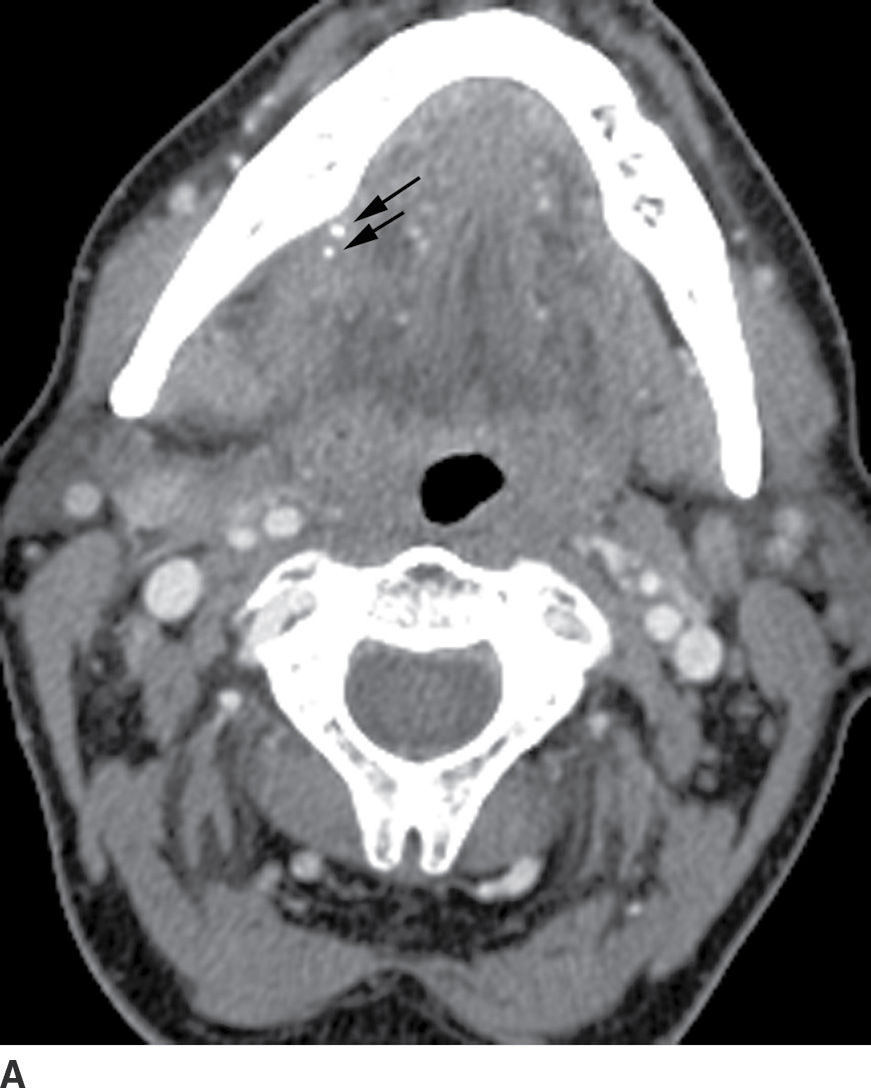
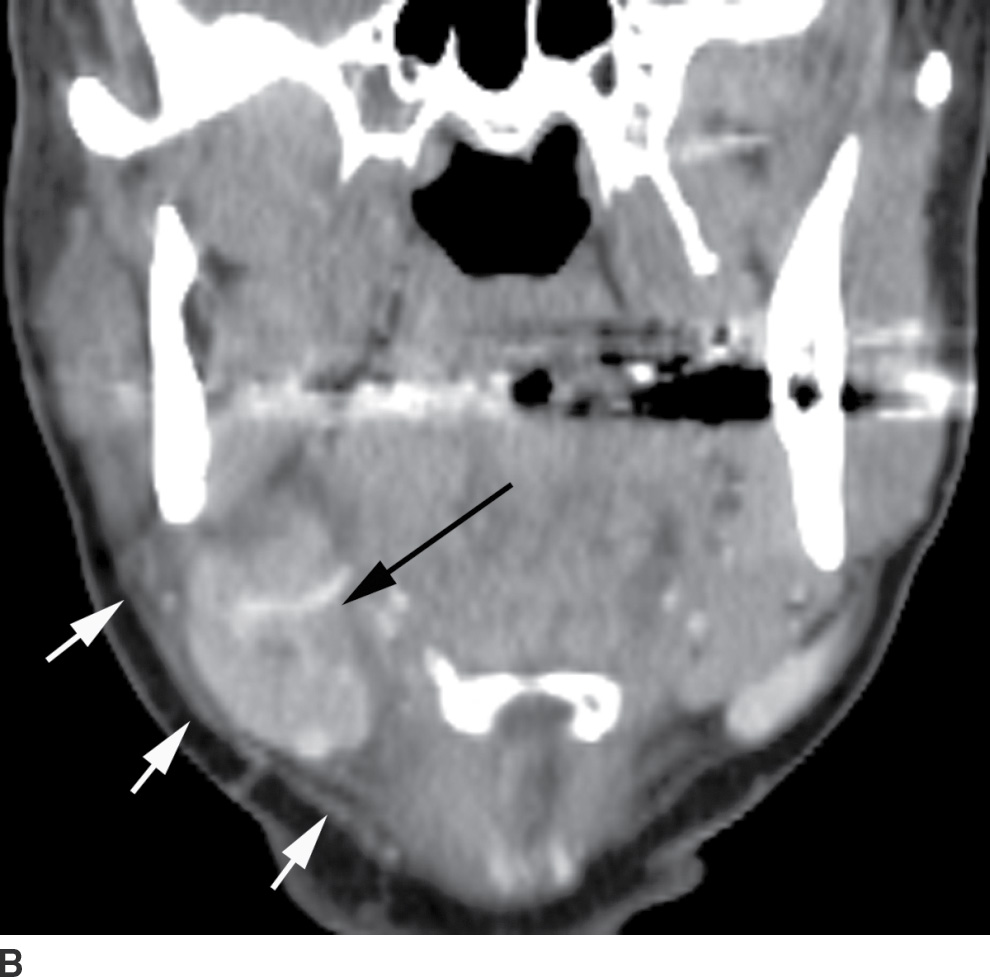
FIG. 21.13 Sialolithiasis with sialadenitis. A: Two calculi (arrows) are seen in the distal submandibular gland duct on axial postcontrast CT image. B: Coronal reformatted postcontrast CT image shows an enlarged, enhancing submandibular gland (black arrow). There is surrounding soft tissue infiltration (white arrows) compatible with adjacent cellulitis.
Complications of sialadenitis include abscess formation, seen as internal areas of low attenuation, which may rupture into the deep spaces of the neck; thrombophlebitis of the retromandibular or facial veins; and rarely cranial nerve VII dysfunction (19). Treatment includes hydration and antibiotic therapy. Drainage may be required if an abscess develops.
Masticator space
Masseter/temporalis abscesses and mandibular osteomyelitis
The masticator space extends craniocaudally between the skull base and the mandibular ramus and transversely between the medial pterygoid and the masseter muscles (Fig. 21.14). The infratemporal fossa is the part of this space between the skull base and zygoma.
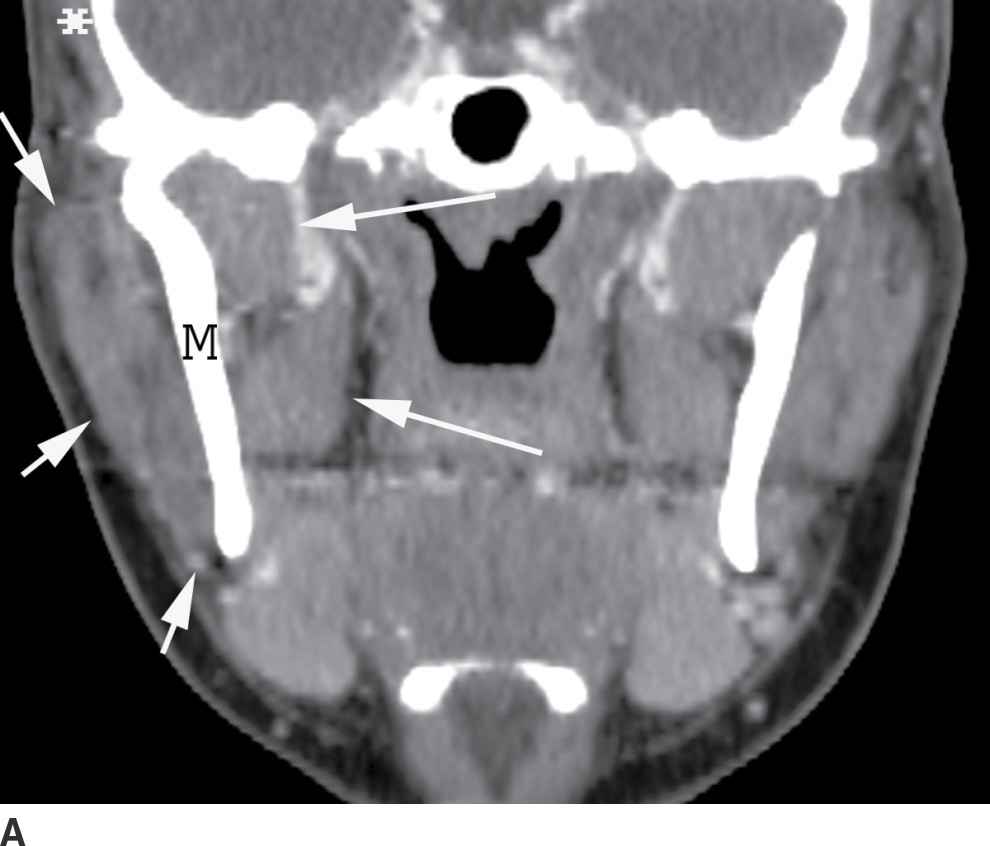
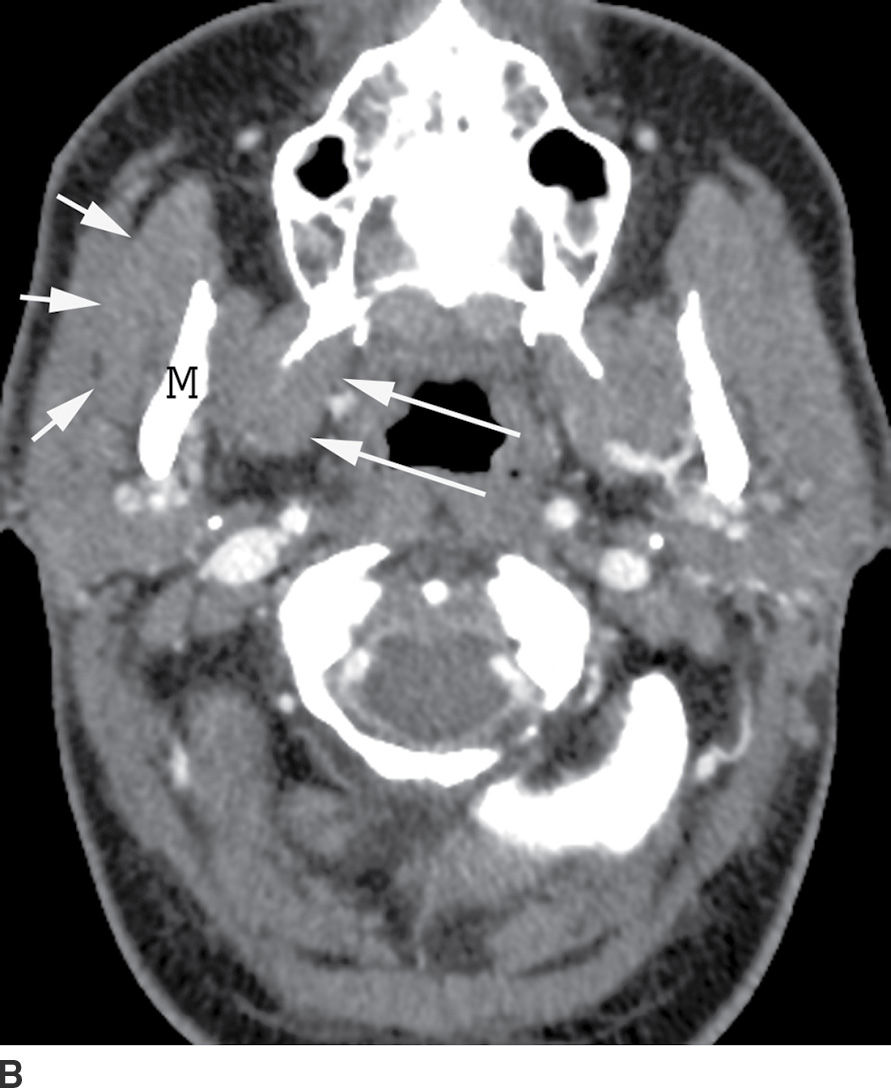
FIG. 21.14 Masticator space. A: Coronal reformatted postcontrast CT image shows the masticator space extending from the skull base to mandibular ramus, bordered laterally by the masseter muscle (short arrows) and medially by the pterygoid muscles (long arrows) with the mandibular ramus (M) in the center. The temporalis muscle (asterisk) portion of this space is partially seen. B: Axial postcontrast CT also shows the lateral (short arrows) and medial (long arrows) borders of the masticator space with the mandibular ramus (M) in the center.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree