Fig. 1
Normal anteroposterior (AP) chest radiograph in a neonate. Symmetrically inflated lungs are seen with sharp costophrenic angles. The configuration of the cardiothymic silhouette is normal
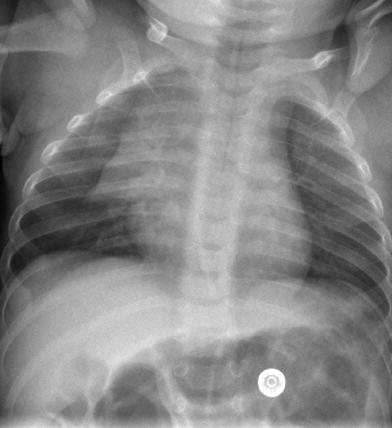
Fig. 2
Thymic wave sign. Undulating lateral border of the soft thymus from deformation by the overlying ribs. The thymus also projects like a sail away from the other mediastinal structures
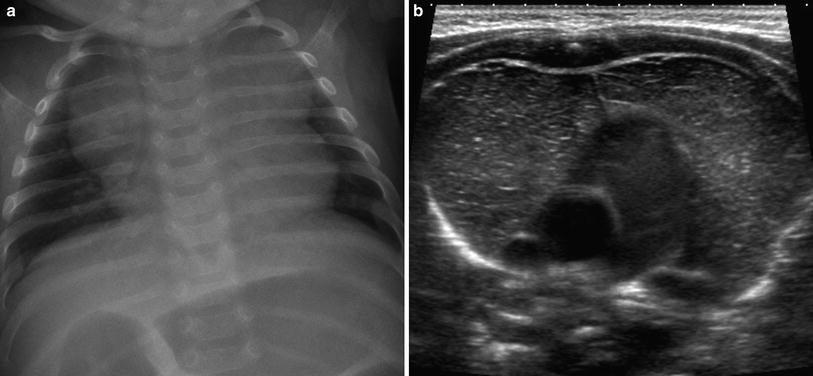
Fig. 3
Prominent thymus confirmed with ultrasound. a Neonate with a prominent lobulated mediastinal contour on a chest radiograph. b Ultrasound demonstrates a normal thymus with the typical appearance of echogenic lines and punctate foci on a hypoechoic stroma
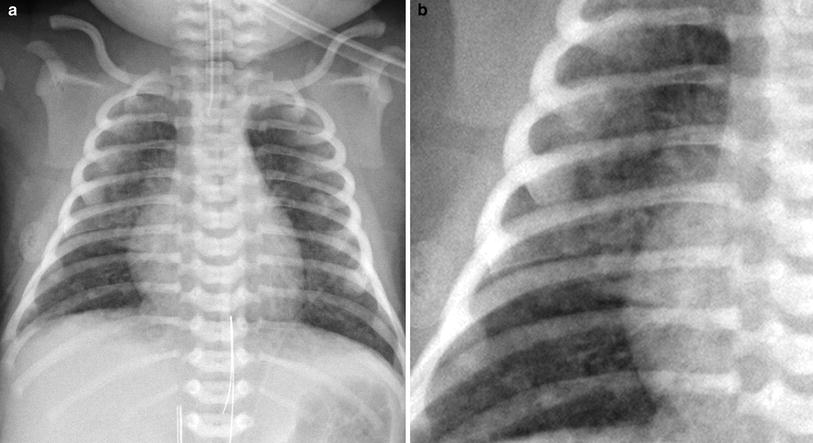
Patients in the neonatal intensive care unit often have catheters and tubes and evaluation of this support apparatus is an important part of reviewing chest radiographs in these infants. Review should include not only the chest cavity, but also the osseous structures and the soft tissues of the chest wall. The upper abdomen should also be reviewed for comorbid conditions such as necrotizing enterocolitis, a common disease in patients with respiratory distress syndrome (RDS). The signs of this condition include pneumatosis intestinalis, portal venous gas, and free intraperitoneal air, all of which may be identified on a chest radiograph (Fig. 4). Abdominal heterotaxy with malposition of the stomach and liver can be accompanied by asplenia or polysplenia and is strongly associated with congenital heart disease. The osseous structures need to be evaluated for vertebral and rib abnormalities. The Vertebral anomalies, Anal atresia, Cardiac anomalies, Tracheoesophageal abnormalities, Renal anomalies, and Limb anomalies (VACTERL) association may be suggested by a dilated esophagus, absent bowel gas, and vertebral anomalies (Fig. 5). Rib anomalies can be associated with conditions with restrictive physiology such as asphyxiating thoracic dystrophy and thanatophoric dwarfism. In cases where the gestational age is unknown or where the provided history is limited, examination of the humeri may help, as the proximal humeral epiphysis is ossified in 80 % of term infants.
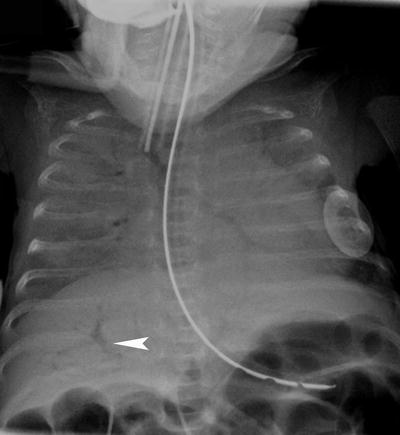
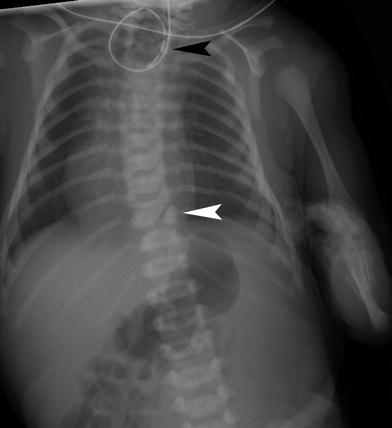

Fig. 4
Abdominal pathology on a chest radiograph. Chest radiograph in a 3 week old former 27 week gestation premature infant with necrotizing enterocolitis demonstrating branching lucent structures in the liver (arrowhead) consistent with portal venous gas

Fig. 5
VACTERL association. Notice the vertebral segmentation anomaly (white arrowhead). An enteric tube is coiled in the atretic proximal esophagus (black arrowhead) and there is gas in the bowel consistent with esophageal atresia with a tracheoesophageal fistula. The patient also has a deformity of the left upper extremity
4 Medical Disease
4.1 Transient Tachypnea of the Newborn
Transient tachypnea of the newborn (TTN), also known as “wet lung,” is a common cause of respiratory distress in infants who are either near-term, term, or postterm. It is secondary to retention of fetal fluid in the newborn’s lung and occurs with an incidence of 5.9 % (Tutdibi et al. 2010).
The production of fetal lung fluid decreases as gestation approaches full term. The remaining fluid is cleared from the lungs during labor by active resorption of fluid from the air spaces through epithelial sodium channels. Hormones, including epinephrine, glucocorticoids, and thyroid hormones released during the stress of labor, as well as inhaled oxygen in the postnatal period trigger this resorptive process (Barker and Olver 2002). The removal of lung fluid is practically completed by 2 h of life (Aherne and Dawkins 1964). If the epithelial sodium transport mechanism is immature or the lungs are not exposed to the stress of labor, fluid resorption may be inadequate. The retained fluid in the interstitium leads to decreased compliance of the lungs. The majority of this fluid is removed from the interstitium via the pulmonary veins and lymphatics (Humphreys et al. 1967; Bland et al. 1982). Epinephrine has also been demonstrated to cause the release of surfactant (Lawson et al. 1978), which increases pulmonary compliance and decreases the effect of any retained fluid. Pressure transmitted to the lungs by compression of the chest wall during delivery with resultant clearance of fetal lung fluid via the tracheobronchial tree, the so-called “vaginal squeeze” contributes minimally to removal of this fluid.
Transient tachypnea of the newborn is seen more often in patients that are born by cesarean section, especially elective cesarean section without preceding labor. It is also seen more commonly in infants of mothers who have been sedated, in infants of diabetic mothers with poor glucose control and in infants of mothers with asthma, in large or small for gestational age infants, and in male infants (Schatz et al. 1991; Persson and Hanson 1998; Tutdibi et al. 2010). The severity of TTN also correlates with elective cesarean section and shorter duration of labor as well as lower gestational age (Tutdibi et al. 2010).
Infants with TTN present with signs of respiratory distress and they may require supplemental oxygen. More severe complications including pneumothorax, the need for positive pressure ventilation or extracorporeal membrane oxygenation, and death have been recorded (Ramachandrappa and Jain 2008). The condition is usually self-limiting, most patients recovering in 24–72 h, but occasionally patients need longer to become asymptomatic. The majority of patients with TTN recover fully without long-term morbidity, although some studies have shown an increased incidence of childhood asthma in these patients (Birnkrant et al. 2006).
Transient tachypnea of the newborn is a clinical diagnosis and radiographs of the chest are primarily performed in these infants to exclude other causes of respiratory distress. On imaging, the lungs are well inflated. Symmetrically prominent interstitial opacities radiating from the hila are most commonly seen (Fig. 6). Diffuse fine granular opacities may be present bilaterally and can be confused with RDS if attention is not paid to the patient’s gestational age and the degree of inflation. Small pleural effusions may be evident, another finding not seen in RDS (Fig. 7). A follow up radiograph is usually not indicated as the patients clinically improve, but if performed it demonstrates rapid clearance of the radiographic abnormalities (Swischuk 1970; Wesenberg et al. 1971).
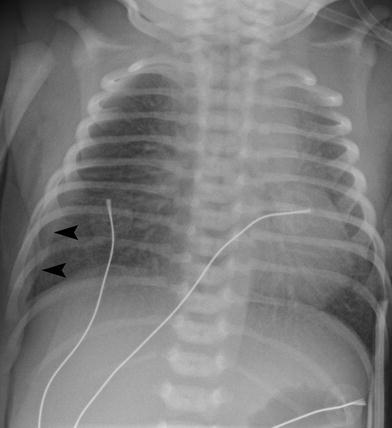
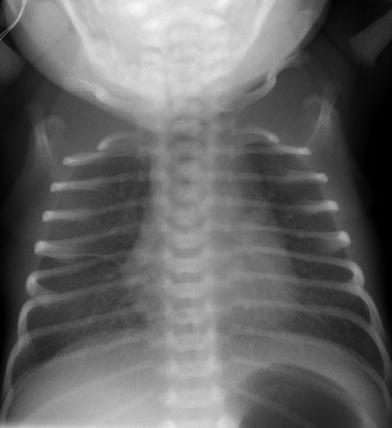

Fig. 6
Term infant with transient tachypnea of the newborn. The lungs are well inflated with prominent interstitial opacities radiating from the hila. There is a small right pleural effusion (arrowheads)

Fig. 7
Term infant with transient tachypnea of the newborn. The appearance could be confused with respiratory distress syndrome as there are fine granular opacities. However, the lungs are well inflated and there is a small amount of pleural fluid along the minor pulmonary fissure
4.2 Respiratory Distress Syndrome
Respiratory Distress Syndrome (RDS) is a disease of neonates resulting from surfactant deficiency. RDS is primarily a disease of premature infants born at less than 36 weeks of gestation, although it can rarely be seen in term infants and also in infants of diabetic mothers with poor glucose control. Other factors that increase the risk of developing RDS include intrapartum asphyxiation, multiple gestation births, sepsis, and maternal and fetal hemorrhage. Male and Caucasian infants have an increased incidence of RDS (Anadkat et al. 2012). The lower the gestational age, the greater the incidence of RDS; it occurs with an incidence of 93 % in infants less than 28 weeks gestation (Stoll et al. 2010), 10 % at 34 weeks, and 0.3 % at greater than 38 weeks gestation (Hibbard et al. 2010).
Alveoli begin to form during the later part of the canalicular stage of lung development, at about 24–28 weeks gestation (Agrons et al. 2005) and are lined by Type II pneumocytes which express surfactant, a complex lipoprotein, into the alveoli. Surfactant becomes activated when it combines with surface surfactant proteins which are also produced by Type II pneumocytes. This activated surfactant reduces surface tension within the alveoli, not only preventing them from collapsing but also allowing collapsed alveoli to expand with less applied force. In preterm infants, an insufficient amount of surfactant results in collapse of the alveoli and atelectasis with resultant development of hypoxia and acidosis. An inflammatory reaction ensues, often exacerbated by oxygen therapy and barotrauma from positive pressure ventilation. This inflammation results in damage to both the respiratory epithelium and capillary endothelium with loss of integrity of these structures. Interstitial edema ensues along with formation of a membrane along the terminal bronchioles and alveolar ducts (Stocker 1992). These hyaline membranes prevent gas exchange and result in ventilation–perfusion mismatching, leading to hypoxia and acidemia. Over time this can lead to arteriolar vasoconstriction and pulmonary hypertension.
Clinically, infants become symptomatic soon after birth and develop progressive worsening of symptoms over the first 48 h of life with signs of respiratory distress. The increased effort required for breathing leads to fatigue often necessitating support with positive pressure ventilation. The natural history for the majority of infants with uncomplicated RDS is gradual improvement as endogenous surfactant production is induced. Superimposed neonatal pneumonia, a patent ductus arteriosus, persistent pulmonary hypertension, and sepsis can all lead to prolongation of symptoms and a more complicated clinical course, including the development of an air leak. Long term, the infants are at risk of developing bronchopulmonary dysplasia.
The administration of steroids to the expectant mother for 12–36 h while in preterm labor has been shown to accelerate lung maturation in fetuses over 28 weeks gestation with decreased severity of RDS (Liggins and Howie 1972). A similar decrease in the severity of RDS has not been shown to occur in infants born between 24 and 28 weeks gestation following prenatal steroid administration to the mother (Garite et al. 1992). However, infants born at this gestation have been shown to have a reduced incidence of other diseases of prematurity following steroid administration, such as necrotizing enterocolitis, high-grade intracranial hemorrhage, and also decreased mortality (Crowley et al. 1990). A further reduction in the severity of RDS can be achieved by the administration of exogenous surfactant to the neonate via an endotracheal tube (Suresh and Soll 2005). Exogenous surfactant therapy results in decreased surface tension in the alveoli and subsequent lessening of respiratory symptoms as the work of breathing lessens. In addition, the infant does not require as high concentrations of therapeutic oxygen or as high positive airway pressures if they are being mechanically ventilated.
The classic description of RDS on radiographs is diffuse fine granularity bilaterally (Fig. 8), effacement of normal pulmonary vascularity, and central air bronchograms. Untreated lungs are low in volume due to the diffuse acinar collapse (Fig. 9), although this is less commonly seen now as patients are usually intubated, receive positive pressure ventilation and are administered surfactant prior to an initial radiograph. Occasionally this may be more severe with near complete whiteout of the lungs (Fig. 10). Following administration of surfactant and application of positive airway pressure, these appearances may change, with improved lung volumes and clearance of the diffuse granularity (Fig. 11). This improvement can be rapid and can result in complete clearance, partial and symmetric clearance, or patchy and asymmetric clearance (Dinger et al. 1997; Slama et al. 1999), which may reflect uneven distribution of the administered surfactant, insufficient surfactant administration, and regional differences in aeration (Slama et al. 1999) (Fig. 12). This uneven clearance can mimic other entities such as neonatal pneumonia. Over time, the radiographic appearance may reflect complications of RDS and prematurity such as an air leak phenomenon, superimposed infection, chronic lung disease related to the surfactant deficiency, i.e., bronchopulmonary dysplasia, persistent pulmonary hypertension, and edema. This edema can sometimes be severe and hemorrhagic and is secondary to a left-to-right shunt through a patent ductus arteriosus. This can occur rapidly as pulmonary arterial blood pressure decreases (van Houten et al. 1992). With treatment, localized hyperinflation can rapidly occur, mimicking air leaks (Cleveland 1995).
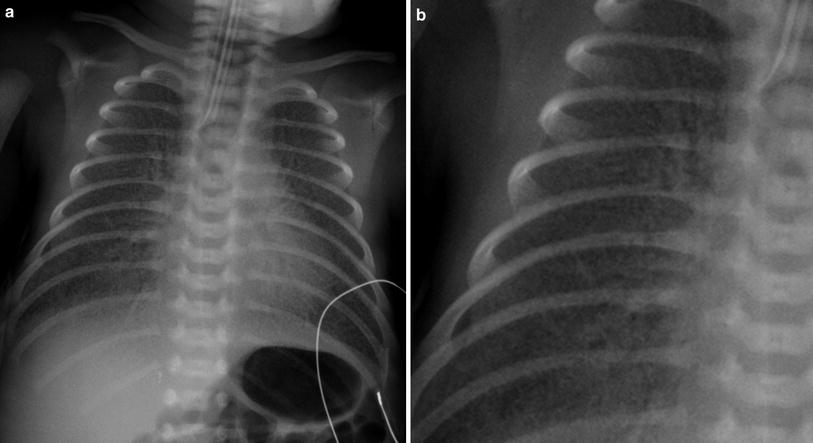
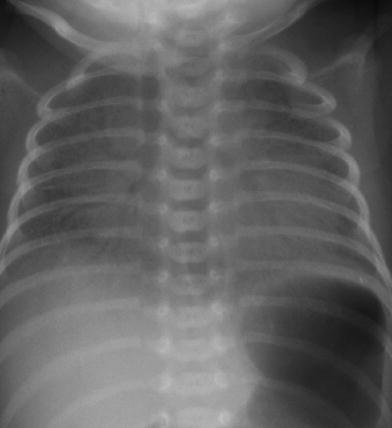
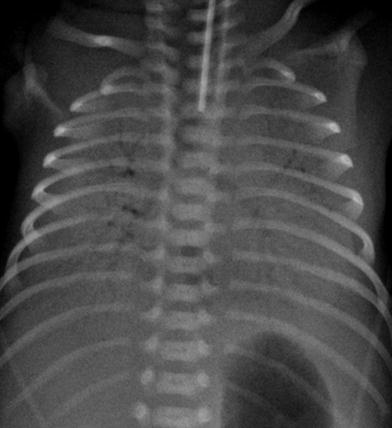
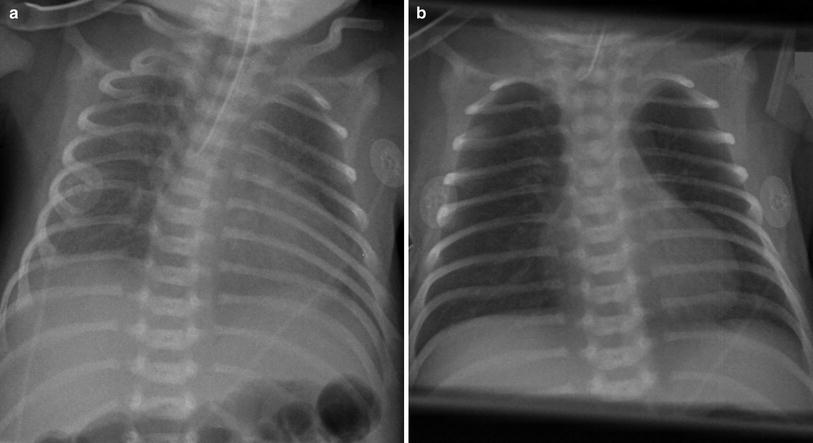
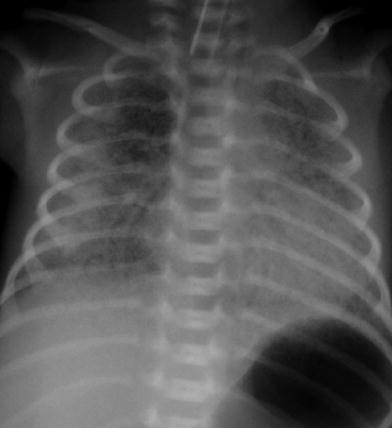

Fig. 8
Newborn with respiratory distress syndrome. a There are hazy opacities diffusely distributed bilaterally. b The granular nature of these opacities is well seen on the magnified image

Fig. 9
Newborn with untreated respiratory distress syndrome. The lungs are low in volume with increased density bilaterally and subtle central air bronchograms

Fig. 10
Newborn with a “whiteout” appearance from respiratory distress syndrome. The lungs are densely opacified, obscuring the cardiac and diaphragmatic contours, and there are prominent air bronchograms bilaterally

Fig. 11
Effect of surfactant administration. a Initial radiograph of a newborn with respiratory distress syndrome (RDS) taken following intubation. Fine granular opacities are present bilaterally. b Radiograph taken 12 h later demonstrates rapid clearing of these opacities following the administration of surfactant

Fig. 12
Newborn premature infant with uneven aeration following the administration of surfactant. There is relative lucency in the right upper lobe and relatively increased density in both lung bases
Very immature infants, less than 27 weeks gestation and weighing less than 1,000 g, may develop mild haziness bilaterally which clears with surfactant therapy. Subsequently, these infants may again develop mild diffuse haziness bilaterally, thought to reflect seepage of lung fluid into the interstitium through damaged arteriolar basement membranes, the so-called “leaky lung” (Swischuk et al. 1996). Because these infants are extremely immature their lungs are hypoplastic with a deficient number of alveoli and as a result they often require prolonged positive pressure ventilation and high oxygen concentrations. The resultant barotrauma can lead to the early development of coarse irregular reticular opacities bilaterally consistent with chronic lung disease (Cleveland 1995) (Fig. 13).
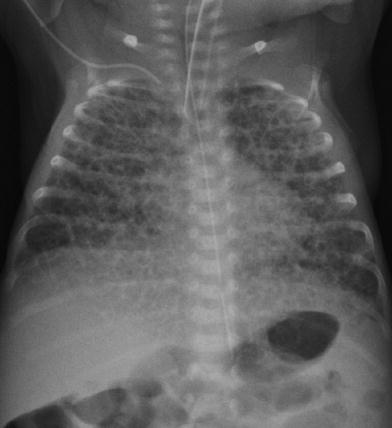

Fig. 13
Early development of chronic lung disease in a premature infant who is now 10 days of age. The lungs are hyperinflated and there are coarse reticular opacities bilaterally with intervening lucencies. These findings are typical of chronic lung disease
Respiratory Distress Syndrome was formerly known as Hyaline Membrane Disease, a term that is no longer favored as hyaline membranes are seen in other neonatal lung diseases and are not specific to RDS. It has been suggested that RDS is also a nonspecific descriptive term that could describe the clinical appearance of a number of respiratory illnesses and it has been proposed that the term Surfactant Deficiency Disease be used as this would more accurately reflect the underlying etiology (Swischuk and John 1996).
4.3 Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is a chronic disorder of the lungs most commonly seen in low birth weight infants who were born prematurely. The pathogenesis of BPD is not completely understood but is complex and multifactorial in origin. Aside from prematurity, mechanical ventilation, and oxygen therapy, a number of other factors are known to contribute to the development of BPD including a patent ductus arteriosus or fluid overload, inflammation (alone or associated with infection), poor nutrition, and genetics (Bancalari et al. 2003). Infection may result from chorioamnionitis, may be acquired during delivery, or may be nosocomial.
Bronchopulmonary dysplasia was originally described as a disease of premature infants who were treated with oxygen and mechanical ventilation (Northway et al. 1967). The effects from the surfactant deficiency coupled with the ventilator-induced lung injury and the further damage from oxygen toxicity resulted in changes of alveolar septal fibrosis, alveolar cell hyperplasia, bronchiolar squamous metaplasia, and necrotizing bronchiolitis (Stocker 1986; Husain et al. 1998). This resulted in heterogeneous airway and parenchymal disease with hyperinflated but otherwise normal alveoli alternating with alveoli that are scarred to varying degrees along with smooth muscle hypertrophy and airway damage.
The patient population in this original description of BPD presented with severe RDS, had an average weight of 1,894 g and a gestational age of 33 weeks (Northway et al. 1967). Radiographically, this “old” BPD has four stages of development. Stage I (at 2–3 days) has changes of RDS with a diffuse granular pattern and central air bronchograms. Stage II (at 4–10 days) demonstrates almost complete opacification bilaterally. Stage III (10–20 days) shows small rounded lucencies with intervening areas of irregular opacification, while Stage IV (>1 month) has further enlargement of the lucent areas and thinning of the intervening linear opacities with hyperinflation of the lungs (Northway and Rosan 1968). These stages are less commonly seen today.
With modern therapy, a similar group of patients to that described in the initial description of BPD would have an excellent prognosis and would be unlikely to develop BPD. However, the incidence of BPD is not decreasing. This reflects significantly increased survival rates of neonates of less than 28 weeks gestation. Now the population that most commonly develops BPD has a gestational age of 22 to 28 weeks and weighs as little as 500 g. The lungs of a patient with this “new” BPD are at a more immature and simplified stage of development with fewer alveoli. Exposure to the extrauterine environment leads to a complete or partial arrest in acinar development (Husain et al. 1998). This extreme immaturity coupled with the frequent administration of prenatal maternal glucocorticoid therapy and postnatal extrinsic surfactant to the infant as well as less aggressive positive pressure ventilation and oxygen therapy, has resulted in a different histological and radiographic appearance and has also resulted in changing definitions of BPD. Currently the most widely accepted definition of BPD was issued in 2001 following a workshop held by the National Institute of Child Health and Human Development, the National Heart, Lung and Blood Institute, and the Office of Rare Diseases (Jobe and Bancalari 2001). This defines BPD as the need for supplemental oxygen for at least 28 days following birth and its severity is graded according to the extent of oxygen therapy and other respiratory support at 36 weeks postmenstrual age (gestational age at birth plus chronologic age) or at discharge, whichever comes first, if born at <32 weeks gestational age, or at 56 days postnatal age or at discharge, whichever comes first, if born at 32 or >weeks gestational age.
The histology of this “new” BPD in these extremely premature neonates demonstrates fewer but larger alveoli which have a more uniform caliber, with less interstitial inflammation and fibrosis. In these very immature neonates, chest radiographs initially may be clear or may demonstrate subtle diffuse hazy opacities bilaterally (Fig. 14). Over time, because they have fewer alveoli than normal, these neonates often require prolonged ventilator support and oxygen therapy. Although minimized, this support leads to chronic lung changes on chest radiographs. Most commonly this is a diffuse haziness bilaterally (Fig. 15). Less commonly they progress from this diffuse haziness to a more heterogeneous appearance with diffuse cystic lucencies and coarse reticular opacities bilaterally giving a “bubbly” appearance to the lungs similar in appearance to stages III and IV of the original radiographic description of BPD. This has been described as developing within a week of birth (Swischuk et al. 1996) but may also occur later as described in “old” BPD (Fig. 16) This appearance is seen when infants have a difficult post natal course that requires intubation and high pressure ventilation and high concentrations of supplemental oxygen.
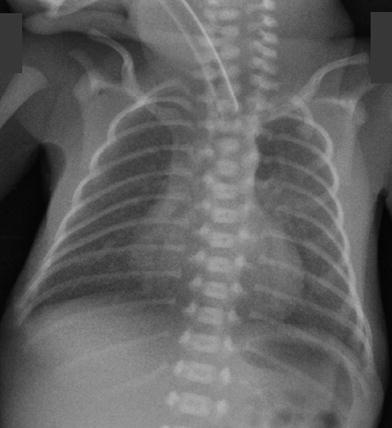
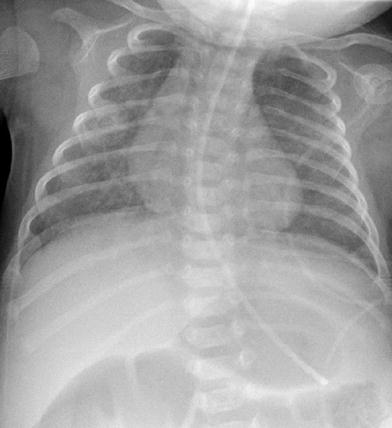
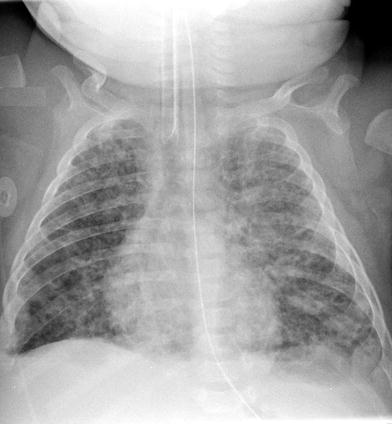

Fig. 14
Newborn 24 week gestation premature infant, first day of life. Mild hazy opacities are seen diffusely throughout both lungs

Fig. 15
Former 25 week gestation premature infant who is now 70 days old. Note the diffuse hazy opacification throughout both lungs. This is consistent with “new” bronchopulmonary dysplasia

Fig. 16
Bronchopulmonary dysplasia in a 3-month-old former 24 week gestation infant. The lungs are hyperinflated. Coarse reticular opacities are present bilaterally with intervening small rounded lucencies giving a somewhat “bubbly” appearance to the lungs
CT is occasionally performed after the neonatal period in patients with BPD. These studies are sometimes requested in patients with ongoing respiratory difficulty in whom there is concern for a comorbid condition such as aspiration. If performed in infancy or early childhood, BPD will appear as hyperinflated lungs with distended secondary pulmonary lobules that are bounded by thickened interlobular septa (Fig. 17). Small subpleural cysts are frequently present. The distended secondary pulmonary lobules demonstrate air trapping on expiratory images. If imaged later in childhood, patients with longstanding BPD demonstrate overall increased lung volumes with mosaic attenuation and air trapping. There are linear and band-like opacities that represent areas of atelectasis and scarring. Subpleural triangular opacities that sometimes are associated with the linear opacities are thought to represent pseudo-fissuring (Oppenheim et al. 1994) (Fig. 18).
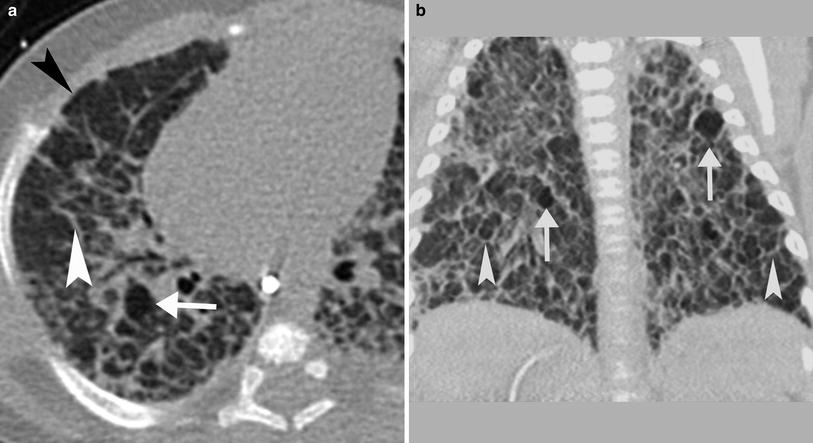
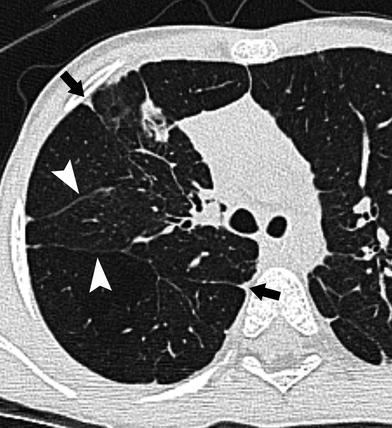

Fig. 17
CT in a 4-month-old former 24 week gestation infant with BPD. a and b There are thickened interlobular septa (white arrowhead) surrounding distended secondary pulmonary lobules, some of which are hyperlucent (arrow). Scattered subpleural cysts (black arrowhead) are also present

Fig. 18
CT in a 5-year-old former 24 week gestation premature infant. Axial CT images demonstrates multiple linear bands (white arrowheads), many extending to the pleural surface and many associated with triangular pleural-based opacities (black arrowheads). Subtle mosaic attenuation of the parenchyma is also present
4.4 Meconium Aspiration Syndrome
Meconium aspiration syndrome (MAS) is defined as respiratory distress in an infant born through meconium-stained amniotic fluid with characteristic radiological changes and whose symptoms cannot be otherwise explained (Wiswell et al. 1990). MAS occurs when meconium is aspirated into the lungs before or during labor and delivery. Staining of amniotic fluid is seen in 8–20 % of births, with MAS developing in approximately 20 % of these cases (Usta et al. 1995).
Meconium aspiration syndrome is a disease of term and especially of postterm neonates and of small for gestational age infants (Clausson et al. 1999). Passage of meconium in utero is rare before 37 weeks of gestation due to immaturity of the colon (Matthews and Warshaw 1979). Infants with MAS born after 40 weeks of gestation are more likely to require intubation than those born earlier (Dargaville et al. 2006). Factors predisposing to in utero passage of meconium include advanced maternal age, placental insufficiency, fetal distress, oligohydramnios, preeclampsia, and maternal drug use, particularly of cocaine (Swarnam et al. 2012).
Aspiration of meconium affects the lungs in a number of ways. If an airway is completely occluded, this can result in atelectasis. Partial occlusion of the airway may result in a ball-valve phenomenon with hyperinflation of the portion of the lung that is distal to the meconium plug. This can result in an air leak phenomenon if there is progressive overinflation. Although the primary constituent of meconium is water, it also contains many compounds such as pancreatic juices, bile, and gastrointestinal secretions that irritate the airways and alveoli, resulting in a chemical pneumonitis. Although the aspiration may be focal, the influx of inflammatory cells and chemical mediators in inflammation such as interleukins and tumor necrosis factors can induce a more widespread pneumonitis involving much of the lung (Cleary and Wiswell 1998). Surfactant lining the alveoli is deactivated by meconium which also may decrease the amount of surfactant produced (Dargaville et al. 2001; Janssen et al. 2006). This combination results in surfactant dysfunction, further promoting atelectasis and diminished aeration. The resultant hypoxia, hypercapnia, and respiratory acidosis lead to pulmonary arterial vasoconstriction. This may be compounded by the effects of preexisting chronic intrauterine hypoxia leading to thickening of the arteriolar walls. The increased resistance in the pulmonary vascular bed can result in persistent pulmonary hypertension of the newborn (PPHN), further worsening the degree of hypoxia and difficulty with ventilation.
Supplemental oxygen is the mainstay of therapy and may be all that is required (Singh et al. 2009) but more aggressive support is not unusual; intubation and mechanical ventilation is required in up to one-third of patients (Cleary and Wiswell 1998). In cases where adequate oxygenation cannot be achieved with mechanical ventilation, extracorporeal membrane oxygenation may be needed. Surfactant therapy, corticosteroids, and nitric oxide have been used as adjuvant therapies (Dargaville 2012).
Over the past decades, a reduction in the risk of MAS has been attributed to better obstetric practices, in particular, to avoidance of postmaturity and to expeditious delivery when fetal distress has been noted (Yoder et al. 2002). However, the mortality in patients with MAS remains considerable at 7 % (Dargaville et al. 2006), usually related to pulmonary hypertension and cerebral hypoxic ischemic injury. Approximately 10 % of patients intubated for MAS will develop a pneumothorax. This is an indicator of disease severity with a mortality rate of 42 % (Dargaville et al. 2006).
The most significant long-term morbidity in MAS is neurological injury. A diagnosis of MAS in the neonatal period confers a considerable risk of cerebral palsy (5–10 %) and global developmental delay (15 %) (Beligere and Rao 2008). Pulmonary sequelae are also seen. In the first year of life, up to half of infants will demonstrate respiratory symptoms with wheezing and coughing (Yuksel et al. 1993). Older children may exhibit evidence of airway obstruction, hyperinflation, and airway hyperreactivity, but appear to have normal aerobic capacity (Swaminathan et al. 1989).
On imaging a variety of appearances may be seen. The lungs are usually well inflated. Some cases may demonstrate symmetric fine reticular opacities bilaterally or may have more streaky opacities in the perihilar regions bilaterally, a radiographic appearance that can be difficult to differentiate from TTN (Fig. 19). In more severe cases, the lungs are hyperinflated with coarser linear and band-like opacities bilaterally reflecting atelectasis alternating with lucent areas corresponding to air trapping (Fig. 20). More ill-defined confluent opacities can be seen and relate to pneumonitis (Fig. 21). Findings may be symmetric but can be quite asymmetric. Small pleural effusions may be present (Gooding and Gregory 1971) (Fig. 22). Because of difficulty with ventilation and adequate oxygenation, air leaks are not uncommon and result in pneumothorax and/or pneumomediastinum in approximately 10 % of patients (Wiswell et al. 1990). Depending on the severity of the aspiration, the findings may resolve within 48 h or they may persist with more gradual improvement. However, the severity of the appearances on imaging does not always correlate with the clinical findings.