Fig. 31.1
Safety protocol for MRI in patients with implanted cardiac devices. AF atrial fibrillation, ATP anti-tachycardia pacing, DDI dual-chamber inhibited pacing without atrial tracking, DOO dual-chamber asynchronous pacing, ICD implantable cardioverter defibrillator, PPM pacemaker, VOO ventricular asynchronous pacing, VVI ventricular inhibited pacing (Adapted from Nazarian et al. [32] with permission)
MRI in Patients with CHD and Cardiac Devices
Pulver and colleagues have recently published their experience in MRI scanning of patients with CHD and implanted cardiac devices using a 1.5-T magnet [33]. They performed 11 MRI scans in eight patients who had undergone device placement for sinus node dysfunction, long QT syndrome, and complete heart block. All patients were non-pacemaker dependent, three of them had dual-chamber devices, and most had epicardial leads. Four of these scans were cardiac, and the rest were non-thoracic scans. Inappropriate pacing or significant changes in generator or lead parameters were absent. The authors concluded that MRI could be safely performed in patients with CHD despite having predominantly epicardial leads [33].
Thoracic Scans
An important aspect of MRI imaging in patients with CHD is related to image quality when performing cardiac MR scans. In patients with implantable devices, metal susceptibility artifacts may potentially limit the image quality especially in higher magnetic fields such as 3-T magnets. Metal artifacts due to magnetic susceptibility effect or induction of eddy currents by radio-frequency pulses appear as bands with increased or decreased signal in the vicinity of the leads or generators. On delayed post-contrast images, artifactual increased signal may be confused with hyperenhancing myocardial scars [24]. In our experience, these artifacts can be reduced by selecting imaging planes perpendicular to the plane of the device generator, shortening the echo time, and using spin echo and fast spin echo sequences (Fig. 31.2). In general, compared to balanced steady-state free-precession (SSFP) sequence, spoiled fast gradient echo sequence demonstrates fewer artifacts from metallic devices. This is likely related to shorter echo times used in the later sequence. Pulver and colleagues have reported that in their experience, in patients with CHD, all MRIs yielded diagnostic quality images with no evidence of major artifacts despite having predominantly epicardial leads [33]. They used routine MR sequences for their cardiac cases. Mild atrial lead artifact was seen in SSFP cine imaging, but double inversion recovery black blood series were essentially artifact-free. Notably, artifacts from leads and pacemaker generators are minimal in size. Moreover, left-sided ICD generators are the primary cause of image distortion in device recipients [34].
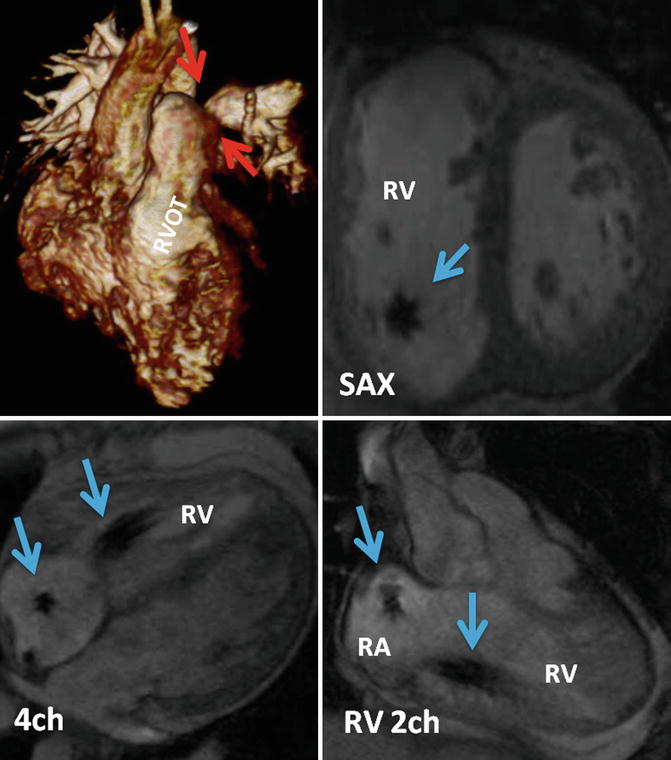

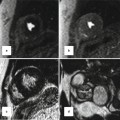
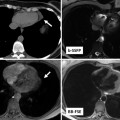
Fig. 31.2
Thoracic MRI of a patient status post complete repair of tetralogy of Fallot and implanted dual-chamber pacemaker. Volume-rendered reconstruction shows left pulmonary artery stenosis (red arrows) and the right ventricle outflow tract patch (RVOT). Cine MR images in short axis (SAX), four chamber (4ch), and right ventricle (RV) two chamber (2ch) showing the lead artifacts in the right atrium(RA) and RV (blue arrows)
MRI-Conditional Cardiac Devices
Given the increased clinical need for MRI scans in patients with implantable cardiac device, implantable cardiac device manufacturers have developed devices that are specifically designed to be safe in the MRI environment. These “MRI-conditional” devices pose no known hazards when MRI is performed using a specific device programming and detailed monitoring conditions [35]. Recently, the United States Food and Drug Administration (FDA) approved the Revo MRI SureScan Pacing System (Medtronic, Inc., Minneapolis, MN), one of the MRI-conditional pacing systems [36]. Additional MRI-conditional devices are available outside of the United States. These devices include the Accent MRI (St Jude Medical, St. Paul, MN) [37], Evia and Estella (Biotronik, Berlin, Germany) [38], as well as Lumax 740 (Biotronik, Berlin, Germany) [38]. Of note, these devices are approved for MRI only when attached to specific leads (e.g., CapSureFix (Medtronic, Inc., Minneapolis, MN), Tendril MRI (St Jude Medical, St. Paul, MN), and Linoxsmart (Biotronik, Berlin, Germany)) and when the MRI field strength is limited to 1.5 T.
Several modifications were made in these devices in order to make them “MRI conditional.” Due to manufacturer proprietary designs, not all changes are disclosed. However, these changes include design features to minimize the use of ferromagnetic materials. Changes in the lead conductor design and filtering are used to alleviate heating. The use of a Hall sensor rather than a reed switch results in more predictable pacemaker performance within the MRI environment [39]. Other changes include modules to simplify MRI safe device programming. For example, in the Revo MRI SureScan Pacing System (Medtronic, Inc., Minneapolis, MN), the MRI mode streamlines the choice between asynchronous and non-stimulation modes, increases pacing output to 5.0 V at 1.0 ms during MRI, and enables MRI mode programming only after system integrity has been checked and approved.
The first feasibility study for such an MRI-conditional device included 107 patients. During this study, patients underwent implantation of the EnRhythm MRI-conditional device or a conventional dual-chamber device. The authors reported no complications during the follow-up period [40]. They observed, however, a trend towards lower successful cephalic vein access when using MRI dedicated leads compared to conventional leads (60.0 % vs. 68.4 %, respectively). Also, subclavian vein access was used more frequently in patients with MRI-conditional leads (40.0 % vs. 31.6 %, respectively, p = NS). Finally, there was no difference in fluoroscopy time (6.0 ± 3.6 min vs. 6.6 ± 3.8 min), overall procedure times (71.7 ± 27.6 min vs. 76.9 ± 30.3 min), or duration of hospitalization. This study suggested that the EnRhythm MRI-compatible pacemaker is safe and could be used for cardiac pacing. Following this study, a larger-scale randomized prospective multicenter study was performed. The study included 464 patients and recruited both pacemaker-dependent and non-pacemaker-dependent patients [39]. Twelve weeks following the procedure, 211 patients underwent MRI examination using a 1.5-T scanner. Other technical parameters included a maximum specific absorption rate of 2 W/kg and a maximum gradient slew rate of 200 T/m/s. During these scans, the isocenter was limited to extra-thoracic region (above the superior surface of C1 vertebra and below the inferior surface of the body of T12). Device interrogation was performed before MRI, immediately after MRI, 1 week after MRI, and at 1 month after MRI. No events were reported during MRI examinations, and no device-related adverse events were attributable to MRI. There were no differences between the study groups with regard to pacemaker measurements including capture threshold, sensitivity, and impedances. Importantly, since this study was limited to specific isocenter locations, the results cannot be generalized to assess safety of this MRI-conditional system with MRI isocenter in the thorax (between C1 and T12 vertebra). This study led to the approval of the Revo MRI Pacemaker System with 5086 MRI CapSureFix MRI Pacing Leads (Medtronic) and the SureScan Software (Medtronic) as “MRI conditional” by the United States FDA [41].
Future Directions
The currently approved MRI-conditional devices were assessed and approved for conditional use in 1.5-T MRI scanners. Higher field strengths are increasingly used in the clinical setting and offer improved signal-to-noise ratio (SNR), spatial resolution, image quality, and diagnostic strength. Gimbel and colleagues observed no arrhythmias or significant changes in device parameters with 3.0-T MRI in 14 patients with cardiac devices. Minor observed events did include, however, one patient with a burning sensation in his chest [42] and two cases of inhibition of pacing during the scan related to both EMI-induced output inhibition [43] and possibly the magnetohydrodynamic effect (i.e., the inhibition of pacing due to current induction from the to-and-fro motion of MRI induced charged ions contained in aortic blood) [44]. Future studies to examine the safety of preforming 3.0-T MRI in patients with implanted MRI-conditional cardiac devices are warranted.
The use of currently approved MRI-conditional devices excludes isocenter localization between C1 and T12 vertebra. The thoracic isocenter restriction could potentially decrease the image SNR and resolution. Since high SNRs are required for various thoracic scans, including assessment for myocardial viability, blood vessels, vascular malformation, and existence of shunts, it is highly important to study the safety of MRI-conditional devices with MRI isocenter located in the thorax and to characterize the extent of artifacts related to the implanted device system.
The population of patients that qualify for ICD and/or CRT devices, including those with CHD, is growing. Many such patients will benefit from an MRI scan at some point after the implant procedure. Hence, it is essential to develop MRI-conditional ICD and/or CRT systems. The “MR-pro” (Lumax 740, Biotronik, Germany) was recently approved in Europe. The data regarding these newly approved devices has not yet been published.
MRI-conditional technologies represent innovation that could facilitate the use of MRIs in patients who require pacemaker or ICD systems. The availability of such technology will surely benefit patients with CHD.Hence, additional studies are needed to investigate safety in the setting of higher static magnetic field strengths, serial MRI examinations, image isocenter located between C1 and T12, and abandoned or epicardial leads.
Acknowledgments
Dr. Nazarian is on the MRI advisory panel (unpaid) for Medtronic. Dr. Nazarian is a scientific advisor to and is principal investigator for research funding to Johns Hopkins University from Biosense Webster Inc. Dr. Nazarian is funded by the National Institutes of Health Career Development Grant K23HL089333.