Fig. 5.1
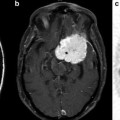
The primary sensory/motor gyrus: The yellow arrow indicates the position of the reverse omega portion of the primary motor gyrus in the posterior frontal lobe. The red arrow shows the position of the sensory gyrus
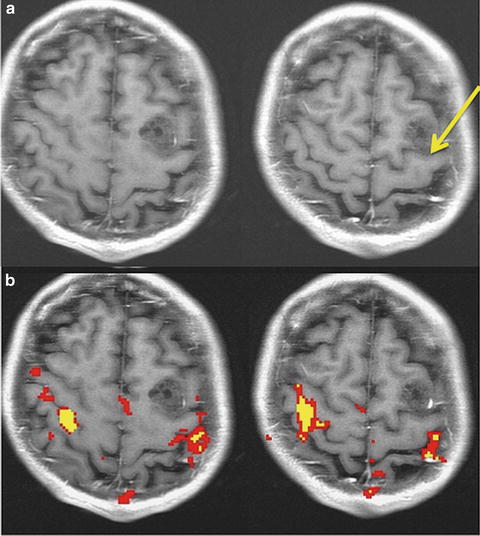
Fig. 5.2
Ambiguous anatomy. In rare instances the reverse omega (yellow arrow) does not indicate the position of the central sulcus. Without fMRI, this lesion would have been assumed to be in the motor gyrus
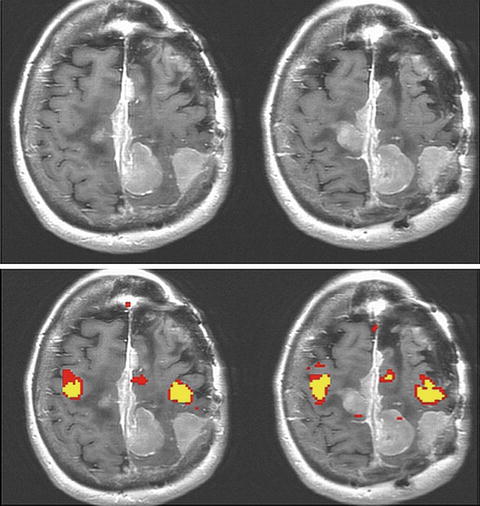
Fig. 5.3
Where is the motor gyrus? fMRI is particularly useful in cases where tumor has obscured normal anatomy. In this case multiple extra-axial lesions make the motor gyrus localization impossible without a technique like fMRI
The face/tongue region of the primary motor gyrus is located on the lateral/inferior aspect of the motor gyrus. This region is anatomically just posterior to Broca’s area in the inferior frontal gyrus. Figure 5.4 shows an fMRI map of both hand and tongue motor movements acquired simultaneously in an intact patient. Of note, finding the tongue motor region by “pulling down” the sulcus, where one first locates the more cephald component of the central sulcus/reverse omega and follows the sulcus inferiorly, can be misleading and inaccurate. The inferior aspect of the central sulcus moves anteriorly as it is traced inferiorly and shortens making precise localization of the inferior aspect of the motor gyrus particularly difficult to discern anatomically alone. For this reason, fMRI is particularly useful for localizing the face/lips/tongue portion of the motor gyrus at its inferior aspect.
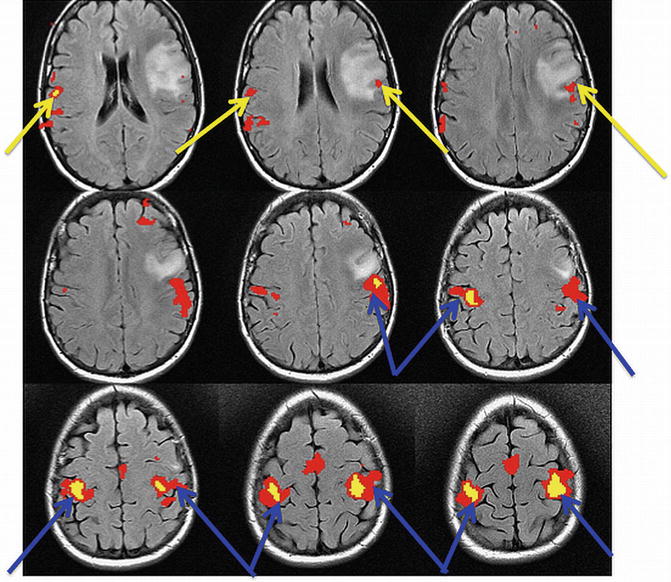

Fig. 5.4
Position of the hand (blue arrow) and tongue (yellow arrow). fMRI signals in the primary motor gyrus
Another way in which fMRI contributes significantly to motor gyrus localization is in the foot motor region. The foot motor region is located most medially just over the interhemispheric fissure. This region is often localized medial and slightly posterior to the hand motor region in the axial plane (Fig. 5.5). Direct cortical stimulation (the surgeon’s intraoperative gold standard for functional mapping) of this region is difficult because the sagittal sinus makes the cortex difficult to access. Therefore, fMRI localization of the foot motor region is valuable for presurgical planning.
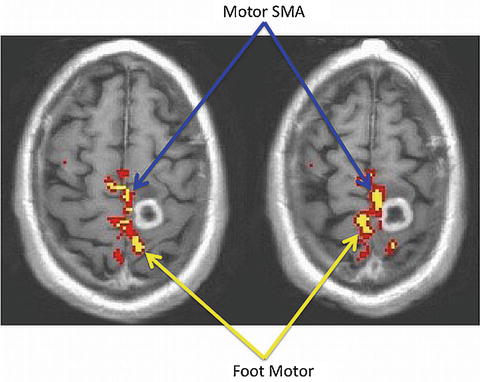

Fig. 5.5
Foot motor and supplementary motor (SMA) regions of the primary motor gyrus
fMRI typically maps these three main motor areas (foot, hand, and face/tongue) for neurosurgical planning. This is partly because these three areas span the gyrus medially to laterally and partly because tasks involving these areas are easily amenable to functional paradigms.
The primary sensory gyrus (also known as the postcentral gyrus) is located just posterior to the precentral gyrus from which it is divided by the central sulcus. Like the primary motor gyrus, the organization of the sensory gyrus is also somatotopically organized (Fig. 5.1).
Secondary Motor Areas
While the primary motor and sensory areas are the main focus of most neurosurgical planning targets, damage to secondary motor areas also carries a risk of morbidity [7–10]. As a result, their precise localization is becoming increasingly important during fMRI exams. The most common secondary motor areas of interest for neurosurgical planning are the supplementary motor area and the pre-motor area. To study the secondary areas, paradigms often focus on a unilateral volitional movement contrasted with rest [11].
Supplementary Motor Area
The SMA is located in the superior frontal gyrus just medial to the superior frontal sulcus (Fig. 5.6). While it is an expansive area with ill-defined anterior borders, the posterior border of the SMA is the foot motor region of the primary motor gyrus. The SMA is made up of an anterior portion (pre-SMA), more active on fMRI during language tasks and a posterior portion, more active on fMRI during motor tasks. The boundary between the pre-SMA and SMA proper has been delineated using a VCA line or a line drawn vertically from the AC/PC line [12].
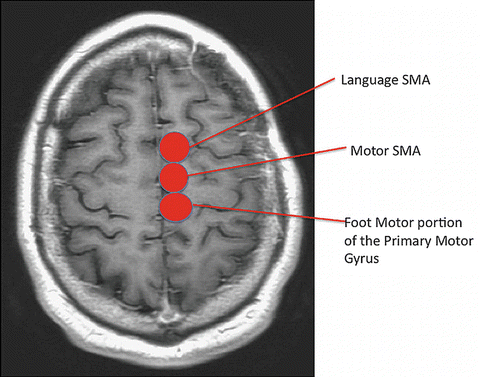

Fig. 5.6
Supplementary motor area: The supplementary motor area located in the superior frontal gyrus. The SMA is functionally segregated into motor (posterior) and speech (anterior) components
Recent studies suggest that the motor portion of the SMA is, like the primary motor gyrus, also somatotopically arranged. In lower animals, it has been shown that the hind limb is located in caudal sites while the forelimb and facial movements are closer to the pre-SMA, the more anterior, language-related portion of the SMA, and are thus more rostral [13–17]. Although somatotopic organization is more commonly associated with the primary motor cortex, the three main motor areas—hand, foot, and face/tongue—may also be somatotopically mapped along the more rostral axis of the SMA [18, 19].
The SMA is broadly responsible for motor planning and activates temporally before the primary motor gyrus [20–22]. Further, it is active when movements are both internally and externally cued [20]. The SMA is best known for being associated with voluntary movement but will also activate on fMRI during passive tasks [23]. The posterior portion of the SMA is more involved in finger movement tasks while the anterior part of the SMA is active during cognitive and language processing [24]. A centralized region of the SMA that is active during both language and motor tasks suggests a region that may be essential. Further investigation is needed to determine whether insult to this centralized region carries an increased incidence or degree of postoperative deficit.
More recent work suggests a role for the SMA in cortical compensation. BOLD fMRI can be used preoperatively to look at the patterns of activation as a tumor invades either the primary motor area or the SMA [25]. The SMA has been shown to be involved in temporal planning and organization of motor movements before execution, as well as sequencing of multiple movements [26]. Peck et al. characterized the role of the SMA in patients with high-grade gliomas and their role in cortical reorganization. The study used fMRI to look at the BOLD hemodynamic responses in the primary motor and SMA of tumor patients. Here, block paradigms were used to assess latency differences so that the more sensitive hemodynamic response would be isolated. This work concluded that patients with glial tumors located within the primary motor cortex experienced lesion-induced compensation in the BOLD magnitude and firing pattern in both the primary motor cortex and the SMA. Cortical reorganization was visibly demonstrated with the SMA’s role assuming functions for which the PMC was likely previously responsible [25, 27].
Why fMRI?
FMRI is especially useful for the purpose of locating the primary sensory motor cortex if normal sulci and/or gyral patterns are distorted or in rare cases when reorganization has occurred secondary to an invasive tumor [28]. fMRI, as a preoperative neuroimaging tool to localize the sensory/motor system, is popular for a variety of reasons. This method is minimally invasive and easily repeatable. Further, given that patients with brain tumors are often impaired, fMRI of sensory motor regions can be acquired with a variety of paradigms that are both volitional and passive. Patients undergo preoperative motor mapping for a multitude of reasons, the majority of which are when anatomical landmarks cannot be identified with certainty by traditional anatomical means. fMRI is also being investigated as a tool to predict deficits [5]. Lastly, fMRI can be used to interrogate cortical reorganization in the motor system, the clinical utility of which is still under investigation [25].
Several studies within the past decade provide evidence for the usefulness of fMRI preoperative sensory motor mapping. In a 2007 study by Pujol et al., patients were examined to identify the sensory motor cortex over a 5-year period. They performed a hand motion paradigm (opening and closing). The fMRI map correctly identified the location of the sensory motor cortex in 96 % of the cases—141 patients out of 147 examined. The 4 % that could not be identified displayed head motion greater than 2 mm. Although both conventional MRI and fMRI were used and compared in this study, fMRI significantly increased the confidence of the motor gyrus identification [29].
Studies investigating the outcomes of fMRI for preoperative identification of eloquent areas are ongoing. An important step in this direction was published by Petrella et al. The study investigated presurgical fMRI in 39 patients who were candidates for tumor resection. Two paradigms were used to map sensory motor areas. Treatment plans following inspection of the fMRI results were altered in 49 % of patients. In this study, fMRI sufficiently changed treatment options offering patients who would have otherwise been deemed inoperable the chance for surgical resection. Additionally, in nine patients for whom surgery was not originally offered, five were reconsidered for a craniotomy with intraoperative mapping. In cases where the course of treatment continued as planned, fMRI provided the neurosurgeons with further confidence in their surgical decision-making [30]. In a 2003 study by Wilkinson et al. [2], preoperative maps created through fMRI data were essential for safe resection by way of allowing for gross total resections and no postoperative deficits in 17 patients mapped with fMRI. Identifying the eloquent areas to prevent damage during tumor removal is of the utmost importance during mapping [31]. Tumors initially believed to be of too high risk for safe resections were now possible to resect since anatomical locations were available through preoperative fMRI scanning. In this study, no patients displayed permanent neurological damage after surgery.
Paradigms
fMRI localization is dependent upon the paradigm used to elicit the activation. In the motor system, these paradigms are relatively straightforward. Common paradigms for the motor area are finger tapping, tongue motion, and foot motion. Finger tapping most commonly involves having patients tap their fingers whilst in the scanner while simultaneously avoiding movement of the arms or the shoulders. During the tongue motion paradigm, patients are asked to keep teeth closed to avoid head motion artifacts and sweep their tongue against the back of their teeth. Motor foot localization consists of repetitive flexion and extension of the toes without moving the ankles. In most cases, small movements of the foot, hand, or tongue provide a significant signal, particularly when head motion is absent [11].
There are a variety of ways to perform motor paradigms with patients. Some are designed to localize the motor gyrus in both hemispheres simultaneously with bilateral finger tapping (to asses motor gyrus displacement) and in some cases patients can be asked to both move their tongue while tapping their fingers to localize both hand and tongue in a single experimental run. Yet another design involves no rest; that is, instead of alternating between a single motor task and rest, patients are instead asked to alternate between finger tapping and tongue motion. In all cases, careful attention should be paid to minimizing head motion. Short scanning time and clear instruction help to minimize artifacts.
fMRI exams of motor function, like any fMRI exam, can be performed using a block design or an event-related design. In many fMRI exams there are two states that are statistically contrasted in post-processing analysis. In an event-related design the patient performs one event (a finger tap for example), which is followed by rest. This type of paradigm allows for detailed estimation of the hemodynamic response [32]. Event-related designs, while possible in patient populations, are arguably preferred in basic science fMRI as more precise neuroanatomic parameters can be extracted from single events. However, this type of design requires many repetitions because the change from baseline for any one event is small (on the order of about 2–6 %). Accordingly, event-related designs tend to be longer than block designs given the need for many repetitions for adequate statistical power. This can be problematic for brain tumor patients who, in our experience, can have trouble keeping still and following complicated instructions with rapid alternations in task demand.
Block designs, by contrast, average the signal from many of the same types of events over a single epoch [23]. For example, a typical block-designed motor task would have the patient resting for five images and finger tapping for five images. This alternating cycle of rest and task would repeat five or six times and last approximately 5 min depending on the time to repeat (TR) of the images [31, 33–35]. The advantage to the block design is that the task-related images and the rest-related images are signal averaged. Therefore, the block design maximizes detection of the signal while the event-related design maximizes estimation of the signal [32, 36]. However, with this said, there is a role for event-related motor paradigms. Work done by Marquart et al. indicated that for finger movement tasks, where head motion artifacts are less likely to occur, a block trial is preferred for greater activation seen within the sensory motor cortex and/or the SMA. However, for toe and tongue movement tasks that are more susceptible to movement artifacts, event related or “single-event” paradigms adequately localized the foot motor areas [37].
There are also many special considerations when using fMRI maps for clinical use. For example, in brain tumor patients it is helpful to have a shorter task or “paradigm” duration as patients have a harder time than normal controls in keeping their head still. While fMRI data is often acquired using an event-related design in healthy control subjects, brain tumor patients as a result often benefit from the signal averaging afforded by block designs. Further, areas can be activated that are associated with the task being investigated but not essential for the task. This of course is an important consideration for BOLD fMRI mapping for neurosurgical planning where the goal is to isolate essential eloquent areas. With this said, fMRI is commonly used to map eloquent areas pre-surgically and has been shown to be sufficiently accurate, particularly in motor areas, for neurosurgical planning in a multitude of studies [2, 23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree