TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ
Tis (DCIS)
Ductal carcinoma in situ
Tis (LCIS)
Lobular carcinoma in situ
Tis (Paget’s)
Paget’s disease of the nipple NOT associated with invasive carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget’s disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget’s disease should still be noted
T1
Tumor ≤20 mm in greatest dimension
T1mi
Tumor ≤1 mm in greatest dimension
T1a
Tumor ≤1 mm but ≤5 mm in greatest dimension
T1b
Tumor ≤5 mm but ≤10 mm in greatest dimension
T1c
Tumor ≤10 mm but ≤20 mm in greatest dimension
T2
Tumor ≤20 mm but ≤50 mm in greatest dimension
T3
Tumor ≤50 mm in greatest dimension
T4
Tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodules). Note: invasion of the dermis alone does not qualify as T4
T4a
Extension to the chest wall, not including only pectoralis muscle adherence/invasion
T4b
Ulceration and/or ipsilateral satellite nodules and/or edema (including peau d’orange) of the skin which do not meet the criteria for inflammatory carcinoma
T4c
Both T4a and T4b
T4d
Inflammatory carcinoma (see rules for classification)
Note: If a cancer was designated as inflammatory before neoadjuvant chemotherapy, the patient will be designated to have inflammatory breast cancer throughout, even if the patient has complete resolution of inflammatory findings
Regional lymph nodes (N)
Clinical
NX
Regional lymph nodes cannot be assessed (e.g., previously removed)
N0
No regional lymph node metastases
N1
Metastases to movable ipsilateral level I, II axillary lymph node(s)
N2
Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted; or in clinically detecteda ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases
N2a
Metastases in ipsilateral axillary lymph nodes fixed to one another (matted) or to other structures
N2b
Metastases only in clinically detecteda ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph node metastases
N3
Metastases in ipsilateral infraclavicular (level III axillary) lymph node(s) with or without level I, II axillary lymph node involvement; or in clinically detecteda ipsilateral internal mammary lymph node(s) with clinically evident level I, II axillary lymph node metastases; or metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement
N3a
Metastases in ipsilateral infraclavicular lymph node(s)
N3b
Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s)
N3c
Metastases in ipsilateral supraclavicular lymph node(s)
a Clinically detected is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination and having characteristics highly suspicious for malignancy or a presumed pathologic macrometastasis based on fine needle aspiration biopsy with cytologic examination. Confirmation of clinically detected metastatic disease by fine needle aspiration without excision biopsy is designated with an (f) suffix, e.g., cN3a(f). Excisional biopsy of a lymph node or biopsy of a sentinel node, in the absence of assignment of a pT, is classified as a clinical N, e.g., cN1. Information regarding the confirmation of the nodal status will be designated in site-specific factors as clinical, fine needle aspiration, core biopsy, or sentinel lymph node biopsy. Pathologic classification (pN) is used for excision or sentinel lymph node biopsy only in conjunction with a pathologic T assignment
Pathologic (pN)b
pNX
Regional lymph nodes cannot be assessed (e.g., previously removed or not removed for pathologic study)
pN0
No regional lymph node metastasis identified histologically
Note: Isolated tumor cell clusters (ITC) are defined as small clusters of cells not ≤0.2 mm, or single tumor cells, or a cluster of fewer than 200 cells in a single histologic cross section. ITCs may be detected by routine histology or by immunohistochemical (IHC) methods. Nodes containing only ITCs are excluded from the total positive node count for purposes of N classification but should be included in the total number of nodes evaluated
pN0(i−)
No regional lymph node metastases histologically, negative IHC
pN0(i+)
Malignant cells in regional lymph node(s) no ≤0.2 mm (detected by H&E or IHC including ITC)
pN0(mol−)
No regional lymph node metastases histologically, negative molecular findings (RT-PCR)
pN0(mol+)
Positive molecular findings (RT-PCR),c but no regional lymph node metastases detected by histology or IHC
pN1
Micrometastases; or metastases in 1–3 axillary lymph nodes; and/or in internal mammary nodes with metastases detected by sentinel lymph node biopsy but not clinically detectedd
pN1mi
Micrometastases (≤0.2 mm and/or more than 200 cells, but none ≤2.0 mm)
pN1a
Metastases in 1–3 axillary lymph nodes, at least one metastasis ≤2.0 mm
pN1b
Metastases in internal mammary nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detectedd
pN1c
Metastases in 1–3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected
pN2
Metastases in 4–9 axillary lymph nodes; or in clinically detectede internal mammary lymph nodes in the absence of axillary lymph node metastases
pN2a
Metastases in 4–9 axillary lymph nodes (at least one tumor deposit ≤2.0 mm)
pN2b
Metastases in clinically detectede internal mammary lymph nodes in the absence of axillary lymph node metastases
pN3
Metastases in 10 or more axillary lymph nodes; or in infraclavicular (level III axillary) lymph nodes; or in clinically detectede ipsilateral internal mammary lymph nodes in the presence of 1 or more positive level I, II axillary lymph nodes; or in more than 3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detectedd; or in ipsilateral supraclavicular lymph nodes
pN3a
Metastases in 10 or more axillary lymph nodes (at least one tumor deposit ≤2.0 mm); or metastases to the infraclavicular (level III axillary lymph) nodes
pN3b
Metastases in clinically detectede ipsilateral internal mammary lymph nodes in the presence of 1 or more positive axillary lymph nodes; or in more than 3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detectedd
pN3c
Metastases in ipsilateral supraclavicular lymph nodes
bClassification is based on axillary lymph node dissection with or without sentinel lymph node biopsy. Classification based solely on sentinel lymph node biopsy without subsequent axillary lymph node dissection is designated (sn) for “sentinel node,” e.g., pN0(sn)
cRT-PCR: reverse transcriptase/polymerase chain reaction
d“Not clinically detected” is defined as not detected by imaging studies (excluding lymphoscintigraphy) or not detected by clinical examination
e“Clinically detected” is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination and having characteristics highly suspicious for malignancy or a presumed pathologic macrometastasis based on fine needle aspiration biopsy with cytologic examination
Posttreatment ypN
Posttreatment yp “N” should be evaluated as for clinical (pretreatment) “N” methods above. The modifier “sn” is used only if a sentinel node evaluation was performed after treatment. If no subscript is attached, it is assumed the axillary nodal evaluation was by axillary node dissection (AND)
The X classification will be used (ypNX) if no yp posttreatment SN or AND was performed
N categories are the same as those used for pN
Distant metastases (M) | |
M0 | No clinical or radiographic evidence of distant metastases |
cM0(i+) No clinical or radiographic evidence of distant metastases, but deposits of molecularly or microscopically detected tumor cells in circulating blood, bone marrow, or other non-regional nodal tissue that are no larger than 0.2 mm in a patient without symptoms or signs of metastases | |
M1 | Distant detectable metastases as determined by classic clinical and radiographic means and/or histologically proven larger than 0.2 mm |
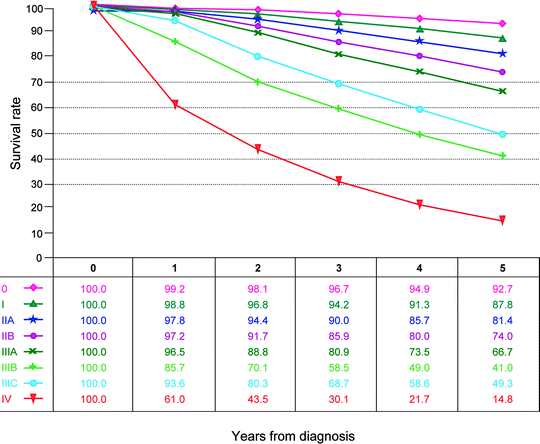
Fig. 12.1
Observed survival rates for 211,645 cases with carcinoma of the breast. Data from the National Cancer Data Base (Commission on Cancer of the American College of Surgeons and the American Cancer Society) diagnosed in years 2001–2002. Stage 0 includes 30,263; stage I, 85,278; stage IIA, 43,047; stage IIB, 17,665; stage IIIA, 13,983; stage IIIB, 4,533; stage IIIC, 6,741; and stage IV, 10,135. (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC http://www.springer.com)
Table 12.2
AJCC anatomic stage and prognostic groups for breast cancer
Group | T category | N category | M category |
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage IA | T1a | N0 | M0 |
Stage IB | T0 | N1mi | M0 |
T1a | N1mi | M0 | |
Stage IIA | T0 | N1b | M0 |
T1a | N1b | M0 | |
T2 | N0 | M0 | |
Stage IIB | T2 | N1 | M0 |
T3 | N0 | M0 | |
Stage IIIA | T0 | N2 | M0 |
T1a | N2 | M0 | |
T2 | N2 | M0 | |
T3 | N1 | M0 | |
T3 | N2 | M0 | |
Stage IIIB | T4 | N0 | M0 |
T4 | N1 | M0 | |
T4 | N2 | M0 | |
Stage IIIC | Any T | N3 | M0 |
Stage IV | Any T | Any N | M1 |
Breast Cancer Treatment
With exception of very early- and late-stage disease, almost all breast cancer care involves a combination of locoregional and systemic treatments. Locoregional therapy includes surgery and radiotherapy, and systemic therapy includes chemotherapy (e.g., taxanes), endocrine (hormonal, e.g., aromatase inhibitors) therapy, and/or targeted (biologic, e.g., anti-HER2 drugs) therapy.
Primary tumor excision remains a key component of the treatment of newly diagnosed breast cancer [13]. This includes mastectomy, removal of the entire breast, and breast conserving surgery, also known as “lumpectomy” of the cancer [13]. Lumpectomy followed by adjuvant radiotherapy provides low rates of local recurrence and survival similar to mastectomy but with preservation of the breast [14]. For invasive disease, axillary nodal staging is routinely performed at the time of surgery. The purpose of axillary sampling is largely for diagnosis; the therapeutic value of lymph node removal remains largely unproven [15]. For this purpose, sentinel lymph node (SLN) biopsy has emerged as the preferred method for patients with clinically negative axillae [16], sparing the patient most of the morbidity of full axillary dissection.
Radiotherapy is commonly used as an adjunct to surgery in locoregional breast cancer treatment [17]. Lumpectomy in the absence of breast radiotherapy leads to unacceptably high rates of local recurrence [14]; therefore, breast radiotherapy is an integral part of breast conservation treatment. In addition to radiation of the breast in patients undergoing breast conservation, adjuvant irradiation can also be used for the chest wall in mastectomy patients, but it is usually reserved for patients with larger tumors or documented chest wall invasion [17]. Adjuvant radiotherapy is also used in the treatment of involved nodal basins and has been shown to enhance survival in those patients with multiple axillary nodal metastases [18].
Breast cancer is one of the solid tumors that is often responsive to systemic therapy. Systemic therapy can be cytotoxic chemotherapy and can also involve more targeted and selective treatments. In all but the earliest cancers, adjuvant systemic therapy is used in addition to locoregional therapy to treat residual microscopic disease [14] and has contributed significantly to reduced breast cancer mortality. Systemic cytotoxic chemotherapy is used in more advanced or aggressive tumors, ER- or PR-negative tumors [19]. In ER-expressing tumors, adjuvant endocrine therapy is used, typically an aromatase inhibitor or tamoxifen [20]. In higher-risk ER-expressing tumors, both chemotherapy and endocrine therapy can be used [19]. Both types of treatment are also primary therapy for metastatic disease [21].
Advances in molecular cancer biology have led to an increased understanding of the biologic factors that contribute to breast cancer pathogenesis and progression. This understanding has already led to new and effective systemic treatments [22], and new targeted therapies continue to be developed and tested [23]. There is also an increasing array of other targeted systemic treatments for breast cancer. HER2 has emerged as an important target. Trastuzumab and more recently lapatinib have become key components of both adjuvant and metastatic systemic therapy for patients with HER2-overexpressing tumors [22, 24]. The antiangiogenesis drug bevacizumab is also under evaluation to treat metastatic breast cancer (MBC) [25]. New breast cancer targets continue to be investigated [23] and may be especially important in triple-negative patients (ER/PR/HER2-negative), where endocrine and HER2-targeted therapies are unlikely to work [26].
Breast Cancer Detection and Diagnosis
For breast cancer, the word detection is most commonly used in the context of breast cancer screening, most commonly using mammography. Breast cancer diagnosis involves the characterization of a suspicious mass or imaging finding and entails tissue sampling to make a definitive diagnosis of cancer versus benign disease. Most primary cancers are detected by physical examination or mammography during screening [27]. Mammography is the primary imaging modality for breast cancer screening, detection, and diagnosis [28]. The average earlier stage and smaller size of lesions detected by mammographic screening, versus symptomatic and/or palpable lesions, reflects the success of mammographic screening, which has been shown to reduce breast cancer mortality [28]. Both ultrasound and MRI are important adjuncts to x-ray mammography for diagnosis, characterization, and determining the extent of breast cancer and are routinely utilized in these roles [29, 30]. Mammography, ultrasound, and breast MRI can all be used to direct tissue sampling by needle biopsy for breast cancer diagnosis. MRI has also shown utility for screening in high-risk women and was recently incorporated in American Cancer Society Recommendations for screening in high-risk patients [31].
Radiotracer methods have also been investigated for primary breast cancer detection and diagnosis. 99mTc-sestamibi and related compounds have been tested for primary breast cancer imaging. The precise mechanisms for 99mTc-Sestamibi uptake in breast tumors are unknown; however, 99mTc-Sestamibi uptake and retention in breast cancer appear to depend on regional blood flow, plasma and mitochondrial membrane potential, angiogenesis, and tissue metabolism [32–34]. 99mTc-Sestamibi imaging was first tested using standard gamma cameras and tables adapted to allow prone imaging. Since the first large-series prone 99mTc-Sestamibi scintimammography in 1994 [35], many studies investigating the diagnostic accuracy of this method in evaluation of suspected breast cancer have been published. Liberman and colleagues [36] performed a meta-analysis of literature published up until 1999 and found overall sensitivity was 85%, specificity 87%, positive predictive value 88%, negative predictive value 81%, and accuracy 86%. However, 99mTc-Sestamibi scintimammography with standard gamma cameras has limited ability to detect lesions less than 1 cm, with sensitivity lower than 50% in some series [37]. 99mTc-Sestamibi scintimammography also has comparably lower sensitivity for non-palpable lesions. As a result of these concerns, 99mTc-Sestamibi scintimammography with conventional gamma cameras has not found widespread use.
More recently, high-resolution small-field-of-view gamma cameras designed for breast imaging have been developed [38, 39], often called breast-specific gamma imaging (BSGI). Early studies had shown improved visualization of non-palpable lesions, lesions smaller than 1 cm, and in situ carcinoma [38, 39]. Larger trials with comparison to standard breast imaging will be needed to support more widespread use of BSGI. These trials are ongoing.
PET has also been tested in application to breast cancer detection and diagnosis, spurred by the finding of increased [18F]fluorodeoxyglucose ([18F]FDG) uptake in cancer compared to normal breast tissue. [18F]FDG uptake in tumors reflects the rate of glucose metabolism and correlates somewhat with histologic type (higher uptake in ductal vs. lobular), tumor histologic grade, and indices of cellular proliferation (higher uptake with higher levels of proliferation) [40, 41]. Overall, the sensitivity of whole-body [18F]FDG-PET in the detection of primary breast cancer ranges 64–96%, specificity 73–100%, positive predictive value 81–100%, and negative predictive value 52–89% [27]. [18F]FDG-PET has low sensitivity for small, well-differentiated, and in situ cancers. [42]. As with 99mTc-Sestamibi scintimammography, this has severely limited the use of whole-body [18F]FDG-PET for primary tumor diagnosis.
Dedicated breast positron emission mammography (PEM) units have been developed [43]. Advantages of PEM include higher spatial resolution, shorter imaging time, and reduced attenuation compared with whole-body imaging. Early studies demonstrated the feasibility of PEM for detecting smaller breast tumors (reviewed in Rosen et al. [43]), and a recent multicenter trial also suggested that PEM may aid in the detection and characterization of both invasive and in situ breast carcinoma [44]. Current limitations for PEM include imaging posterior lesions, variable [18F]FDG uptake in small tumors, and false-positive findings from prior biopsy [44, 45]. Recently, PEM with capability of biopsy has been developed and is being tested [46]. The clinical data regarding dedicated positron breast imaging devices are still limited, particularly compared to the large amount of data supporting and validating screening mammography and other adjunctive primary breast imaging modalities like ultrasound and breast MRI. The utility of PEM compared to existing breast imaging methods awaits validation in larger prospective trials before more general acceptance and more widespread clinical use.
Breast Cancer Staging
Regional Lymph Nodes
Staging is typically divided into regional lymph nodes, especially axillary nodes, and distant or systemic staging of sites beyond regional lymph nodes. Almost all patients with invasive breast cancer will undergo axillary nodal staging since the presence or absence of axillary lymph nodes is an important consideration for further treatment after surgery [16]. The traditional approach to axillary nodal staging was the axillary nodal dissection, where axillary contents were removed and examined; however, the morbidity of this process, including a significant incidence of lymphedema, led to the search for a less morbid but equally accurate approach [16]. This consideration motivated SLN mapping methods, designed to identify and sample the node(s) that are the first draining nodes, that is, “sentinels” in the axillary drainage basin for the breast lesion. SLN biopsy is a shorter surgical procedure than full axillary nodal dissection with much less morbidity [16]. If sentinel nodes are negative, then the chance of “downstream” nodal metastases is quite small, and the patient can be spared the full dissection. If the sentinel node shows evidence of metastasis, then an axillary dissection may be needed to determine the extent of axillary spread. SLN biopsy has been shown to be highly accurate compared to axillary dissection [47–49] and has emerged as the standard of care for axillary nodal sampling in patients with clinically negative axillae [50]. SLN biopsy has been thoroughly tested in large clinical trials and shown to be safe and accurate, with comparable relapse and survival compared to axillary nodal dissection [51, 52].
Radionuclide methods play a key role in SLN biopsy. Typically, for SLN mapping, a colloidal radiopharmaceutical (99mTc-sulfur colloid in the USA; albumin or antimony nanocolloid in Europe) is injected, and sentinel nodes are identified through imaging (lymphoscintigraphy, Fig. 12.2), gamma probes, or both [53]. Other, more specific agents have been developed and are undergoing testing [54]. Some centers use both radiocolloid and visible blue dye to guide SLN sampling, while some centers use one of the methods without the other [16].
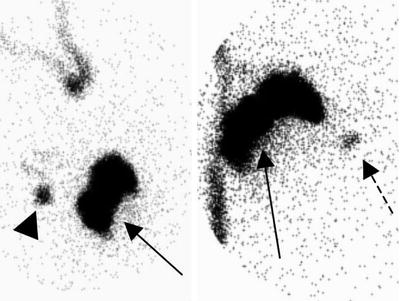

Fig. 12.2
Lymphoscintigraphy for breast cancer SLN mapping. Images (oblique, left, and anterior, right), with the body contour outline drawn using a radioactive marker, show a perilesional injection (solid arrows), an axillary sentinel lymph node (arrowhead), and drainage to an internal mammary lymph node (dashed arrow)
Early studies used perilesional injection of radiotracer or blue dye with the goal of mapping lymphatic drainage pathways arising from the tumor site [55]. Subsequent studies showed that intradermal injection over the lesion site or peri-areolar injection identified the same axillary SLN found by perilesional injection [56] and provided accuracy for axillary SLN sampling [57]. However, most studies done using radiocolloid and lymphoscintigraphy suggest that only perilesional injection depicts extra-axillary drainage, especially to internal mammary (IM) nodes [55]. Studies have shown that approximately 15–20% of non-upper outer quadrant (UOQ) lesions drain to IM lymph nodes after perilesional injection [58]; however, this rate is much smaller for other injection methods [55]. The importance of sampling IM nodes is a point of considerable debate [59]. Studies using SLN mapping and lymphoscintigraphy to sample IM lymph have demonstrated the feasibility of this approach [60–62]; however, the overall percentage of positive IM nodes is on the order of 5% or less, and it is rare to have IM lymph node metastasis in the absence of axillary nodal metastases [63]. As a result, most practices in the USA do not routinely sample IM nodes. Yao et al. [64] found that patients with positive axillary nodes and IM nodal drainage, even in the absence of IM nodal sampling, were significantly more likely to relapse and die of breast cancer than those without drainage. Another study found a higher rate of nodal relapse outside the axilla, versus in the axilla, after sentinel node biopsy [65]. Such studies prompt careful consideration of the approach to SLN mapping in breast cancer and the potential benefit of perilesional injection and lymphoscintigraphy in selected patients to identify extra-axillary drainage.
There has been considerable interest in noninvasive imaging to detect axillary nodal spread in breast cancer and avoid the need for invasive biopsy. This has been best studied for [18F]FDG-PET (reviewed in Crippa et al. [66]). Early studies showed high sensitivity (also reviewed in Eubank et al. [67]), suggesting feasibility of [18F]FDG-PET as a noninvasive method for axillary staging and potential substitute for axillary nodal dissection. However, more recent studies with larger fraction of smaller primary cancers, comparable to the size found in screened populations, demonstrated sensitivities as low as 20–30% for axillary nodal metastases (reviewed in Rosen et al. [43]). In a prospective multicenter trial representing one of the largest patient cohorts to date [68], [18F]FDG-PET was performed in 360 women with newly diagnosed invasive breast cancer, and the results were interpreted blindly by three experienced readers and compared to pathologic analysis of axillary nodes. Overall, [18F]FDG-PET was 61% sensitive and 80% specific for axillary metastases, with a positive predictive value of 62% and a negative predictive value of 79%. Further studies comparing [18F]FDG-PET to SLN biopsy support SLN biopsy for early-stage disease and confirmed limited sensitivity of [18F]FDG-PET for axillary nodal metastases in this clinical setting compared to SLN biopsy (as low as 20–40%), particularly in small (ranging from 1 to 15 mm) and isolated lesions [43 69]. These results indicate the limitation of [18F]FDG-PET in detection of micrometastases and small tumor-infiltrated axillary lymph nodes and indicate that [18F]FDG-PET is not sufficiently sensitive to replace SLN biopsy in patients with early-stage breast cancer.
Although [18F]FDG-PET has limited sensitivity for axillary nodal metastases in early breast cancer, it has high specificity; most studies demonstrate specificities in the range of 93–100% (reviewed in Rosen et al. and Eubank et al. [43 70]). This high specificity has led some to suggest that [18F]FDG-PET may be useful in patient at higher risk for axillary nodal spread, including those with palpable nodes, avoiding the need for SLN biopsy in patients shown to have definitive evidence of axillary spread [70–75]. In such patients, a positive [18F]FDG-PET would direct the patient to full axillary dissection, while a negative [18F]FDG study would be followed by SLN biopsy to rule out small-volume disease missed by PET. In current practice, however, this role has largely been taken over by axillary ultrasound, which can also be used to direct nodal sampling for confirmation of nodal metastases [76, 77].
[18F]FDG-PET has performed well in regional nodal staging in patients with more advanced cancers, including patients with palpable axillary disease, plexopathy, or symptomatic metastases [78]. This is an important use of [18F]FDG-PET/CT for breast cancer and can be helpful for directing treatments such as regional radiotherapy [79].
[18F]FDG-PET may be helpful in assessing breast cancer spread to regional nodal sites outside of the axilla, especially the IM chain (Fig. 12.3). [18F]FDG uptake in IM nodes has been reported in some studies of nodal staging in patients with more advanced disease, showing IM nodal uptake as high as 25% [80, 81]. Some reports suggest that IM [18F]FDG uptake predicts treatment failure patterns of disease consistent with IM nodal involvement and progression [81]. Tran et al. showed that the likelihood of extra-axillary lymph node finding on [18F]FDG-PET was dependent upon the position of tumor (medial vs. lateral), and the presence of extra-axillary nodal uptake on [18F]FDG-PET combined with medial tumor location indicated a high risk of subsequent disease progression [82]. An early study by Jones and colleagues showed the feasibility of detecting IM nodal metastases in early-stage patients using [18F]FDG-PET [83], although more data are required to further validate these results.
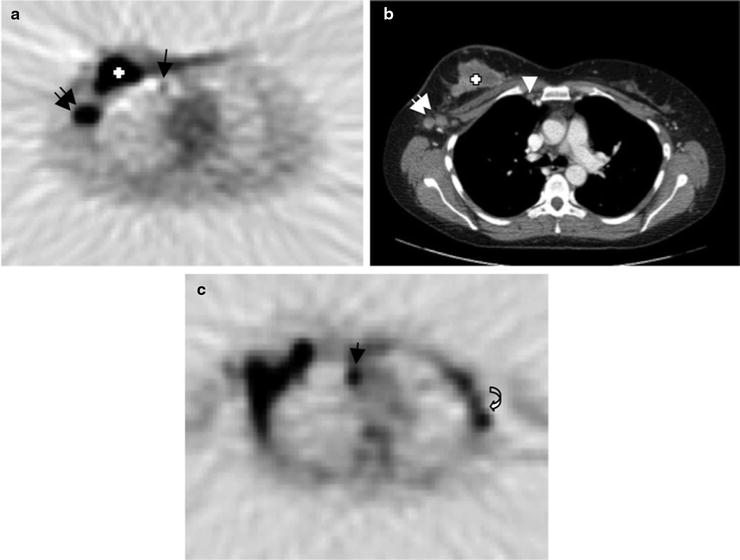

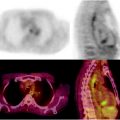
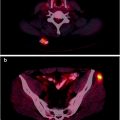
Fig. 12.3
Internal mammary nodal metastases detected by [18]FDG-PET/CT—A patient with right locally advanced breast cancer underwent pre-therapy PET/CT which shows a small hypermetabolic focus (arrow) in the right internal mammary (IM) region (a) which corresponds to small lymph node (arrow head) on contrast-enhanced CT image (b). There are larger hypermetabolic masses in the right breast, which corresponds to known triple-negative breast cancer (cross) and axillary nodal metastasis (double arrows). Follow-up [18]FDG-PET image after clinical evidence of disease progression (c) demonstrates worsening of IM metastasis (arrow) with new involvement of left axillary (contralateral) lymph nodes (curved arrow)
Distant Metastases
For patients with early-stage breast cancer and without evidence of regional nodal spread (stage I or low end of stage II), systemic staging is not recommended unless there are symptoms [84] since the chance of distant metastases is low and the chance of false-positive findings in staging studies is considerably higher than the chance of true positive findings [85, 86]. For recurrent breast cancer or breast cancer with known metastases, systemic staging is recommended [84]. For locally advanced breast cancer (LABC), especially for patients with advanced axillary nodal disease, the risk of systemic metastases is high enough that systemic staging is recommended as part of the initial evaluation [87].
Important areas of more distant spread for breast cancer include nodal beds outside the axillary and IM chain, for example, in the mediastinum, the lungs, the liver, and bones [88]. In patients with symptoms, other sites, for example, the brain, may be investigated. While anatomic imaging (largely using CT of the chest, abdomen, and possibly pelvis) plays a large role in systemic staging, radionuclide methods, especially PET and bone scintigraphy, also play key roles.
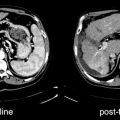
Between 30 and 85% of patients with metastatic breast cancer (MBC) have bone metastases during the course of disease, and bone is the first site of metastasis for 26–50% of patients with metastatic breast carcinoma [89, 90]. Bone metastases in breast cancer can be both osteoblastic and osteolytic. Breast cancer preferentially metastasizes to the spine and pelvis, followed by ribs, skull, and femur [90]. Bone scanning is the most commonly used method for breast cancer bone metastasis staging [90] (Fig. 12.4). The reported sensitivity ranges from 62 to 100% and specificity ranges from 78 to 100% [90, 91]. False positives can be caused by trauma, degenerative changes, and other benign conditions, while false negatives can occur in presence of predominantly osteolytic metastases, low bone turnover, or in avascular regions (such as necrosis) [90]. Single-photon emission computed tomography (SPECT) may improve the performance of bone scanning for bone metastasis staging [92]. SPECT is particularly helpful to characterize solitary lesions in the spine and/or to differentiate bone metastases from degenerative lesions. Salvelli et al. reported the sensitivity and specificity of SPECT to be over 90% [92].
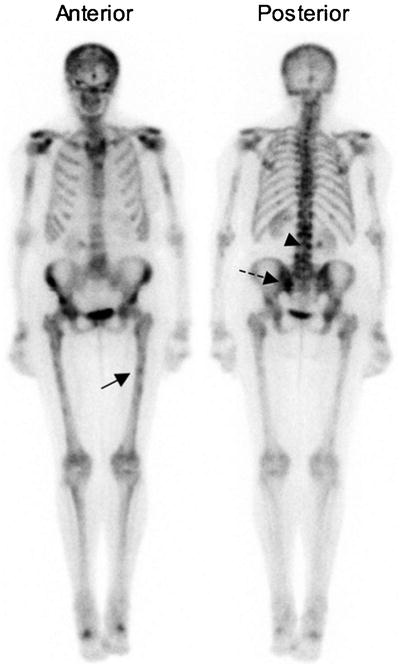

Fig. 12.4
Bone metastases identified by bone scan. 99mTc-methylene diphosphonate (MDP) bone scan shows multiple metastases, including, for example, a femoral lesion (arrow), a sacral lesion (dashed line), and a spine lesion (arrowhead)
Some studies shown that fluoride (18F−) PET (Fig. 12.5) is superior to bone scan and SPECT in detecting bone metastases and differentiating malignant from benign lesions [93–95]. The mechanism of localization of fluoride in bone and uptake in bone metastases is similar to diphosphonates in that both are deposited in newly mineralizing bone accompanying osteoblastic activity [96]. 18F− has practical advantages over 99mTc-labeled bone scan agents that include faster clearance and higher contrast [96]. 18F− PET/CT may be particularly helpful in relating biochemical changes in bone detected by PET to anatomic abnormalities seen on CT, helping with specificity [93, 94]. Sensitivity may also improve using fluoride PET compared to bone scan. Schirrmeister and colleagues found more metastatic lesions on 18F− PET compared to bone scan with additional SPECT, impacting clinical management in 12% of patients [93]. Although recent studies have successfully shown 18F− PET to have higher diagnostic accuracy in assessment of bone metastases, long-standing familiarity with bone scan, concerns regarding cost, and a lack of data on the clinical impact of improved diagnostic accuracy of 18F− PET compared to bone scan have somewhat limited the use of 18F− PET, although its use can be expected to increase with increased experience and data.
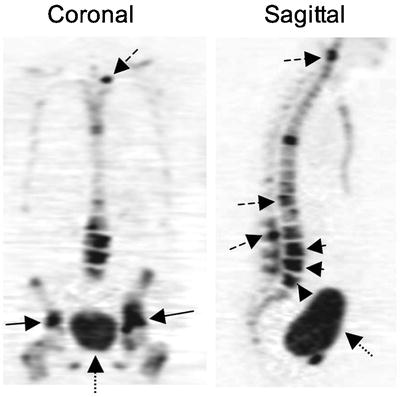

Fig. 12.5
Bone metastases identified by 18F-fluoride PET. Fluoride PET images of a patient with multiple bone metastases demonstrate the increased detail and tomographic information by PET. Images show bilateral pelvic metastases (arrows) and multiple spine metastases (dashed arrows). The band-like appearance of pathologic compression fraction in the lower lumbar spine is also demonstrated (arrowheads). Normal bladder excretion (dotted arrows) is also seen
[18F]FDG-PET and PET/CT have been increasingly used in systemic staging for recurrent or MBC (Fig. 12.6). The reported sensitivities and specificities of [18F]FDG-PET for detection of distant metastases are 80–100% and 50–97%, respectively (summarized in Eubank et al. [97]). In an early study of [18F]FDG-PET for breast cancer systemic staging, Moon and colleagues performed [18F]FDG-PET in 57 patients suspected of having recurrent or MBC and demonstrated that [18F]FDG-PET was 93% sensitive and 79% specific [98]. [18F]FDG-PET is also reported to be more sensitive compare to conventional imaging modalities; Mahner and colleagues showed that [18F]FDG-PET was 87% sensitive and 83% specific while combined conventional imaging modalities were 43% sensitive and 98% specific [99]. The addition of CT for [18F]FDG-PET/CT improve performance further [100] and is now widely used for systemic breast cancer staging in patients with advanced disease [101].
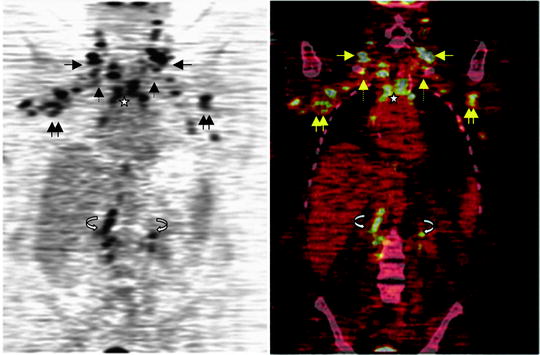

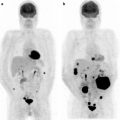
Fig. 12.6
Breast cancer distant metastases identified by [18F]FDG-PET/CT. Coronal [18F]FDG-PET (left) and PET/CT (right) fusion images are shown for a patient with an unusual pattern of widely metastatic disease to multiple lymph node beds. Images demonstrate multiple hypermetabolic lesions in the cervical (arrow), axillary (double arrows), supraclavicular (dashed arrow), mediastinal (star), and retroperitoneal (curved arrow) nodal beds, found to be widespread metastases from breast cancer
The additional sensitivity of [18F]FDG-PET/CT for regional nodes and distant disease may be particularly important in staging for locally advanced breast cancer (LABC). LABC, including primary tumor larger than 5 cm, skin or chest wall involvement, fixed axillary nodes, positive supraclavicular/infraclavicular/IM nodes and inflammatory cancer, has a poor prognosis because of the high incidence of distant metastases during follow-up [87, 102]. Currently, the standard approach is neoadjuvant chemotherapy followed by surgery with axillary nodal dissection and radiation with the intent of cure. However, some patients with apparent LABC may have occult distant metastases, in which case aggressive therapies with curative intent may not be indicated. Thus, detecting distant metastases in the patients with LABC is crucial for determination of treatment. There is evidence to support the utility of [18F]FDG-PET in LABC at diagnosis. Recent studies have shown that [18F]FDG-PET and PET/CT will detect additional sites of regional nodal spread and/or distant metastases not found by standard imaging in up to 25% of patients (Table 12.3) [80, 81, 99, 103–106]. The rate of detection is especially high in inflammatory breast cancer [106]. Thus, [18F]FDG-PET/CT may be increasingly used as part of the initial evaluation of LABC.
Table 12.3
Contribution of [18F]FDG-PET and PET/CT to staging of locally advanced breast cancer (LABC)a
Study | N | Extra-axillary nodes | Distant metastases | Comments |
|---|---|---|---|---|
Danforth et al. [80] | 28 | 25% | – | Stage II and III |
Bellon et al. [81] | 28 | 25% | – | LABC |
44 | – | 10% | LABC; FP common | |
Mahner et al. [99] | 69 | – | 21% | LABC subset |
Fuster et al. [104] | 60 | 7% | 13% | PET/CT; LABC; specificity >98% |
Groheux et al. [105] | 39 | 8% | 10% | PET/CT; stage II and III |
Carkaci et al. [106] | 41
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|