Fig. 1
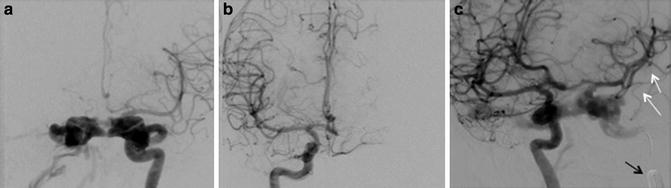
Venous drainage pathways. (a) Frontal projection and (b) lateral projection of a right internal carotid artery angiogram demonstrating a direct right-sided CCF with bilateral venous drainage via the circular sinus. Multiple venous drainage pathways are illustrated, including the left superior petrosal sinus (thin black arrows), left inferior petrosal sinus (thick black arrows), right superior ophthalmic vein (thin white arrows), right inferior ophthalmic vein (thick white arrows), right superficial middle cerebral vein (thin blue arrows), right deep middle cerebral vein (thin red arrow), and pterygoid venous plexus (thick red arrow)
Potential Routes of CCF Venous Drainage
Anterior drainage via the orbital veins
Eventually draining into angular and facial veins
Posterior drainage
Superior and inferior petrosal sinuses
Contralateral drainage
Circular sinus
Superior drainage
Superficial middle cerebral vein
Inferior drainage
Pterygoid venous plexus & emissary veins
Classification
CCFs can be categorized using several criteria [5, 13]. These include classifications systems based on etiology (i.e., traumatic vs. spontaneous), hemodynamics (high-flow vs. low-flow shunts), as well as angiographic criteria [1, 5, 8, 13]. The latter was characterized by Barrow et al. [5] and is based on both the hemodynamics of the shunt as well as the arterial supply to the fistula as demonstrated on angiography [5].
Type A CCFs are high-flow direct shunts between the ICA and the cavernous sinus (Fig. 2). Type B CCFs are dural shunts between meningeal branches of the cavernous ICA (arising from the meningohypophyseal or ILT trunks) and the cavernous sinus (Fig. 3). Type C CCFs are dural shunts between external carotid meningeal branches and the cavernous sinus (Fig. 4). Finally, type D CCFs are dural shunts with arterial supply from meningeal branches of both the ICA and ECA (Fig. 5) [5]. Rarely, a direct fistula between the ophthalmic artery and an ophthalmic vein can present in a similar fashion to a CCF, a so-called orbital shunt [1] (Fig. 6; Table 1).
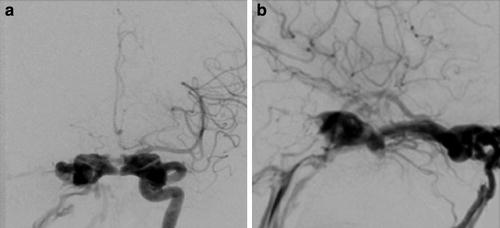
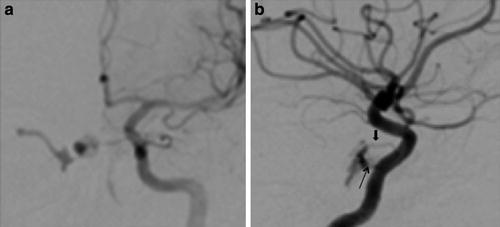
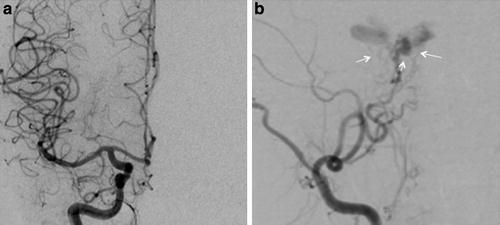
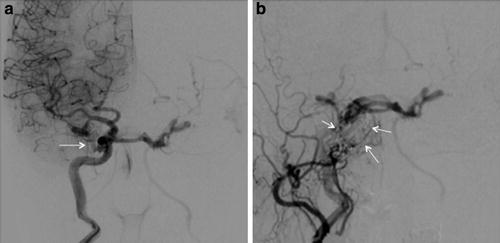
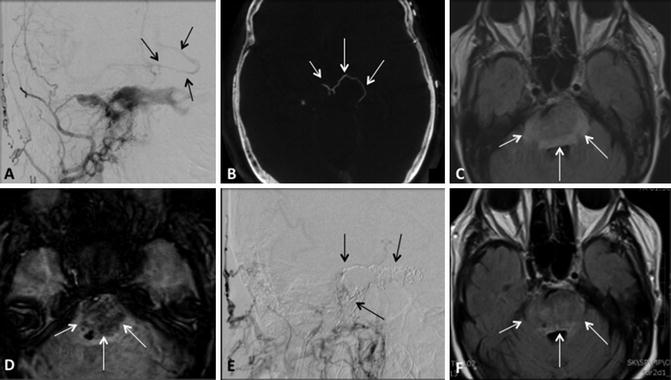

Fig. 2
Type A direct CCF. (a) Frontal projection and (b) lateral projection of a left internal carotid artery angiogram demonstrating a high-flow direct shunt between the left internal carotid artery and the ipsilateral cavernous sinus. Venous drainage is primarily via the circular and contralateral cavernous sinuses

Fig. 3
Indirect type B CCF. (a) Frontal projection and (b) lateral projection of a left internal carotid artery angiogram demonstrating an indirect CCF supplied by meningeal branches of the ICA arising from the meningohypophyseal trunk (thin black arrow) and inferolateral trunk (thick black arrow)

Fig. 4
Indirect type C CCF. (a) Frontal projection of a right internal carotid artery angiogram demonstrating no evidence of arteriovenous early cavernous shunting. (b) Frontal projection right external carotid artery angiogram demonstrating dural shunts between external carotid meningeal branches and the ipsilateral cavernous sinus (white arrows)

Fig. 5
Indirect type D CCF. (a) Frontal projections right internal carotid and (b) right external carotid artery angiograms demonstrating an indirect CCF with arterial supply from meningeal branches arising from both internal and external carotid arteries (white arrows)

Fig. 6
Cortical venous reflux. (a) Frontal projection right external carotid artery angiogram and (b) flat panel computed tomography demonstrates a type C indirect CCF with cortical venous reflux (black arrowsa, white arrowsb). (c) Axial FLAIR and (d) susceptibility MR imaging demonstrates a resulting hemorrhagic brainstem venous infarct (white arrowsc, d). (e) Lateral right external carotid artery angiogram and (f) axial FLAIR imaging following coil embolization demonstrates no residual arteriovenous shunting (black arrowse) as well as resolution of ischemic changes in the brain stem (white arrowsf)
Table 1
Barrow angiographic classification of carotid cavernous fistulas
Fistula type | Arterial supply | |
|---|---|---|
Type A | Direct | Cavernous ICA |
Type B | Indirect | Meningeal ICA branches |
Type C | Indirect | Meningeal ECA branches |
Type D | Indirect | Meningeal ICA and ECA branches |
Etiology and Epidemiology
Direct Fistulas: Direct CCFs often result from blunt or penetrating trauma that results in a tear in the cavernous segment of the ICA, with resulting rapid arteriovenous shunting into the cavernous sinus [8, 10, 14]. These lesions are most commonly found in young males, presumably due to the increased prevalence of head trauma in this population [13–15]. Debrun et al. [10] found that the arterial tear in traumatic fistulas can vary in size anywhere from 1 to 5 mm, and rarely multiple rents may be present. Despite the high-flow arteriovenous shunting associated with direct fistulas, patients often present in a somewhat delayed fashion, anywhere from days to a few weeks following the inciting trauma [16]. The mechanism of vessel injury in many of these cases is likely a sudden increase in intraluminal arterial pressure associated with the traumatic event [17]. Penetrating trauma is another possible cause, as well as rarely arterial injury during skull base surgery [14–16, 18]. Finally, traumatic direct CCFs have been associated with fractures involving the skull base, with Liang et al. [16] in a retrospective review reporting an 8.3 % incidence of direct fistulas in patients with fractures involving the middle cranial fossa, particularly those with a transverse or oblique orientation.
Direct CCF fistulas can also be encountered in patients without a history of preceding trauma [8, 19]. These lesions are often encountered in middle-age women, but are not exclusive to this age group [20]. These spontaneous direct fistulas have several potential etiologies, the most common of which is rupture of a cavernous segment ICA aneurysm [18, 19]. Cavernous ICA aneurysms represent somewhere between 1.9 % and 9 % of all intracranial aneurysms, are more commonly associated with CCF development as opposed to subarachnoid hemorrhage, and give rise to roughly 20 % of direct CCFs [14, 19, 21]. In a retrospective review of all cavernous ICA aneurysms presenting to a single medical center, Kupersmith et al. [21] found that 13 of 193 lesions were associated with a CCF (6.7 %). However, it is important to remember that a predisposing cavernous aneurysm may not be identified following direct fistula formation due to the presence of high-flow arteriovenous shunting [22].
Etiologies of Direct Carotid Cavernous Fistulas
Traumatic
Blunt
Penetrating
Iatrogenic
Spontaneous
ICA aneurysm rupture
Collagen vascular disorders
Ehlers-Danlos type IV syndrome
Pseudoxanthoma elasticum
Fibromuscular dysplasia
Minor episode of trauma or valsalva
Coughing and sneezing
An additional cause of spontaneous direct CCFs includes various genetic syndromes that can weaken the arterial wall and predispose to rupture after minor trauma or episodes of valsalva, including coughing or sneezing [1, 18, 19]. Specific genetic conditions that can affect vascular wall structural integrity and have been associated with CCF include fibromuscular dysplasia, Ehlers-Danlos type IV syndrome, and pseudoxanthoma elasticum [23–25]. Finally, a direct CCF can be associated with a persistent trigeminal artery extending from the cavernous ICA to the basilar artery [22]. These so-called trigemino-cavernous fistulas may arise either from an inherent weakness in the aberrant vessel wall or due to aneurysm formation at the ICA-trigeminal artery branch point [22].
Indirect or Dural Fistulas: Indirect CCFs represent a subset of dural arteriovenous fistulas involving the cavernous sinus, which receive blood flow from meningeal branches of the internal and/or external carotid arteries that normally supply the cavernous sinus dura [2, 26]. These lesions most often occur spontaneously in postmenopausal women [15, 26, 27]. However, as is the case with direct CCFs, these lesions may present in all age groups, including children and infants, and can develop following minor trauma or episodes of valsalva [12]. Altogether, dural arteriovenous fistulas represent approximately 10–15 % of intracranial vascular malformations [28]. Potential arterial feeders to indirect CCFs include branches of the external carotid artery, such as the internal maxillary, middle meningeal, accessory meningeal, and ascending pharyngeal arteries, as well as internal carotid artery branches [26]. The latter include meningohypophyseal, capsular, inferolateral trunk arteries, as well as ethmoidal branches arising from the ophthalmic artery [26].
As is the case with dural arteriovenous fistulas found elsewhere in the intracranial compartment, the etiology of indirect CCF remains uncertain [12, 15]. One theory speculates that these fistulas may result from breakdown in small thin-walled dural arteries that normally cross the cavernous sinus [12]. Alternatively, venous thrombosis and/or elevated venous pressure may result in the opening of normally closed, small anastomotic channels in the dura [8, 12]. Risk factors for the development of these dural fistulas include atherosclerosis, hypertension, diabetes, sinusitis, pregnancy, and collagen vascular disease [8, 27, 29].
Etiology of Indirect Carotid Cavernous Fistulas
Uncertain, two possible mechanisms
Breakdown of small thin-walled dural arteries that transverse the cavernous sinus
Opening of small anastomotic dural channels
Venous sinus thrombosis
Elevated venous pressure
Risk factors
Atherosclerosis, diabetes, hypertension, and pregnancy
Clinical Presentations
The majority of symptomatic CCFs have anterior drainage via the ophthalmic veins, with resulting reflux of high-pressure arterial blood into the ipsilateral orbit [3, 12, 15]. This arteriovenous shunting results in orbito-ocular congestion and is responsible for the classic clinical triad associated with CCFs, namely, pulsatile exophthalmos, orbital bruit, and chemosis of the ipsilateral globe [1, 3, 4, 14, 19]. The contralateral orbit may also be affected due to reflux of blood across the circular sinus, occasionally in the absence of ipsilateral orbital symptoms depending on the pattern of venous drainage [1, 9, 12]. Other signs and symptoms of orbito-ocular congestion include medically refractory glaucoma, ophthalmoplegia, retro-orbital pain, vision loss, as well as dilatation and arterialization of conjunctival and episcleral veins on ophthalmologic exam [1, 12]. Although this latter finding may be seen in the setting of other diseases such as conjunctivitis, a tortuous corkscrew appearance of these vessels is highly specific for the diagnosis of CCF [12]. General differential considerations for physical exam findings suggestive of orbito-ocular congestion include vascular malformations involving the orbit or cavernous sinus, cavernous sinus thrombosis, as well as inflammatory processes such as scleritis, with vortex vein blockage [1].
Some CCFs may present without classic symptoms, depending on the pattern of venous drainage as well as the degree of arteriovenous shunting [3, 11, 15, 18, 28]. For example, low-flow indirect CCFs with exclusively posterior venous drainage characteristically lack signs and symptoms of orbito-ocular congestion, including pulsatile exophthalmos and orbital bruit [3, 4, 12, 29, 30]. Instead, these lesions often are either asymptomatic or associated with nonspecific symptoms including headache, tinnitus, trigeminal neuropathy, facial nerve palsy, or isolated oculomotor palsy due to the involvement of the corresponding cranial nerves [11, 12, 30]. In these instances, patient symptoms may be due, at least in part, to brainstem congestion resulting from the posterior venous drainage [12, 31]. The atypical presentation of these posteriorly draining fistulas often leads to a delay in their diagnosis, which is unfortunate given the association between drainage via the superior petrosal sinus and cortical venous reflux, a high-risk feature discussed subsequently in this chapter [1, 4].
Clinical Presentation of Carotid Cavernous Fistulas
Fistulas with anterior drainage via orbital veins
Pulsatile exophthalmos
Chemosis
Glaucoma
Vision loss
Ophthalmoplegia
Fistulas with exclusively posterior drainage via petrosal sinuses
Asymptomatic
Headache
Tinnitus
Trigeminal neuropathy
Isolated oculomotor palsy
Finally, symptom progression in CCFs is often determined by the degree of associated arteriovenous shunting, with high-flow lesions often presenting acutely with rapid deterioration, while low-flow fistulas may demonstrate an insidious onset and subsequent benign course [1, 15]. Low-flow shunts are most often encountered in the setting of an indirect, dural CCF, although direct lesions may also behave in a similar manner if the tear in the cavernous ICA is relatively small or if there is partial thrombosis of the involved cavernous sinus [1]. Recurrent fistulas following treatment may also present insidiously, again depending on the amount of residual arteriovenous shunting and available venous drainage pathways [22].
Complications and High-Risk Features
Ophthalmologic complications of CCFs include vision loss, ophthalmoplegia, and medically refractory glaucoma [1, 12]. Ophthalmoplegia can result from either entrapment of the extraocular muscles due to swelling of these structures or due to cranial nerve palsy secondary to mechanical compression of the corresponding nerve(s) [1, 12]. Glaucoma in CCFs may develop from high orbital venous pressure, congestion of the choroid or iris, displacement of the iris-lens diaphragm, or neovascularity secondary to chronic ischemia [12]. Finally, loss or deterioration of vision in the involved eye can be secondary to venous stasis retinopathy with resulting retinal ischemia, secondary glaucoma with optic nerve damage, or spontaneous choroidal detachment [1, 11, 12]. Vision loss is more commonly seen with direct CCF, although this finding may be encountered in as many as 20–30 % of indirect fistulas, particularly chronic lesions [12].
Ophthalmologic Complications of Carotid Cavernous Fistulas
Glaucoma
Elevated orbital venous pressure
Choroid or iris congestion
Iris-lens diaphragm displacement
Neovascularity
Ophthalmoplegia
Extraocular muscle swelling
Cranial nerve palsy
Vision loss
Venous stasis retinopathy
Glaucoma with optic nerve damage
Spontaneous choroidal detachment
Additional complications of CCFs include intracranial hemorrhage, both intraparenchymal and subarachnoid, venous infarct, epistaxis (which may be fatal), as well as increased intracranial pressure with venous hypertension [18, 26, 32]. Intraparenchymal hemorrhage is often a result of reflux of high-pressure arterialized blood into cortical veins overlying adjacent brain parenchyma [1, 15, 18]. Posterior venous drainage of a CCF via the superior petrosal sinus has been associated with the development of this high-risk feature and may be precipitated by the spontaneous thrombosis or iatrogenic occlusion of alternative venous drainage pathways [1, 4, 15, 18]. Overall, cortical venous reflux has been reported in anywhere from 10 % to 55 % of CCFs, is often symptomatic due to increased intracranial pressure, and carries up to a 30–40 % chance of hemorrhagic stroke if untreated [15, 18, 26, 33]. Furthermore, the reported annual mortality rate of intracranial dural arteriovenous fistulas with cortical venous reflux, regardless of location, is 10.4 % [33].
Subarachnoid hemorrhage from a CCF has been associated with the development of a cavernous sinus varix or pseudoaneurysm (Fig. 7) [18]. In a retrospective review of the angiographic features of 155 patients with CCFs, Halbach et al. [18] found that cavernous sinus venous varix was present in three of four patients who presented with subarachnoid hemorrhage, all of which were fatal. Although cavernous sinus varices were encountered in patients without intracranial hemorrhage, the authors argued the risk of fatal subarachnoid hemorrhage warranted emergent fistula treatment with this finding is present [18]. Finally, drainage into the sigmoid and transverse sinuses has been associated with elevation of intracranial pressure from resulting venous hypertension [18].
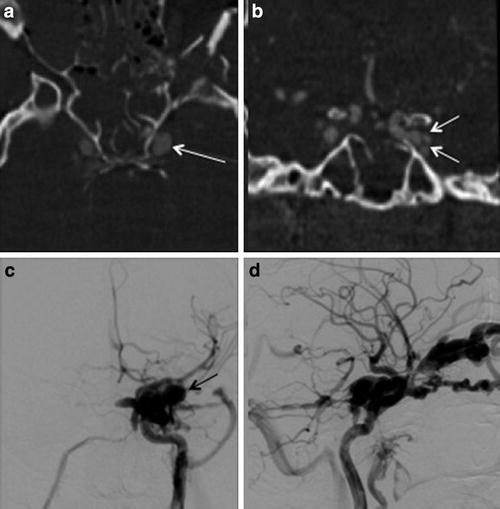

Fig. 7
Cavernous sinus pseudoaneurysm. (a) Axial and (b) coronal reconstructed CT angiography images show an ectatic left cavernous sinus (white arrowa) with multiple lateral projecting outpouchings (white arrowsb), which may represent possible pseudoaneurysm and/or draining venous tributaries. (c) Frontal projection and (d) lateral projection of a left internal carotid artery angiogram demonstrate a direct CCF with associated pseudoaneurysm (black arrowc)
High-Risk Features of Carotid Cavernous Fistulas
Cortical venous reflux
Between 10 % and 55 % of CCFs
Up to 30–40 % risk of hemorrhagic stroke untreated
Associated with superior petrosal sinus drainage
Can arise from blockage of venous drainage pathways
Subarachnoid hemorrhage
Rare, but often fatal
Associated with cavernous sinus varix
Drainage into sigmoid and transverse sinuses
Risk of elevated intracranial pressure from resulting venous hypertension
Imaging Evaluation
Noninvasive Cross-Sectional Imaging: Although many patients with a CCF present with classic signs and symptoms suggestive of the diagnosis, others may demonstrate either atypical symptomatology and/or an insidious, slowly progressive course [26]. In these instances, conventional MR, time-of-flight (TOF) and contrast-enhanced MR angiography (MRA), Doppler ultrasound, as well as CT angiography (CTA), may be used to screen patients for the presence of an arteriovenous fistula involving the cavernous sinus [34–36]. Conventional MR imaging finding suggestive of a CCF includes prominent flow voids in the involved cavernous or inferior petrosal sinuses on spin-echo sequences, dilated intercavernous venous channels, as well as the sequelae of orbito-ocular congestion including enlargement of the superior ophthalmic vein and extraocular muscles and proptosis [37–39]. However, these findings may only be present in high-flow fistulas, with conventional MR imaging being relatively insensitive for lesions demonstrating less rapid arteriovenous shunting [40].
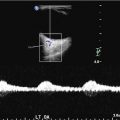
3D TOF MRA can increase the sensitivity for detection of CCF by depicting flow-related enhancement in the involved cavernous sinus as well as arterial feeders in the setting of an indirect fistula [35, 37, 40]. It is important to note however that venous flow signal can be normally seen in the cavernous and inferior petrosal sinuses on 3D TOF MRA in the absence of a CCF [41]. In addition, 3D TOF MRA is limited by the lack of temporal resolution, which precludes full characterization of dynamic shunting lesions such as a CCF [40]. However, time-resolved contrast-enhanced MRA can help to overcome some of these challenges by imaging the suspected diseased cavernous sinus during passage of a gadolinium contrast bolus [40]. This technique has been shown to be both sensitive and specific for the diagnosis of CCF and is a promising tool both for screening patients as well as surveillance for fistula recurrence following treatment (Fig. 8) [40].
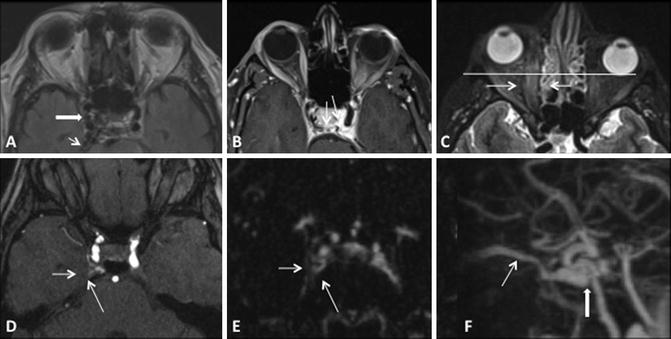

Fig. 8
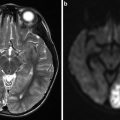
Conventional MR and MRA finding suggestive of a right CCF. (a, b) Prominent flow voids are noted on spin-echo sequences in the right cavernous and inferior petrosal sinuses (white thick and thin arrows respectively a) as well as dilated intercavernous venous channels (thin black arrowsb). (c) Sequelae of orbito-ocular congestion are present in the ipsilateral orbit with enlargement of the extraocular muscles (white thin arrows) and proptosis (horizontal white line). (d) Post-contrast MRA demonstrates enhancement of the involved cavernous sinus (white arrows). (e, f) Time-resolved contrast-enhanced MRA demonstrates early opacification of right cavernous sinus (thin white arrowe) as well as the fistulous shunt and drainage via the superior ophthalmic vein (thick and thin white arrows, respectively f)
CT angiography has also been demonstrated to be a promising technique for screening patients suspected of having a CCF [42]. With the availability of 256 and 320 slice CT scanners, excellent quality dynamic studies can be performed with high spatial and acceptable temporal resolution. CT and CT angiography findings of a CCF include an enlarged cavernous sinus that demonstrates early enhancement in the arterial phase, as well as dilatation of draining venous tributaries (e.g., superior ophthalmic vein) [42, 43]. Chen et al. [37] performed a retrospective study comparing noninvasive 3D TOF MRA and CTA to the gold standard of catheter angiography. They found that CTA performed better at detecting CCF than TOF MRA, particularly for fistula involving the more proximal aspect of the cavernous ICA (Table 2).
Table 2
Noninvasive imaging modalities for evaluation of CCFs
Modality | Findings | Potential disadvantages |
|---|---|---|
MRI | Flow voids, cavernous and petrosal sinuses | Insensitive for moderate- to low-flow fistulas |
Distension of the cavernous sinus | ||
Dilated intercavernous channels | ||
Sequelae of orbito-ocular congestion | ||
TOF MRA | Flow-related enhancement in cavernous sinus | Not 100 % specific |
No temporal resolution | ||
CE MRA | Flow-related enhancement in cavernous sinus | Improved temporal resolution |
May not detect high-risk features | ||
CTA | Distension of the cavernous sinus | Poor temporal resolution |
Early sinus enhancement in arterial phase | May not detect high-risk features | |
Dilatation of draining venous tributaries |
Catheter Angiography: Despite advances in cross-sectional imaging of CCF, catheter angiography remains the gold standard for the diagnosis and characterization of these lesions due to its superior spatial and temporal resolution [26, 40]. The diagnosis of a CCF is readily made on catheter angiography by the demonstration of abnormal arteriovenous shunting into the cavernous sinus from either the ipsilateral cavernous ICA or meningeal branches to the sinus wall in the case of an indirect fistula. Goals of catheter angiography when evaluating a CCF include the identification of the exact site of fistulization, evaluation of the degree of arteriovenous shunting, determination of arterial supply to the lesion, evaluation of the pattern of venous outflow, the presence of high-risk features including cortical venous reflux and venous varix, potential dangerous external or internal carotid artery anastomoses, as well as the presence of atherosclerotic disease if carotid compression is contemplated as a potential treatment [26].
Catheter Angiography Evaluation of Carotid Cavernous Fistulas
Determination of type of fistula
Arterial supply with indirect lesions
Localization of fistulous tear with direct lesions
Evaluation of degree of arteriovenous shunting
Analysis of venous drainage pathways
Identification of high-risk features, including cortical venous reflux
Huber’s Maneuver: In the setting of a high-flow CCF, the tremendous arteriovenous shunting may obscure the underlying ICA tear, and the carotid artery more distally may not fill due to essentially complete diversion of blood flow into the cavernous sinus [2, 10, 22]. In these instances, injection of a vertebral artery during manual compression of the ipsilateral ICA may allow for better characterization of both the location and the size of the fistulous communication (Fig. 9) [10, 44]. This technique works by allowing a limited amount of contrast to reach the shunt via a posterior communicating artery with subsequent retrograde flow down the ipsilateral supraclinoid ICA [10]. Other maneuvers that can help delineate the fistula size and location include very high frame rate imaging and 3D subtraction angiography. Finally, C-arm angiographic CT (flat panel rotational computed tomography) is another exceptional technique for locating and studying the anatomy of fistula.
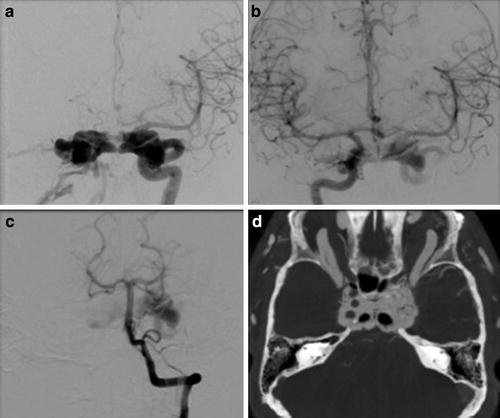

Fig. 9
Huber maneuver. (a) Frontal projection left internal carotid artery angiogram demonstrates a direct CCF with the high-flow arteriovenous shunting obscuring the underlying tear in the carotid artery. (b) Injection of the contralateral ICA or (c) vertebral artery during manual or balloon compression of the ipsilateral ICA (Huber’s maneuver) may allow for better characterization of both the location and the size of the fistulous communication. (d) C-arm angiographic CT (flat panel rotational computed tomography) is also a exceptional technique for locating and studying the anatomy of fistula as well
Balloon Occlusion Test (BOT): As a significant minority of direct CCFs may require ipsilateral ICA sacrifice for successful closure, performing a balloon occlusion test during the diagnostic workup can provide invaluable information to the treating physician (Fig. 10). First, a guide catheter is placed in the ipsilateral common carotid artery. Next, following full heparinization, a soft, compliant balloon (typically a HyperForm or HyperGlide Occlusion Balloon, ev3, Irvine, California) is navigated into the cervical ICA proximal to the fistula. The balloon is then carefully inflated under fluoroscopic imaging, and a gentle injection is performed through the guide catheter to confirm vessel occlusion. Continuous neurologic monitoring, including testing of patient speech and contralateral strength, is then performed for a total of 30 min. The test is immediately stopped if the patient develops symptoms suggestive of ischemia involving the ipsilateral cerebral hemisphere. The patient can be further challenged during a balloon occlusion test by purposefully dropping the systolic blood pressure by 30 %, although all operators do not routinely perform this maneuver. Finally, collateral flow to the affected vascular territory via the Circle of Willis (i.e., an angiographic balloon occlusion test) can be performed during balloon inflation by injection of the contralateral carotid and vertebral arteries using a second diagnostic catheter.