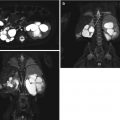
Fig. 14.1
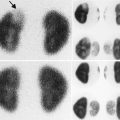
Normal bladder. Normal bladder full and after voiding: transverse views of the bladder before (a) and after voiding (b) demonstrate the normal appearance of the bladder. Notice how the normal bladder wall when decompressed appears thicker. This demonstrates how it is normal for the degree of distention of the bladder to influence wall thickness
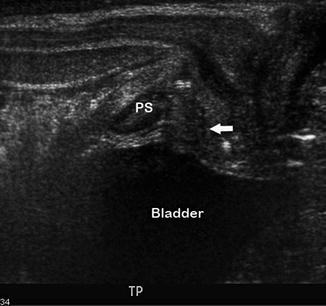
Fig. 14.2
Transperineal ultrasound. This transperineal ultrasound, performed with the transducer on the perineum in the sagittal plane, demonstrates the bladder, posterior urethra (arrow) surrounded by the echogenic prostate, and pubic symphysis (PS), among other structures. The appearance of the bladder base and posterior urethra is normal
Although CT is commonly used in adults to evaluate the genitourinary tract, its emission of ionizing radiation makes it less attractive in children. CT scans without contrast are commonly used in the evaluation of urinary tract stones. CT scans with contrast are used to evaluate masses (parenchymal phase) and the collecting system (excretory phase).
Magnetic resonance imaging (MRI) is inherently suited to evaluating the urinary tract as the fluid in the dilated ureter and bladder is easily distinguished from adjacent soft tissues. Contrast-enhanced MR urography is also useful in evaluating the functional contribution of each kidney. The main problems are that these examinations often take an hour or more, necessitating the use of sedation for young children, are generally less available, and more expensive than other examinations.
Nuclear medicine studies DMSA and MAG-3 each show the function of the urinary tract. The agent used for DMSA is a cortical agent that allows for the precise determination of differential renal function, renal shape, and renal position. MAG-3 uses an excretory agent to determine the speed of contrast excretion, measure the degree of urinary tract obstruction, and show the general appearance of the collecting system.
The voiding cystourethrogram (VCUG) is the most common fluoroscopic examination to evaluate the lower urinary tract. After contrast is instilled into the bladder, the appearance of the bladder and filling defects such as ureteroceles are apparent. The ureters are evaluated if reflux is present. Retrograde ureterograms and antegrade pyelograms also use contrast to opacify and evaluate the ureters. Intravenous pyelograms are used rarely but continue to provide useful information regarding the function and appearance of the urinary tract.
Normal Imaging Anatomy: Bladder
The bladder resides in the pelvis where it serves its dual functions of urinary storage and emptying. When it is decompressed, it is usually contained entirely within the pelvis below the pubic symphysis, although in young children the bladder extends superiorly to the lower abdominal region. Anterior to the bladder, there is a potential space called the prevesical or retropubic space. The median umbilical ligament, the vestigial remnant of the fetal urachus, tethers the bladder apex to the abdominal wall as it attaches to the umbilicus (Fig. 14.3). The bladder is an extraperitoneal structure with the visceral peritoneum covering its superior-lateral aspects.

Fig. 14.3
Normal urachal remnant. This sagittal view of the bladder demonstrates a normal diverticular outpouching (arrow) at the anterior dome of the bladder, which is a normal urachal remnant
In boys, two seminal vesicles and the ampullae of the vasa deferentia lie posteriorly at the base of the bladder in the rectovesical space. In girls, this rectovesical space is occupied by the vagina and the uterus, which rests on the bladder dome (Fig. 14.4). The ureters enter the bladder at its base, posterior-laterally inserting into the trigone. The bladder neck rests directly on the pelvic floor muscles. However, in boys, the prostate gland lies between the bladder neck and the pelvic floor.
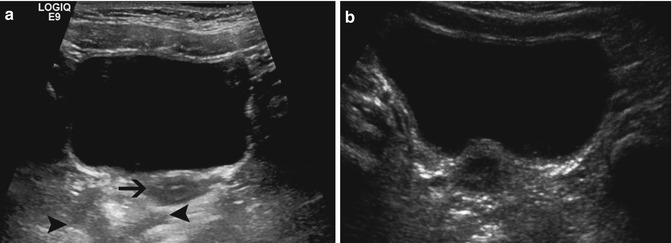

Fig. 14.4
Normal bladder: These images demonstrate the normal appearance of the bladder in girls. (a) The normal hypoechoic uterus is adjacent to the posterior wall of the bladder (arrow). The rectum is filled with gas and stool and has a variable appearance depending on its contents (arrowhead). (b) The hypoechoic uterus often indents the posterior wall of the bladder and should not be confused for a mass
The wall of the bladder consists of layers of perivesical fat, serosa, smooth muscle (i.e., detrusor muscle), and mucosa (urothelium). As the bladder distends, the detrusor muscle is stretched, and the mucosa, which normally has folds, flattens causing the bladder wall to appear thinner.
Bladder Duplication
Pathogenesis
Bladder duplication is a rare clinical entity with few cases reported in the literature. When it does occur, the degree of septation of the bladder may be partial or complete and in either the coronal or sagittal plane. Complete duplication in the sagittal plane has been reported as the most common combination [1]. In complete duplication, the two resulting bladders can be functionally independent with their own respective ureter and urethras [2]. However, one bladder may not have a urethra, resulting in obstruction of its corresponding kidney, ultimately causing renal dysplasia [3]. The luminal compartments of incomplete or partially duplicated bladders are shared at some point above the bladder neck and therefore drain into a single urethra.
Duplication should be distinguished from septation, in which the bladder is divided into two separate (complete) or almost separate (partial) compartments that share a common wall (the septum). As with duplication, complete septation may form a compartment with no outlet, leading to an abnormal ipsilateral kidney.
Embryologically, the cause of duplication is unclear, although some have postulated that it may represent a defect in cloacal septation with the urorectal fold [4] or even partial twinning of the early embryo, a theory taken from its occasional association with duplication of the bowel or spine [5].
Clinical Presentation
In accordance with the variability of the abnormality in bladder duplication, the presentation and diagnosis of the disorder are equally variable. Early discovery usually arises from outward manifestations of duplication, i.e., duplicated external genitalia or spine. In the absence of symptoms such as recurrent urinary tract infections or urinary incontinence, some children are diagnosed incidentally when they are imaged radiographically for other clinical reasons.
Bladder duplication is often associated with other abnormalities related to the genitourinary system such as the genital duplication noted above [6], spinal duplication, and a variety of gastrointestinal tract anomalies, including fistulous communication with the urogenital tract and lower intestinal tract duplication [7].
Imaging
Complete understanding of the patient’s anatomy is crucial to management and often requires a combination of imaging studies. Ultrasound is the initial imaging modality, which helps to define the presence of two kidneys, a solitary bladder separated by a septum, or two separate bladders (Fig. 14.5). Transperineal scanning may give further definition of urethral anatomy. Voiding cystourethrograms in combination with retrograde urethrograms, in which the suspected urethral orifices are catheterized and further defined by instillation of contrast, can also be useful to define the appearance of the urethra(s), blind channels, and bladder(s). Studies such as CT urography (CTU), MR urography (MRU), and IVP can also define the functional and anatomical relationships between kidney(s), ureters, and bladder(s). Unlike CT and IVP, MR has the benefit of the lack of ionizing radiation which needs to be weighed against the cost of the examination and potential need for sedation in younger children. Renal scans and MRU can both estimate renal function. Video-urodynamics will give much of the same information as a VCUG and will also provide functional information.
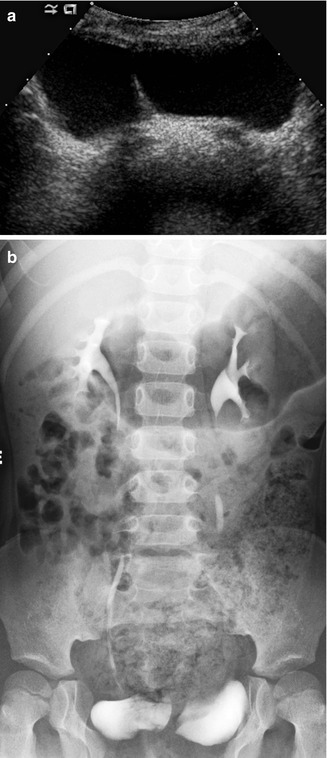

Fig. 14.5
Bladder duplication. A transverse view of the bladder (a) demonstrates complete bladder duplication with a septation dividing each bladder lumen. The IVP (b) demonstrates that each bladder is supplied by its own ureter and kidney
Treatment
Obstruction of a kidney should be relieved surgically to prevent infection and preserve renal function. Patients with the associated abnormalities discussed above will have individualized treatment based on their disease pattern. Long-term goals include urinary continence and functional genital reconstruction.
Urachal Anomalies
Pathogenesis
The allantoic stalk serves as the epithelial-lined conduit between the bladder and the umbilical cord in fetal life. As the bladder descends and matures, the allantois narrows and thickens into the urachus. The lumen of the urachus normally obliterates and becomes the median umbilical ligament [8]. However, by mechanisms still unclear, a portion or the entire urachus can remain patent.
Clinical Presentation
The most common symptomatic urachal anomaly is the entirely patent urachus, accounting for more than half of all disorders of the urachus. In the patent urachus, urine freely passes from the bladder and out of the umbilicus, and the child usually presents with persistent leakage. Leakage may be exacerbated with voiding or in situations of increased abdominal pressure such as with crying or straining. Symptoms can also include abdominal pain, umbilical mass, or dysuria [9].
Occasionally, the urachus can partially obliterate with varying features depending on which portion of the urachus remains open. When the patent end is distal, 15 % of cases, it is labeled as an umbilical-urachal sinus. This can also present with umbilical discharge, but since there is no communication with the bladder, it is less copious. When the patency lies at the proximal end, communicating with the apex of the bladder, it is referred to as a vesico-urachal diverticulum. Usually these do not cause any clinical symptoms since it is widely open to the bladder.
Thirty percent of urachal anomalies consist of a urachal cyst, where the urachus is patent along its middle portion and there is no communication with the umbilicus or bladder. However, sometimes these cysts can drain intermittently. The diagnosis of these may be delayed since often they are not discovered unless they become infected or are discovered incidentally during abdominal imaging or surgery. Some reports have suggested that there may be an increased incidence of vesicoureteral reflux associated with urachal anomalies [10].
Imaging
Imaging for a suspected urachal abnormality typically occurs during the newborn period. Ultrasound is the initial study for urachal anomalies [11]. Ideally, imaging should be performed when the bladder is full to better show the close relationship between the dome of the bladder, umbilicus, and urachal remnant. A patent urachus can be seen on ultrasound located at the dome of the bladder, as a fluid-filled tubular structure when viewed in the longitudinal plane. This should be distinguished from a patent vitelline, or omphalomesenteric, duct which may have a similar appearance. Urachal cysts can also be demonstrated with ultrasound with features of any cyst of the body, hypoechogenicity with variable degrees of debris, and noncommunication with the umbilicus or bladder. Vesico-urachal diverticula appear as protruding fluid-filled sacs not in continuity with the umbilicus [12]. An obliterated urachal remnant is a common finding in newborn children and warrants no further evaluation or treatment [13, 14].
A VCUG is indicated in patients who have fluid draining from their umbilicus, and a patent urachus needs to be distinguished from a urachal sinus. Contrast can be seen flowing through a patent urachus when the bladder is full (Fig. 14.6), whereas a urachal sinus will not demonstrate communication from the bladder to the umbilicus. If a patient has periumbilical drainage, a retrograde fistulogram can also determine the diagnosis of either a patent urachus or urachal sinus [9]. Retrograde injection of the contrast will demonstrate either filling of the bladder in a patent urachus or opacification of just the sinus tract in the case of the urachal sinus. If it is a patent vitelline duct, contrast will fill loops of bowel.
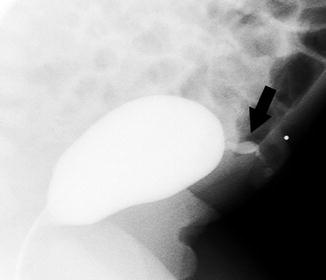

Fig. 14.6
Patent urachus. This newborn boy had clear leakage per urethra. This lateral view during a VCUG shows contrast flowing from the patent urachus at the dome of the bladder (arrow)
Urachal remnants are more common in patients with certain abnormalities. Children with prune belly syndrome often have urachal diverticula. When wide mouthed, these are asymptomatic and appear as large diverticula at the dome of the bladder. Urachal diverticula can also be found during the evaluation of recurrent cystitis. Infected urachal cysts can present with fever and abdominal pain, mimic abdominal infections such as appendicitis, and be found during radiological evaluations for more common causes of abdominal pain (Fig. 14.7). These cystic structures in the pelvis can be distinguished from other cysts, e.g., enteric duplication cysts and ovarian abnormalities, by its location near the dome of the bladder.
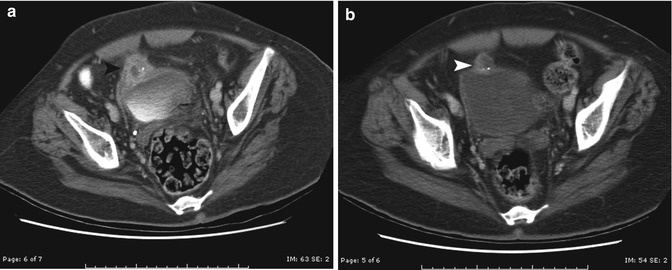

Fig. 14.7
Infected urachal cyst. This boy presented with right lower quadrant pain and fever and was thought to have appendicitis. (a) Axial contrast-enhanced CT scan demonstrates an irregular thick-walled circular structure adjacent to the dome of the bladder (black arrowhead). (b) After treatment with antibiotics, the appearance of the wall is smoother due to decreased inflammation (white arrowhead). Surgery confirmed a urachal diverticulum
Treatment
A patent urachus or umbilical-urachal sinus should be excised surgically. If a preceding infection is present, antibiotics and drainage if appropriate should be undertaken prior to surgery. There is a risk of malignant transformation of the urachus, so it should be removed in its entirety with a cuff of bladder [15–19]. Observation is an option in the case of the asymptomatic urachal cyst [20–22] or vesico-urachal diverticulum. However, if resolution does not occur or if symptoms arise, complete excision, cyst and tract, should be considered. In a large series from the Mayo clinic, urachal carcinoma appeared in a large proportion of the adults (51 %), with the majority of them having urachal cysts. Traditionally, the prognosis for urachal cancer is abysmal with low survival rates, leading most surgeons to recommend complete excision of all urachal anomalies in childhood [19].
Bladder Diverticulum
Pathogenesis
In the adult, most bladder diverticula are secondary processes stemming from bladder outlet obstruction. In children the cause is congenital, and these mucosal outpouchings herniate through weak areas of the muscle wall of the bladder. The reported incidence is 1.7 % in a pediatric population undergoing radiologic evaluation for symptoms [23]. Diverticula can occur around the bladder’s natural defects, namely, the ureters. Hutch described in 1961 two kinds of paraureteral diverticula: primary and secondary [24].
Primary or congenital diverticula occur in isolation in what is otherwise a normal-appearing bladder with an absence of bladder outlet obstruction. The mucosal herniation occurs through a deficient portion of the bladder wall between the intravesical ureter and the roof of the hiatus where the ureter enters the bladder [25, 26]. As the diverticulum grows larger, the ureterovesical junction is distorted, causing either reflux [27, 28] or, worse yet, obstruction [28, 29]. Primary diverticula have been reported in children with connective tissue diseases such as Ehlers-Danlos, Menkes’ kinky hair syndrome, and Williams syndrome (Fig. 14.8) [23, 30].
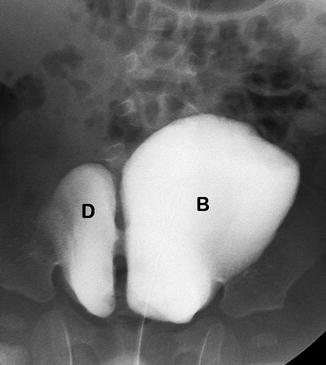

Fig. 14.8
Bladder diverticulum in a patient with Menkes disease. This 6-year-old boy has seizure disorder and red hair and a diagnosis of Menkes disease. This VCUG shows a large right-sided bladder diverticulum (D) communicating with the lumen of the bladder (B). Patients with Menkes disease, Ehlers-Danlos syndrome, and Williams syndrome frequently have multiple bladder diverticula
Secondary diverticula are acquired as a result of bladder outlet obstruction as in posterior urethral valves, detrusor sphincter dyssynergia, or neurogenic bladder. The increased intravesical pressure causes the bladder mucosa to bulge between hypertrophic muscle fibers. Diverticula that occur following bladder surgery also fall within this category.
Clinical Presentation
Presenting symptoms are varied dependent on the size and location of the diverticulum. Urinary stasis can lead to infection, the most common presenting complaint [31–33]; inflamed mucosa can cause hematuria; and mass effect can cause obstruction of the ureter or, if large enough, the bladder [32, 34–37].
Imaging
A bladder diverticulum is typically found incidentally, often during the evaluation of a child with a urinary tract infection. Large bladder diverticula can be detected by ultrasound (Fig. 14.9) [42]. The bladder has to be adequately filled to allow for sonographic determination. The diverticulum typically fills during bladder distention and can enlarge further with voiding. Using 3-dimensional ultrasound, the mouth of the diverticulum can be seen [43]. If the diverticular neck is narrow, bladder air/CO2 contrast sonography has been shown to aid in the differentiation of a diverticulum with other cystic pelvic structures by demonstrating communication [44]. Also, color Doppler can be used to demonstrate alternating, bidirectional, “jet-like” flow between the diverticulum and the bladder [45]. After voiding, residual urine is often seen within the diverticulum. Primary diverticula can appear intermittently on evaluation. If the bladder appears trabeculated, the patient is likely to have secondary diverticula and underlying causes should be sought out (e.g., posterior urethral valves, neurogenic bladder). Large bladder diverticula may also be seen by CT and MRI.
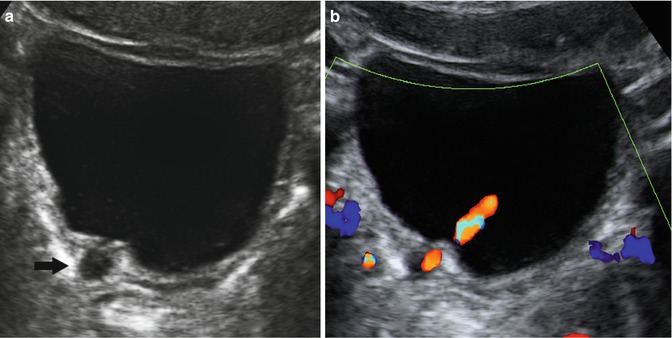

Fig. 14.9
Bladder diverticulum. This patient initially presented with recurrent urinary tract infections. (a) Transverse view of the bladder demonstrates an anechoic circular structure adjacent to the right posterolateral bladder wall (arrow). (b) A ureteral jet of flowing urine is present adjacent to the paraureteral diverticulum or so-called Hutch diverticulum
Voiding cystourethrogram is probably the sensitive test for finding bladder diverticula because the bladder is visualized dynamically during filling and voiding. Changes in intravesical pressure are more likely to elicit the intermittently filling diverticula. The study not only demonstrates the diverticulum but also reveals associated vesicoureteral reflux when the diverticulum is located near the ureterovesical junction [44, 46, 47]. Meticulous technique is important when performing a VCUG including beginning the study with an empty bladder, real-time fluoroscopic monitoring with oblique and lateral views, and visualization during and immediately post-void [46]. This is done since the appearance of diverticula may vary with the phases of voiding and may not be present on the same study at different times. Therefore, a repeat VCUG may be warranted if clinical suspicion remains after previous negative studies.
Treatment
Symptomatic diverticula should be excised with ipsilateral ureteral reimplantation if the ureter is nearby or involved. Many surgeons correct paraureteral diverticula if it is associated with VUR. Otherwise, if the diverticulum is small, found incidentally without symptoms, or is not accompanied by VUR, observation is reasonable. In the case of secondary diverticula, bladder outlet obstruction should be remedied first, since direct treatment for the diverticulum may become unnecessary.
Bladder Rhabdomyosarcoma
Pathogenesis
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children, with 20 % arising from the genitourinary (GU) tract. The most common GU sites are prostate, bladder, and paratesticular. With a bimodal age distribution, peak incidence of the disease occurs in the first 2 years of life and again in adolescence. Children under the age of 6 years comprise the majority of cases. There is a high association with certain syndromes such as Li-Fraumeni syndrome and neurofibromatosis, and the subtypes of RMS (embryonal vs. alveolar) have been shown to have distinct cytogenetic abnormalities. These abnormalities (loss of heterozygosity on chromosome 11, translocation between chromosomes 1 or 2 and 13, etc.) may not only be involved in the pathogenesis but may also have prognostic implications. Most GU RMS are of the embryonal variety and when they occur in hollow organs, such as the bladder or vagina, they present as sarcoma botryoides. Fortunately, the botryoid variants portend a more favorable prognosis.
Clinical Presentation
Presentation is dependent on the site of the primary tumor. When found in the bladder, patients often present with urinary retention and hematuria. Urinary retention, in turn, causes abdominal pain and distention and urinary tract infection. Irritative voiding symptoms such as dysuria and frequency can also occur. In a thin child, a palpable abdominal mass can sometimes be found on physical exam either from the mass itself or from a distended bladder.
Imaging
The imaging features of RMS of the bladder are nonspecific, and tumor diagnosis is via direct biopsy and tissue confirmation with cystoscopy. Intravenous urography was the imaging modality of choice even well into the late 1970s, with images of pelvic masses causing mass effect on nearby pelvic structures [48]. Filling of the bladder with contrast will reveal an irregular filling defect if the mass is indeed in the bladder. Otherwise, cystography can show extrinsic compression of the bladder from an adjacent RMS. However, this has been supplanted by sonography, CT, and MRI [49].
Ultrasonography is the screening study of choice for abdominopelvic masses. This usually demonstrates a large, heterogeneous, solid mass within the lumen [50] (Fig. 14.10a). The botryoid variant will appear as a cluster of “grapes” with its polypoid configuration. Hypoechoic foci can be seen within the mass representing hemorrhage or necrosis [49]. Spatial relationships with adjacent pelvic structures can be determined. In girls who have RMS arising from the uterus or vagina, one can visualize a pelvic mass that displaces the bladder, and invasion into the bladder wall can sometimes be seen on ultrasound. If the mass is sufficiently large, obstruction of the ureter(s) may cause hydroureter detected on US. One has to be wary for other disease processes that can mimic RMS such as inflammatory masses, such as inflammatory pseudotumor or post surgical masses. If history, laboratory values, and comparison to prior studies do not reveal the cause, cystoscopy and biopsy may be needed to distinguish these entities.
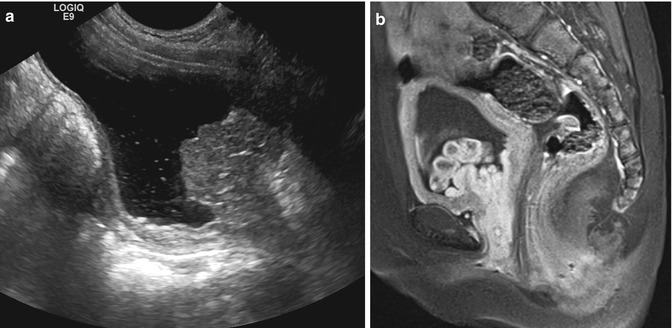

Fig. 14.10
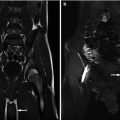
Rhabdomyosarcoma. This 3-year-old female presented with bloody underwear and frond-like soft tissue within her perineum. (a) Sagittal US view of the bladder shows an echogenic lobulated mass extending from the posterior wall of the bladder to the bladder base and into the urethra. (b) Sagittal T1 FS postcontrast MRI demonstrates the enhancing bladder mass has multiple lobules and extends into the urethra. These appearances are classic for sarcoma botryoides
After ultrasound, MRI is the best imaging modality to assess the site of origin for a pelvic rhabdomyosarcoma and the sites of local invasion. Tumors which involve the bladder may actually have arisen from adjacent organs such as the prostate or vagina. MRI is superior to CT in tissue contrast and distinguishing the mass from adjacent organs (Fig. 14.10b).
Both CT and MRI are useful in the evaluation for enlargement of the pelvic and retroperitoneal lymph nodes and metastases. Contrast enhancement for either study is necessary for best visualization.
Treatment
Treatment of RMS has shifted from radical cystectomy to bladder preservation. Unfortunately, those tumors arising from the bladder trigone are not amenable to partial cystectomy as are those located at the dome or sides of the bladder. For those children, neoadjuvant chemotherapy and radiation can sufficiently shrink the tumor enough to allow for partial cystectomy. This approach has allowed for an increased rate of bladder function with reasonable overall survival (78 %) [51, 52]. A caveat is that patients with residual disease can have relapse of their disease, which has shown to have high mortality [52]. Also, patients who received radiation therapy have compromised functional bladder capacity and abnormal voiding patterns [53]. To address this dilemma, one group recommends a risk-based approach, taking into account the relative response of the tumor to chemotherapy for risk stratification and reserving radiation for relapse in low-risk patients (high-risk patients undergo radical cystectomy) [54].
Urinary diversion is needed following cystectomy, and these come in the form of conduits or continent reconstructions. Early reconstruction, however, should be performed when no further local therapy is required.
Neurogenic Bladder
Pathogenesis
Neurogenic bladder incorporates a rather wide range of clinical manifestations dependent on the level and extent of the lesion along the pathway between the brain and sacral spinal cord. Indeed, even similar neural injuries at the same location may result in different types of dysfunction. The complexity of diagnosis of the neurogenic or neuropathic bladder depends on a complete history and physical/neurologic examination. Radiographic studies, including video-urodynamics, are to provide a complete picture of the functional deficit.
In general, lesions above the sacral cord produce a low-capacity, high-pressure, and overactive bladder. This is demonstrated with urodynamic studies as well as anatomic studies such as VCUG and US. Lesions of this nature are usually from trauma, spinal dysraphism such as spina bifida, cerebral vascular accidents, Parkinson’s disease, multiple sclerosis, infection, or tumor. Associated effects from this bladder dysfunction can include VUR from the increased bladder pressures, ureteral obstruction due to the thickened and hypertrophic bladder wall, or progressive renal insufficiency from lower urinary tract dysfunction. These effects are the result of the derangement of the neural control of urinary storage and emptying. Specifically, detrusor sphincter dyssynergia, or discoordination between the bladder and the external urethral sphincter, is thought to be the most deleterious factor.
Lesions at or below the sacral spinal cord typically result in a large capacity, areflexic, and low-pressure bladder. Not only can this dysfunction occur from direct spinal cord lesions, it can also occur from other medical conditions such as diabetes mellitus and pernicious anemia. The underlying pathology is often a deficit in sensory input to the bladder.
Clinical Presentation
Returning to the broad categorization above, patients with suprasacral injuries can present with urinary incontinence, irritative symptoms such as urgency or frequency, recurrent urinary tract infection, or upper tract changes. Patients with sacral lesions often have urinary retention and overflow incontinence.
Imaging
Since neurogenic bladder dysfunction is often a progressive disorder, initial radiologic evaluation includes an assessment of baseline renal and bladder anatomy and structure with ultrasound. The imaging findings of patients with neurogenic bladder depend on the level of the spinal cord abnormality. Abnormalities which lead to a small-capacity high-pressure bladder result in a thick-walled, trabeculated bladder. The combination of a hypertrophied bladder wall and high-pressure voiding may cause hydroureteronephrosis due to ureterovesical junction obstruction, reflux, or both. Patients with large areflexic bladders have smooth-walled bladders with greater than expected capacity for age. These patients may also have hydroureteronephrosis.
The redundant undulating mucosa and hypertrophied muscularis seen in patients with thick bladder walls can be visualized by all imaging modalities, but the most common imaging study to evaluate the bladder is ultrasound (Fig. 14.11). One measure for the degree of bladder wall hypertrophy is AP bladder wall thickness. However, the thickness of the bladder varies by the degree of bladder distension and location of measurement. Tanaka et al. determined that bladder wall thickness of greater than 3.3 mm correlated with a higher risk for unfavorable urodynamic (UDS) risk patterns (i.e., parameters most likely to lead to upper urinary tract deterioration) among patients with neurogenic bladder from spina bifida. Specificity for the diagnosis of unfavorable UDS was 75 % and sensitivity was 95.1 % [55]. Leonardo et al. demonstrated that bladder wall thickness was the only sonographic parameter that was a marginal risk factor (p = 0.07) for the finding of renal scarring on DMSA renal scan in neurogenic and nonneurogenic bladder dysfunction [56]. Some groups have advocated for routine baseline DMSA scans in patients with neurogenic bladder dysfunction given their high risk of renal function deterioration.
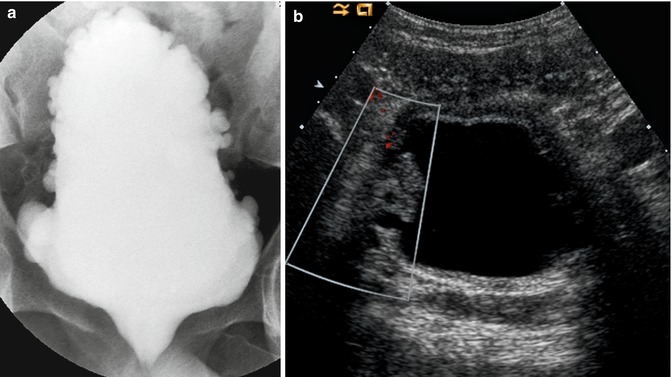

Fig. 14.11
Neurogenic bladder. This boy was born with a terminal lipomyelomeningocele and had previously undergone cord detethering. He has persistent incontinence. (a) AP supine image of the bladder during a VCUG demonstrating the typical “Christmas tree appearance” of a neurogenic bladder, characterized by hypertrophied muscularis and contrast-filling multiple large saccules. The trabeculation spares the bladder base. (b) Ultrasound image demonstrates a thick-walled bladder and multiple diverticula in this patient with a neurogenic bladder
UDS has been an integral component of the evaluation of children with NGB, as it allows for identification of those at high risk for urinary tract deterioration. If implemented early, aggressive treatment can be initiated for preventive, renal preservation measures [57, 58].
VCUG is important as the incidence of VUR has been reported as high as 50 % in children with spina bifida [59, 60] which may lead to renal deterioration in the presence of high-pressure voiding [61]. In general, we reserve VCUG for patients with poor bladder dynamics, patients with DSD, and/or hydro- or hydroureteronephrosis. DeLair et al. identified high-grade VUR as an independent risk factor for cortical loss, especially in girls [62]. Shiroyanagi et al. demonstrated, in their small cohort of myelodysplastic patients, that all of their patients with an abnormal DMSA scan had a history of VUR [63]. The technique of VCUG and UDS can be combined in video-urodynamics (V-UDS) so that the patients are subjected to one invasive procedure rather than two. However, UDS, much less V-UDS, does not have widespread availability, and some have proposed a modified VCUG to give anatomic as well as functional data [64, 65]. This technique entails an in-depth understanding to bladder physiology/dynamics.
Since neurogenic bladder dysfunction can be progressive, ultrasonography should be performed on a regular basis, with the addition of VCUG if there is new hydroureteronephrosis or febrile UTI. The frequency of UDS varies according to patient age and clinical status.
It should be noted that the optimum position for UDS is in the sitting position if the patient is able to tolerate it. Lorenzo et al. reported that although there were no differences in maximum cystometric capacity, detrusor leak point pressure, or pressure-specific volumes at 20 and 30 cm water, based on patient positioning (supine vs. sitting), volume of first sensation and detection of detrusor overactivity was significantly lower in the sitting position. Incontinence was also detected more readily in the sitting position [66].
Treatment
As with diagnosis, treatment is individualized to the patient depending on the nature and severity of his/her dysfunction. Goals of therapy are directed towards preservation of renal function and alleviation of symptoms. Maneuvers should be done to simulate the normal functions of the bladder, namely, low-pressure storage of urine and complete emptying of urine. This is normally accomplished by the use of anticholinergic medication to lower intravesical pressure and decrease overactive contractions and clean intermittent catheterization (CIC) for bladder emptying. Early implementation of these treatment regimens in patients at risk is associated with more favorable outcomes [57, 58, 62, 63].
Normal Imaging Anatomy: Ureter
The ureter stretches from the renal pelvis of the kidney to the bladder for a length of about 25–30 cm. Along its course, there are 3 areas of relative narrowing: the ureteropelvic junction (UPJ), where the ureter crosses the iliac vessels as it dips into the pelvis, and the ureterovesical junction (UVJ). These areas are important as they are the locations where urinary calculi can become lodged. Both proximal ureters run along the psoas muscles. They are initially lateral to the gonadal vessels but switch positions just prior to the bifurcation of the aorta and vena cava as the gonadal vessels cross the ureter anteriorly. In the pelvis, the ureters course medially as they enter the bladder.
The ureter has a robust muscular layer of circular and longitudinal muscle fibers that are responsible for peristaltic propulsion of urine. Since imaging techniques are snapshots in time, relative dilations and constrictions as a result of propulsion of the bolus of urine can be misconstrued as pathologic.
Preureteral Vena Cava (Retrocaval/Circumcaval Ureter)
Pathogenesis
The normal course of the right ureter is lateral to the inferior vena cava (IVC). However, in certain circumstances (estimated 1 in 1,100) [67], the ureter takes an anomalous course, wrapping around the vena cava posteriorly, exiting between the IVC and aorta, and proceeding caudad to cross the iliac vessels anteriorly as it enters the pelvis to insert into the normal position in the bladder. This occurs because of a divergence of the normal embryologic development of the IVC. The IVC develops through a series of fusion events between multiple inchoate venous drainage systems of the 6th–8th-week embryo. Those segments that do not fuse to form the IVC selectively degenerate. One such drainage system is the posterior cardinal veins which drain the inferior portion of the embryo. The right posterior cardinal vein lies anterior to the developing ureter and with renal ascent becomes situated lateral to them. The subcardinal veins, situated medial to the ureter, are the venous segment that fuses to normally become the renal segment of the IVC. However, if the right posterior cardinal vein does not degenerate, it forms the renal segment of the IVC, and its anterior and lateral position to the ureter persists. In this conformation, the ureter may become obstructed by the overlying vena cava. Since the condition is due to a disorder of vascular development and not ureteral development, it has been suggested that it be referred to as “preureteral vena cava.”
Clinical Presentation
If the ureter becomes obstructed, patients typically present with symptoms indicative of ureteral obstruction. Symptoms may be intermittent or may not surface until a diuretic event occurs, as with the ingestion of caffeinated or alcoholic beverages. Given this, the condition is often not diagnosed until early adulthood. These symptoms may very well exist in the pediatric population, but the similarities with symptoms of gastrointestinal origin probably delays diagnosis. Conversely, the diagnosis is sometimes made incidentally during radiographic evaluation of other disorders.
The signs and symptoms are of typical renal colic, usually abdominal or flank pain and nausea and vomiting. Urinary stasis can predispose to urinary tract infections or pyelonephritis, which will prompt evaluation and intervention. Hydronephrosis can also result in gross hematuria. Urinary calculi formation and deterioration of renal function are also other presentations that lead to evaluation and diagnosis.
Imaging
Retrocaval ureters can exhibit two clinical types [68, 69]: Type 1, the more common one, is the classical obstructed ureter with hydronephrosis. Type 2 has minimal to absent hydronephrosis, suggesting a non-obstructive pattern. The diagnosis of either type of retrocaval ureter is made radiographically. However, given the sometimes absence of symptoms, early diagnosis is contingent on high clinical suspicion. In the past, as was the case in many urologic maladies of the upper tract, initial evaluation was with intravenous pyelography (IVP). One would typically see right-sided hydronephrosis tapering to a “sickle”- or “fish-hook”-shaped ureter just proximal to the IVC (Type 1). The ureter is medially deviated, and the point of obstruction appears to occur at the edge of the iliopsoas muscle, typically around the transverse process of the third lumbar vertebrae. As it arises from under the IVC, distally, the ureter may have incomplete opacification. With retrograde pyelography, one can see the above findings, except the distal ureter is more clearly opacified. Of note, mid-ureteral valves, another exceedingly rare congenital entity, can masquerade as a retrocaval ureter with similar radiographic findings due to obstruction.
The contemporary modality for diagnosis is contrast-enhanced CT (Fig. 14.12) since the ureter and IVC can be visualized simultaneously, thus clinching the diagnosis [70–74]. As expected, the retrocaval ureter passes posterior and medial to the IVC, and if the ureter is obstructed, like on IVP, the distal ureter may not fill with contrast. However, the anatomical advantage of CT clearly delineates the necessary spatial relationship of the two structures for diagnosis.
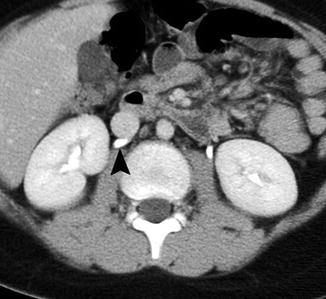

Fig. 14.12
Retrocaval ureter. This CT scan performed for trauma demonstrates that the right ureter is posterior to the inferior vena cava (arrowhead). This retrocaval ureter or preureteral vena cava configuration was not associated with obstruction or hydronephrosis
The use of MRI has been also reported, in which multiplanar reconstruction images can map out the entire course of the retrocaval ureter with the same findings as in CT imaging [75, 76]. The advantage of lack of radiation or iodinated contrast medium makes MRI an attractive alternative for older children, who do not require sedation.
Treatment
If ureteral obstruction is present, intervention is warranted. Open ureteroureterostomy [77] has largely been replaced by laparoscopic reconstruction given its decreased postoperative convalescence and relative technical ease [78–88]. The retroperitoneal laparoscopic approach [89–95] as well as robotic-assisted laparoscopic approach have also been reported in the literature [96–101]. Regardless of the modality, the basic steps of the reconstruction are the same: division of the ureter on either side of the IVC, repositioning of the ureteral ends lateral to the IVC, and reanastomosis to restore continuity.
Ureteral Polyps
Pathogenesis
Fibroepithelial polyps (FEP) have unclear etiology. As the name implies, these benign soft tissue masses consist of mainly fibrous stromal tissue, vasculature, and normal urothelium. Inflammation is usually not present. They may develop anywhere along the urothelial-lined urinary tract. These polyps can be obstructive, but the combination of ureteral peristalsis and urine flow produces persistent traction causing the peduncle of the mass to grow and elongate. When they are situated proximally (most common) and have not undergone significant longitudinal growth, they are a rare cause of ureteropelvic junction (UPJ) obstruction.
Clinical Presentation
Fibroepithelial polyps are uncommon in children. In one large series of all UPJ cases requiring pyeloplasty in a 35-year period, fibroepithelial polyps occurred 0.5 % of the time [102]. Cassar Delia et al. performed a literature review of polyps causing UPJ obstruction in children showing that the ratio of boys to girls was 13.5:1. The left side was involved in 67 % of cases and bilateral occurrence was 7 % [103]. Abdominal or flank pain was the presenting symptom in 86 % of cases. These polyps can be quite long, even prolapsing into the bladder or urethra. They may also present clinically with hematuria or as an incidental finding after workup for hydronephrosis. Reports of metachronous FEPs have also been reported after excision and pyeloplasty [104]. There has been one report of FEP causing complete obstruction and leading to complete loss of renal function [105].
Imaging
A recent series of 35 children with confirmed fibroepithelial polyps reported a 62 % accuracy rate of establishing the diagnosis on ultrasonography, as well as inferior accuracy with intravenous pyelography (IVP) (24 %) [106]. Younger and thinner children were examined with higher frequency transducers (L5-12, 5–8 MHz) while lower frequency transducers (C8-5, 5–8 MHz) were utilized in older and larger children. Echogenic non-shadowing, well-defined masses were discovered with distention of the renal pelvis. If the mass is obstructing, hydronephrosis or hydroureter is present. Doppler detected blood flow [106].
In a review of the literature, Cassar Delia et al. found that a filling defect is only discovered 36 % of the time on IVP (Fig. 14.13) but 70 % of retrograde pyelograms (RPG) [103]. Consequently, preoperative recognition of these polyps is uncommon in the absence of RPG. The ureter usually appears narrow at the point of attachment of the polyp stalk with hydroureter distally around the body of the polyp secondary to partial obstruction. The mass presents as a long, slender filling defect with surrounding contrast. These filling defects can be mistaken for radiolucent urinary calculi or blood clots, especially in patients with a history of hematuria.
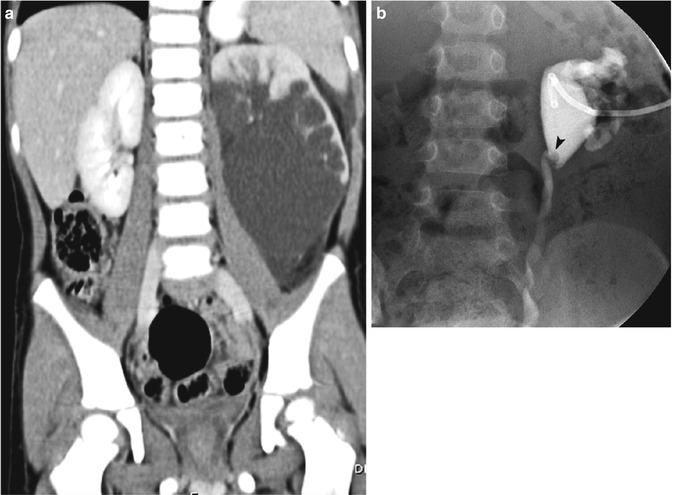

Fig. 14.13
Ureteral polyp. (a) This coronal contrast-enhanced CT scan performed for flank pain demonstrates severe hydronephrosis of the left lower pole. The right kidney is normal. (b) After a nephrostomy tube was placed, antegrade nephrostogram demonstrates a circular filling defect at the ureteropelvic junction (arrowhead), a ureteral polyp, which was causing the ureteropelvic junction obstruction
On computed tomography (CT), FEPs appear as a soft tissue mass in a dilated system with contrast surrounding it circumferentially [107, 108]. Polyps can appear as either a long, pedunculated, smooth-walled soft tissue mass or a short mass with multiple, polypoid projections [109]. CT ureteroscopy is a method of CT imaging using special surface-rendering techniques and endoscopic software that has been reportedly used to diagnose FEP [110]. An obvious disadvantage is the increase in radiation exposure associated with CT-based imaging.
Magnetic resonance imaging (MRI) characterization of FEP has been described as a ureteral lesion hyperintense to the skeletal muscle on T2-weighted images and hypointense (to muscle) on T1-weighted images. There is intense post-gadolinium enhancement (T1), and its lower signal intensity relative to high-intensity urine on T2-weighted images allows for visualization in a dilated collecting system [111, 112]. Short of the lack of radiation, this imaging modality does not appear to garner additional useful information compared to the “simpler” methods described above, especially given its added expense.
Treatment
Management is dependent on location of the polyp. Since the majority of the polyps are located around the UPJ, and preoperative diagnosis of FEP is relatively uncommon, a significant proportion are discovered and treated while undergoing open [109, 113–115] or laparoscopic and robotic-assisted laparoscopic pyeloplasty [104, 116–118]. Historically, nephroureterectomy was performed when polyps were mistaken for malignancy. Since then, different open surgical options have also be described, including ureterotomy [119–123] or pyelotomy with excision [124]. However, in the contemporary literature, numerous reports describe successful endoscopic treatment with ureteroscopy [125–137] or percutaneous nephroscopy [131] for lesions at the UPJ. Recurrence should be on the differential diagnosis for patients who have had a previous FEP and have reappearance of symptoms [104].
Ureteral Duplication
Pathogenesis
Embryologically, abnormalities of the ureteric bud, sprouting off the caudal end of the mesonephric ducts to interact with the metanephric blastema, can lead to ureteral duplication. It is a relatively common congenital malformation, occurring in approximately 1 in 125 based on autopsy studies. It is found slightly more often in girls and unilaterally. When two separate ureteric buds sprout from the mesonephric duct, each induces different portions of the metanephric mesenchyme, dividing the eventual kidney into upper and lower poles. When the separate buds incorporate into the bladder, the more cranial bud (serving the upper 1/3 of the kidney) terminates in a location more caudal and medial than expected. Conversely, the most caudal bud (which will drain the lower pole of the kidney) ends more cranial and lateral. This phenomenon is known as the Weigert-Meyer rule and holds true in all but the rarest exceptions. The lower pole ureter tends to have a shorter course within in the bladder wall due to its lateral positioning, predisposing it to vesicoureteral reflux (VUR), whereas the upper pole ureter with a long course within the bladder wall is more often associated with obstruction or ureterocele (see next section). Partial duplications will have convergence of the ureters into a single system prior to its entry into the bladder (ureterovesical junction, UVJ). These duplications, a result of branching of the ureteric bud prior to interaction with the metanephric blastema, often are associated with normal renal systems.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree