Fig. 1
a Otoscopic view of the myriongotomy tube of the left tympanic membrane before positioning of the applicator tube. b Otoscopic view of the ideal position of the applicator tube in the myriongotomy tube of the same patient at the moment of radionuclide application (courtesy Godbersen, G/Kiel)
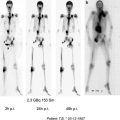
For this purpose, winged infusion sets are used after cutting off the needle top. The small activity volumes of approximately 50 μL are preloaded into the transparent tube, seen as a small bolus. Local anesthesia should be performed using lidocaine spray. The positioning of the tube is done by the otorhinolaryngologist, with the tip of the tube kept strictly in sight during application of the radionuclide. Biokinetics as well as whole-body distribution studies should be accomplished by static images or SPECT or SPECT/CT of the head- and neck-region 1, 24, and 72 h after application, shown in Fig. 2, with ROI analysis as described above. Planar whole-body images can be acquired to evaluate unwanted tracer deposition outside the region of application, especially in cervical lymph nodes or the gastrointestinal tract, demonstrated in Fig. 3.
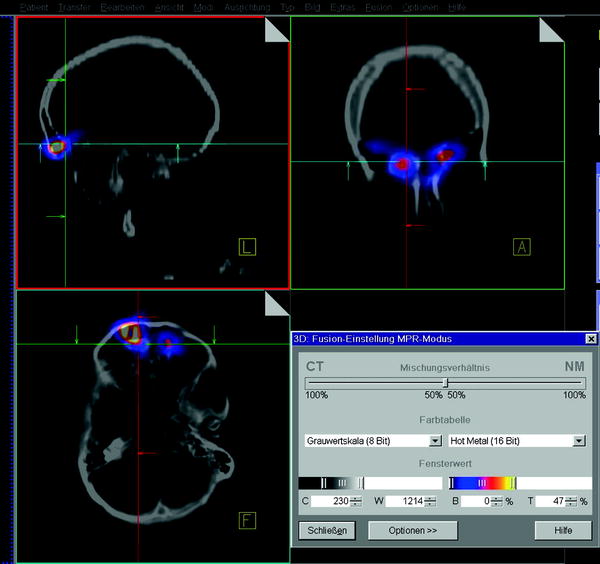
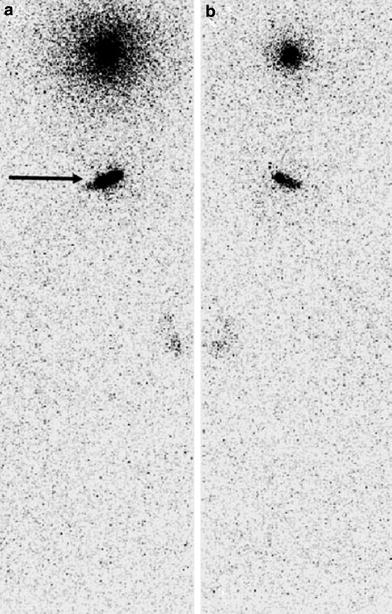

Fig. 2
SPECT/CT images of the head-and neck-region after application of 30 MBq 186 Re-colloid in the frontal sinuses on both sides 24 h after radionuclide instillation (courtesy Lützen, U/Kiel)

Fig. 3
Whole-body images from ventral a and dorsal b view, 30 min after application of 20 MBq 186Re-colloid (each) in both frontal sinuses. Extracranial tracer accumulation is seen in stomach (arrow), which was estimated to be approximately 6 % of instilled activity (courtesy Henze, E/Kiel)
2.4 Optional Diagnostic Pre-Investigation/Planning
To review the feasibility of this treatment approach, an initial estimation of the time of persistence of a colloidal radionuclide at the application site can be performed using 99mTc-colloid scintigraphy of tympanic cavities and the paranasal sinuses. One and 24 h after injection of 200 MBq 99mTc-nanocolloid (Nanocoll; Nycomed Amersham, Buchler, Braunschweig, Germany; particle diameter, <80 nm) through tympanostomy and endoscopic tubes, static images, SPECT, and additional coregistered CT of the head can be acquired using a double-head gamma-camera system (e-cam or Symbia T; Siemens, Erlangen, Germany). Time–activity plots of the application sites can be obtained using the regions-of-interest (ROI) technique. The biological t1/2 of the radionuclide is calculable by exponential curve fitting.
2.5 Therapeutic Activity of 186Re-Colloid
An activity of 20–30 MBq should be chosen for the first application. Re-RTSO is possible. Increasing activities, reaching a maximum of 35 MBq, can be used for repeated applications under regular follow-up, unless hearing impairment, pain, or worsening of audiometric data are seen or the clinical success is poor. It must be noted that a minimum time interval of 2 weeks is kept between two successive applications at the same region.
3 Results
3.1 Biological Half-Life
In a study performed by Kampen and coworkers (2004) the mean biological t1/2 of the diagnostic 99mTc-nanocolloid for the maxillary sinuses was 29.6 h. In the case of middle ear involvement, 36.1 h was calculated for the right and 30.6 h for the left tympanon.
For 186Re-colloid, a mean biological t1/2 of 27.5 ± 13.2 h was measured in the frontal and 25.6 ± 10.9 h in the maxillary sinuses, respectively. Radionuclide retention was always of longer duration in the tympanic cavities with a t1/2 of 47.8 ± 15.9 h on the right and 47.6 ± 10.7 h on the left side as shown in Table 1.
Table 1
Mean biological half-life values in different application sites
Application Site | Radiopharmaceutical | Biologic t1/2 (h) |
|---|---|---|
MSR or MSL | 99mTc-Nanocolloid | 29.6a |
TYR | 99mTc-Nanocolloid | 36.1a |
TYL | 99mTc-Nanocolloid | 30.6a |
TYR | 186Re-Colloid | 47.8 ± 15.9 |
TYL | 186Re-Colloid | 47.6 ± 10.7 |
FSR or FSL | 186Re-Colloid | 27.5 ± 13.2 |
MSR or MSL | 186Re-Colloid | 25.6 ± 10.9 |
3.2 Extracranial Tracer Dispersion
Whole-body images can sometimes show extracranial tracer deposition. For example, a slight radionuclide deposition can be seen in the gastrointestinal tract after swallowing due to drainage out of the application sites into the nasopharynx as shown in Fig. 3. ROI estimation in a whole-body scan 30 min after RTSO resulted in this fraction to be a maximum of approximately 6 % of the total activity applied. Fortunately, measurable tracer accumulation in regional cervical lymph nodes is rarely detectable.
3.3 Aftercare and Treatment Check-Up
The effects of RTSO should be evaluated approximately 3–6 months after the last radionuclide application by otorhinolaryngologic follow-up. A scoring regimen is set up concerning the changes of both objective findings and subjective symptoms, using a standardized questionnaire concerning secretion of ear and nose, intensity of nostril obstruction and breathing impairment, pain, or degree of deafness in case of middle ear affection. Objective re-evaluation can be performed by standard otorhinolaryngologic examination, including otoscopy and endoscopy of the endonasal cavities, audiometry, standard radiographs or CT scans, and sampling of tissue specimens in some cases.
In the treatise of Kampen et al. (2004), the overall subjective success was proven by a cumulative mean self-evaluation score in all application sites of +1.44 ± 0.5, thus representing slight (+1) to excellent (+2) improvement. No significant differences in clinical improvement were detected between the mean score values of the tympanic cavity (+1.6 ± 0.5), the frontal sinuses (+1.5 ± 0.7), and the maxillary sinuses (+1.3 ± 0.6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree