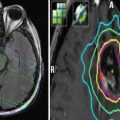
Fig. 1
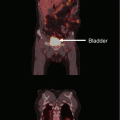
Example of an internationally used systematic biopsy scheme which can be used to prove a local recurrence. The number of cores depicted here is 10
Next, the evaluation of the biopsies is difficult. In early reports, little was known about the rate of histological clearance of irradiated tumor, and failures were determined on biopsies even 6 months after radiotherapy (Scardino 1983). More recent analyses have shown that early biopsies have a high chance of being false positive. Radiation causes postmitotic cell death. Therefore, fatally damaged cells may even survive a limited number of cell divisions before dying off (Mostofi et al. 1992). As there is no visible difference between these dying cells and viable tumor cells, it is impossible for a pathologist to predict whether visible tumor cells are really clonogenic and need treatment (Crook et al. 1997). Approximately 30 % of biopsies positive in the first year after radiation treatment will be negative for tumor in 24–30 months (Crook et al. 1995, 2000). Crook et al. (1995) therefore determined that the optimal time to biopsy would be 30–36 months post radiotherapy. Of course, even 36 months after treatment biopsies may be false positive, although chances are reduced at that moment. As postradiation pathology is difficult to interpret, experienced uropathologists are required to evaluate these biopsies. An extra difficulty is the interpretation of biopsies. The currently used Gleason system is based on the glandular pattern of the tumor seen at relatively low magnification. As an irradiated prostate will show a significantly changed anatomy, especially on glandular level, Gleason score seems less useful after radiation treatment. Still, to evaluate the possibilities for salvage, it will be required to have some information on the potential aggressiveness of the visible tumor cells. Pathologists will need to be cautious here as one of the problems with evaluating carcinomas that have been treated with radiotherapy is that the grade often appears higher (Bostwick et al. 1982). Although post-RT prostate biopsies are burdened with problems of timing, interpretation, and sampling error, they are required as evidence before radical local salvage for radiation failure is considered.
Imaging to visualize the local recurrence is regularly performed using TRUS. Unfortunately, sensitivity is very poor for the primary setting (Crook et al. 1993; Onur et al. 2004). In radio-recurrent disease, accuracy may even be further decreased due to fibrosis of the prostatic tissue. Therefore, current clinical practice lacks a proper imaging tool to detect a local recurrence. Promising local imaging techniques still under development are dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and 18F or 11C-choline positron emission tomography (PET) (Rouviere et al. 2004; Haider et al. 2008; Moman et al. 2010; Wang et al. 2009; Breeuwsma et al. 2010; Barentsz et al. 2012; de Rooij et al. 2015; Umbehr et al. 2013). More recent developments have provided prostate-specific membrane antigen (PSMA) PET-CT using various tracers (Jadvar 2015; Rybalov et al. 2014). However larger series evaluating these techniques and assessment with the pathology reference standard is needed. Recent diagnostic meta-analyses have shown that the use of MRI (using the prostate imaging reporting and data system (PI-RADS)) can help in assessing localized disease, capsular extension, and the exclusion of clinically significant disease with a negative predictive value up to 95 % (Hamoen et al. 2015; Fütterer et al. 2015; de Rooij et al. 2015).
Imaging to exclude possible distant metastases should be performed when evaluating the possibilities for salvage. But as a bone scan, pelvic computed tomography (CT), and MRI need a significant tumor load to show metastases (Hovels et al. 2008; Abuzallouf et al. 2004), it is unlikely they will detect small metastases present in patients being selected for salvage treatment, e.g., with a PSA less than 10 ng/ml (Zagars and Pollack 1997; Nguyen et al. 2007a, b). Next, a pelvic lymph node dissection may be performed to exclude metastases. As a limited pelvic node dissection of the obturator fossa will only detect approximately 30 % of lymph node metastases, the value of a lymph node dissection is questioned (Heesakkers et al. 2008). For early metastases detection in the future, the role of choline PET with various tracers (Breeuwsma et al. 2010) and magnetic nanoparticles in MRI (Barentsz et al. 2007) needs to be further evaluated. PET-CT imaging seems to be able to provide more accurate assessment of metastatic disease and assessment of prostate-confined recurrences. Recent overviews have shown promising results regarding lymph node involvement, prostate-confined recurrences, and distant metastatic disease (pooled sensitivity and specificity of 85.6 and 92.6 % for all three sites) (Evangelista et al. 2013). However, the spread across series is still large and standardization is necessary to achieve this high diagnostic accuracy in every center.
Of course, clinical patient characteristics before primary treatment and salvage are of importance when evaluating the possibility of successful salvage. Patients with a high initial chance of developing distant metastases are unlikely to have a local recurrence only (Nguyen et al. 2007a, b). An initial high Gleason score, high T-stage, high PSA value, and high PSA kinetics (before primary treatment) are possibly also risk factors post treatment to evaluate the likelihood of effective salvage. Therefore, salvage especially is advocated in primary low-risk clinical features (Nguyen et al. 2007a, b). However, evidence in this area from multivariable models is generally lacking and pre-salvage characteristics seem to have the highest predictive ability for cancer control outcomes (Chade et al. 2011, 2012; Wenske et al. 2013; Murat et al. 2009). From these larger salvage series, it seems pre-salvage PSA, PSADT, and pre-salvage Gleason score are most often associated with cancer control outcomes.
Next, the chance of having toxicity from a local salvage treatment will be increased if a patient had severe toxicity during or after primary radiation treatment. Also, other additional treatments may attribute to the possible toxicity from salvage, like a transurethral resection (TURP), high-intensity focussed ultrasound (HIFU) treatment, or ADT use. These treatments will produce extra scar tissue and, therefore, will reduce the repair capacity of normal tissue in case of re-irradiation of the prostate. So far, however, no current salvage re-irradiation literature exists, which models these characteristics in a multivariable manner to the probability of developing toxicity.
Based on the above, a list of preliminary selection criteria for local salvage can be completed. Still, it is of major importance to use such a list reasonably, as many clinical aspects, like age, medical history, and performance score contribute in the decision for a local salvage treatment. The list below partially uses the criteria as described in the currently active radiation therapy oncology group (RTOG) trial 0526 (principle investigator: J. Crook, MD). These selection criteria were also mostly verified in a recent international collaboration on selection for salvage treatment (van den Bos et al. 2015).
Biopsy-proven local recurrence at least 3 years after primary radiation treatment
Posttreatment PSA <10 ng/ml, PSA doubling time (PSADT) >12 months
Bone scan and CT abdomen or lymph node dissection without evidence of metastases
Primary tumor characteristics preferably low to intermediate risk
Acceptable toxicity of primary radiation treatment
No other prostate treatments performed (like TURP, HIFU, etc.)
Additional to RTOG: verification of tumor localization with multiparametric MRI (consisting at least of a 1.5 Tesla T2-weighted, dynamic contrast-enhanced, and diffusion-weighted imaging sequence). Exclusion of metastatic disease using PET-CT (18F or 11C choline PET or Ga-68-PSMA)
3 General Results of Prostate Cancer Salvage
Different salvage treatment modalities are currently clinically practiced. Radical prostatectomy, external beam radiotherapy, cryosurgery, additional I125/Pd103 or Ir192 brachytherapy, and HIFU are used (Pisters et al. 2000; Lee et al. 2008; Grado et al. 1999; Murat et al. 2009; van der Poel et al. 2007; Nguyen et al. 2007a, b; Chade et al. 2011, 2012, Paparel et al. 2009 Wenske et al. 2013, Williams et al. 2011, Spiess et al. 2010, Chen et al. 2013, Peters et al. 2013, Burri et al. 2010; Henríquez et al. 2014; Yamada et al. 2014). Literature is scarce and shows discordant results in terms of treatment effect and toxicity. Furthermore, few large series have been published; most studies are retrospective and contain less than 100 patients. No head-to-head comparisons have been performed. As a consequence, current treatment decisions often depend on patients’ and doctors’/institutional preferences. An overview of the literature is presented below.
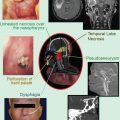
Salvage radical prostatectomy has been described in several articles and has been performed in these series since the 1960s. Five-year biochemical no evidence of disease (bNED) ranges from 50 to 60 % (Nguyen et al. 2007a, b, Chade et al. 2012). In most series pre-salvage PSA was <10 ng/ml. Only a few rather large series have been described. Chade et al. (2011) described the largest salvage radical prostatectomy series with 404 radio-recurrent prostate cancer patients, treated since 1985. Ward et al. (2005) described the results of 199 patients, treated since 1967. In addition, Paparel et al. (2009) described 146 patients from a single institution. Finally, Stephenson et al. (2004) described results of 100 patients (Stephenson et al. 2004). The other published studies contain between 6 and 51 patients (Sanderson et al. 2006; van der Poel et al. 2007; Vaidya and Soloway 2000). The definition of bNED after prostatectomy was most commonly defined as a rise in PSA of >0.2 ng/ml. It was concluded that patients with a pre-salvage PSA of <10 ng/ml did significantly better compared to patients with a PSA >10 ng/ml (Ward et al. 2005; Bianco et al. 2005). In addition, a lower pre-salvage Gleason score was shown to improve biochemical disease-free survival and reduced the development of metastases in multivariable analysis (Chade et al. 2011). Multivariable analysis was not performed for mortality in this largest series. However, these factors were associated with prostate cancer-specific survival after multivariable analysis in the series of Paparel et al. (2009). Neoadjuvant hormonal therapy did not seem to improve outcome (Ward et al. 2005; van der Poel et al. 2007). Also a cystoprostatectomy did not seem to improve oncologic outcome compared to a radical prostatectomy alone (Ward et al. 2005). Although preoperative morbidity, lasting from the previous radiotherapy treatment, was low, urinary incontinence had a weighted average of 41 %, bladder neck strictures of 24 %, and rectal injury of approximately 5 % (Nguyen et al. 2007a, b).
Salvage cryotherapy studies have been described with patients treated since the 1990s (Nguyen et al. 2007a, b). Five larger series have been published. The largest series describes 797 patients from 6 tertiary centers, of the basis of which a pretreatment nomogram to predict biochemical recurrence was created (Spiess et al. 2010). Wenske et al. (2013) described 328 patients after either primary EBRT (n = 259), primary I-125 brachytherapy (n = 49), or primary cryotherapy (n = 20). Furthermore, Williams et al. described 187 patients, Izawa et al. (2002) showed outcomes of 131 patients, and Chin et al. (2001) described results of 125 patients. Other studies contained 59 patients or less (Bahn et al. 2003). Most series used a double freeze-thaw therapy (Izawa et al. 2002; Chin et al. 2001; Bahn et al. 2003, Williams et al. 2011). Unfortunately, the series published on salvage cryotherapy used different definitions for a relapse after salvage, which hampers a decent comparison. In addition, there were also large differences in prognostic characteristics between cohorts. From their overview Nguyen et al. (2007a, b) concluded that outcome of salvage cryotherapy is comparable with the outcome reported from the prostatectomy data above. This is confirmed in the larger series, although the spread across series remains significant. Toxicity again is severe, with a weighted average of urinary incontinence of 36 %, urinary sloughing 11 %, bladder neck stricture or retention 36 %, perineal pain 44 %, and fistulas approximately 3 % (Nguyen et al. 2007a, b).
Salvage brachytherapy series also describe patients treated since the 1990s (Nguyen et al. 2007a, b). Compared to the salvage prostatectomy and salvage cryotherapy series, far less and smaller brachytherapy series have been published. The largest I-125 salvage brachytherapy study contained 49 patients (Grado et al. 1999). Recently, Chen et al. (2013) reported on their HDR-salvage brachytherapy patients (n = 52), and the results of a somewhat larger combined I-125 salvage brachytherapy (n = 37) and HDR-salvage brachytherapy (n = 19) cohort have been published (Henríquez et al. 2014). In addition, a phase II study of salvage HDR brachytherapy (n = 42) has recently been reported (Yamada et al. 2014). Other series reported 31 patients or less (Nguyen et al. 2007b; Wallner et al. 1990; Beyer 1999; Lee et al. 2008; Battermann 2000; Moman et al. 2010). Again, varying definitions of PSA relapse after treatment hamper comparison between the different series. Still, midterm outcomes can be considered comparable to salvage prostatectomy and salvage cryotherapy series (Nguyen et al. 2007a, b). However, multivariable analyses do not provide uniform parameters associated with biochemical failure or survival due to insufficient sample size and sometimes methodological limitations. Weighted incontinence rate was 6 % and other grade 3–4 toxicities were weighted 6 % for the gastrointestinal (GI) tract and 17 % for the genitourinary (GU) tract. Fistulas averaged 3 % (Nguyen et al. 2007a, b). Table 1 shows an overview of published salvage brachytherapy series.
Table 1
Overview of clinical outcome and severe toxicity of published series on salvage brachytherapy
Reference | iPSA (ng/ml) | N | HDR/seeds | HT (%) | FU (months) | bNED, Kaplan-Meier estimates | % GI ≥ grade 3 | % GU ≥ grade 3 |
|---|---|---|---|---|---|---|---|---|
Yamada et al. (2014) | Median 3.5 | 42 | HDR | + (43) | Median 36 | 69 % (5-year) | 0 | 8 |
Henríquez (2014) | Median 3.7 | 56 | HDR (n = 19), seeds (n = 37) | + (27) | Median 48 | 77 % (5-year)a,b | 4 | 23 |
Chen et al. (2013) | Median 9.3 | 52 | HDR | + (46) | Median 60 | 51 % (5-year)a | 0 | 2 |
Burri et al. (2010) | Median 5.6 | 37 | Seeds | + (84) | Median 86 | 54 % (10-year) | 3 | 8 |
Moman et al. (2010) | Mean 11.4 | 31 | Seeds | + (16) | Mean 110 | 20 % (5-year)a | 0 | 6 |
Lee et al. (2008) | Median 3.8 | 21 | Seeds | NA | Median 36 | 38 % (5-year)b | 0 | 0 |
Median 7.5 | 25 | Seeds | − | Median 47 | 70 % (4-year)a | 24 | 16 | |
Lee et al. (2007) | Median 5.9 | 21 | HDR | + (52) | Median 19 | 89 % (2-year)b | 0 | 14 |
Wong et al. (2006) | Median 4.7 | 17 | Seeds | + (88) | Median 44 | 75 % (4-year)b | 6 | 47 |
Lo et al. (2005) | NA | 30 | Seeds | − | Median 59 | 57 % (5-year)b | 3 | 10 |
Koutrouvelis et al. (2003) | NA | 31 | Seeds | + | Median 30 | 87 % (3-year)b | 5 | 13 |
Grado et al. (1999) | Median 5.6 | 49 | Seeds | + (16) | Median 64 | 34 % (5-year)c | 4 | 20 |
Beyer (1999) | Median 2.2 | 17 | Seeds | + (47) | Median 62 | 53 % (5-year)b | 0 | 24 |
Loening and Turner (1993) | NA | 31 | Seeds | − | Median 23 | 67 % (2-year)c | 0 | 0 |
Data on other modalities for local salvage are very limited. One study on HIFU has a mean follow-up of 15 months and showed an incontinence rate of 7 %, bladder neck stenosis 17 %, and fistulas 6 % (Gelet et al. 2004). A more recent and larger series in 290 patients has shown a 5-year biochemical recurrence-free rate in 43, 22, and 17 % in D’Amico low-, intermediate-, and high-risk patients, respectively. Grade 3 urinary incontinence occurred in approximately 10 % of patients; 46 % of patients had a bladder outlet obstruction for which intervention was needed, of which four patients (1.3 %) required urinary diversion. Rectourethral fistulas occurred in 2 % and pubic osteitis in 2.7 % (Crouzet et al. 2012).
Ferromagnetic thermal ablation has been described in 14 patients (Master et al. 2004) and external beam radiotherapy (30.6–50 Gy) combined with external hyperthermia (5–8 treatments) in three patients (Kalapurakal et al. 2001). Of course, further more extensive studies are required to be able to judge these new developments regarding tumor control and toxicity.
In the studies above outcome is mainly described as bNED. Still, survival data are required to judge whether salvage is worthwhile performing. Salvage is often performed in an older patient population. Because of the competing risks in these patients, the potential survival benefit is therefore not automatically derived from the benefit in bNED. However, ADT use can be postponed or prevented with postponing the moment of biochemical failure, thereby reducing side effects from this palliative strategy and possibly increasing cost-effectiveness. Therefore, a generally accepted primary goal of a salvage approach can be to postpone hormonal therapy. This leads to a discussion whether also patients with oligo-metastases in slowly progressing disease might benefit from a local salvage treatment.
The high severe toxicity rates of the varying salvage modalities make salvage unpopular and this probably causes the absence of large randomized studies. Still, these are required to evaluate whether salvage has a future. A randomized study would preferably consist of comparing hormonal treatment alone to one type of salvage and would have to prove a gain in survival. Next to toxicity scoring, quality of life will be essential to monitor (Nguyen et al. 2009) to judge the actual influence of severe toxicities and compare the difference between ADT use and salvage therapy.
4 Re-irradiation of Prostate Cancer
Post-radiotherapy local salvage is historically performed by brachytherapy (LDR, PDR, or HDR) or EBRT (Kimura et al. 2010). There is a preference for brachytherapy, as it can be expected that the surrounding area which receives a relatively high dose will be larger after EBRT, and therefore EBRT can be expected to cause more severe toxicity. This explains why there is hardly any literature on EBRT for recurrent local disease after primary radiotherapy. In a national disease registry, the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE), 935 men were described who received a salvage treatment after primary EBRT. Of these men only eight received salvage by EBRT (Agarwal et al. 2008). In their article, Agarwal et al. (2008) did not notice any survival benefit for any particular combination of primary and salvage therapy. The inability to protect the bladder and the anterior rectal wall without blocking a part of the prostate/tumor was largely caused by the limitations of the EBRT technique. Current EBRT techniques have improved enormously, e.g., by daily position verification techniques (van der Heide et al. 2007) and by the introduction of intensity-modulated radiotherapy (IMRT). Passive-scattering proton therapy has recently been associated with a potential increase in GI toxicity in a large propensity score-matched analysis (Sheets et al. 2012). Advanced forms of proton therapy delivery with intensity-modulated proton therapy (IMPT) may provide better salvage outcome and toxicity data, but IMPT will need IGRT and daily position verification. Although most articles and textbooks discourage the use of EBRT for salvage after primary radiotherapy, these new techniques indeed might lead to comparable results as described above. But as the current standard salvage techniques already show hardly acceptable severe toxicity rates, the further evaluation of EBRT for this purpose seems not the way forward.
In salvage brachytherapy permanent seeds are commonly used. Both I125 and Pd103 have been described (Wallner et al. 1990; Beyer 1999; Grado et al. 1999; Koutrouvelis et al. 2003; Lee et al. 2008; Battermann 2000; Nguyen et al. 2007b; Moman et al. 2010). More recently, HDR brachytherapy is being adopted in the salvage setting (Chen et al. 2013; Henríquez et al. 2014; Yamada et al. 2014). Again, comparison in outcome is hampered by differences in study populations, treatment methods, and the definition of failure (Nguyen et al. 2007a, b). In patients who meet the criteria described in part 2, it can be expected to achieve a 5-year bNED rate of approximately 50–70 % (Table 1). Five-year overall survival rates ranging from 80 to 90 % have been described (Beyer 1999; Lee et al. 2008). Still, the severe toxicity rate is high, up to approximately 10–30 % for GU and GI combined (Moman et al. 2010; Nguyen et al. 2007b, Peters et al. 2013). Currently, an RTOG trial (no. 0526) is ongoing for the prospective evaluation of brachytherapy after EBRT. The results of this trial can be expected to give more insight in the toxicity risk profiles of patients receiving salvage brachytherapy. This can improve patient selection and may augment the benefit/risk ratio (Nguyen et al. 2007a, b; Moman et al. 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree