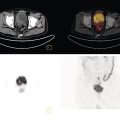
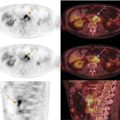
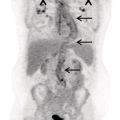
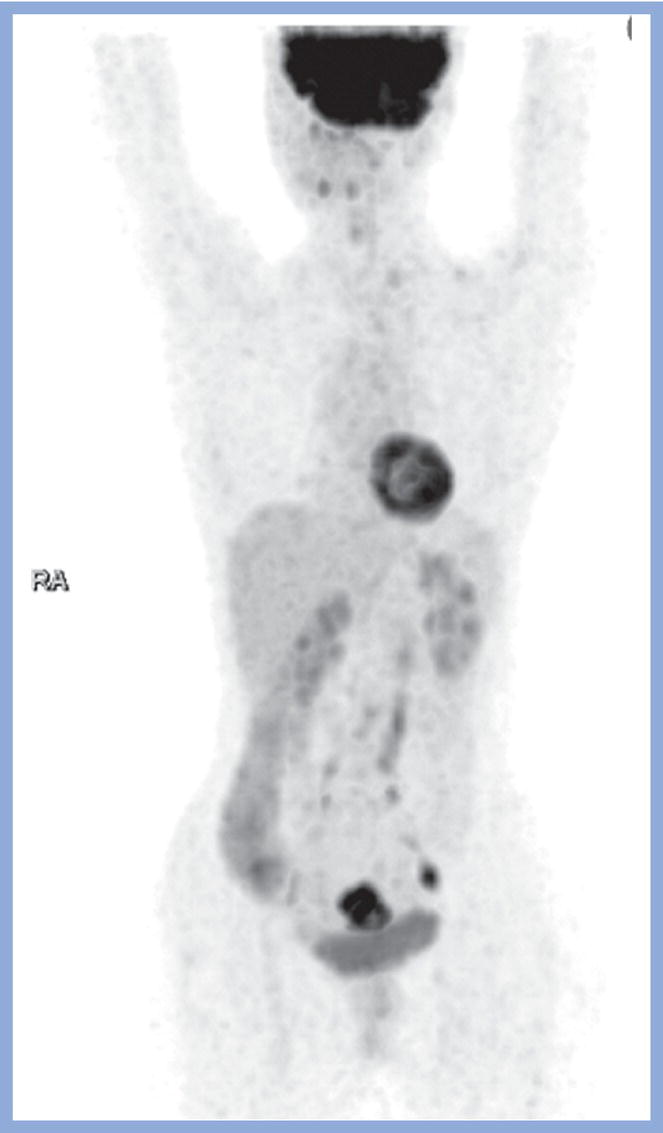
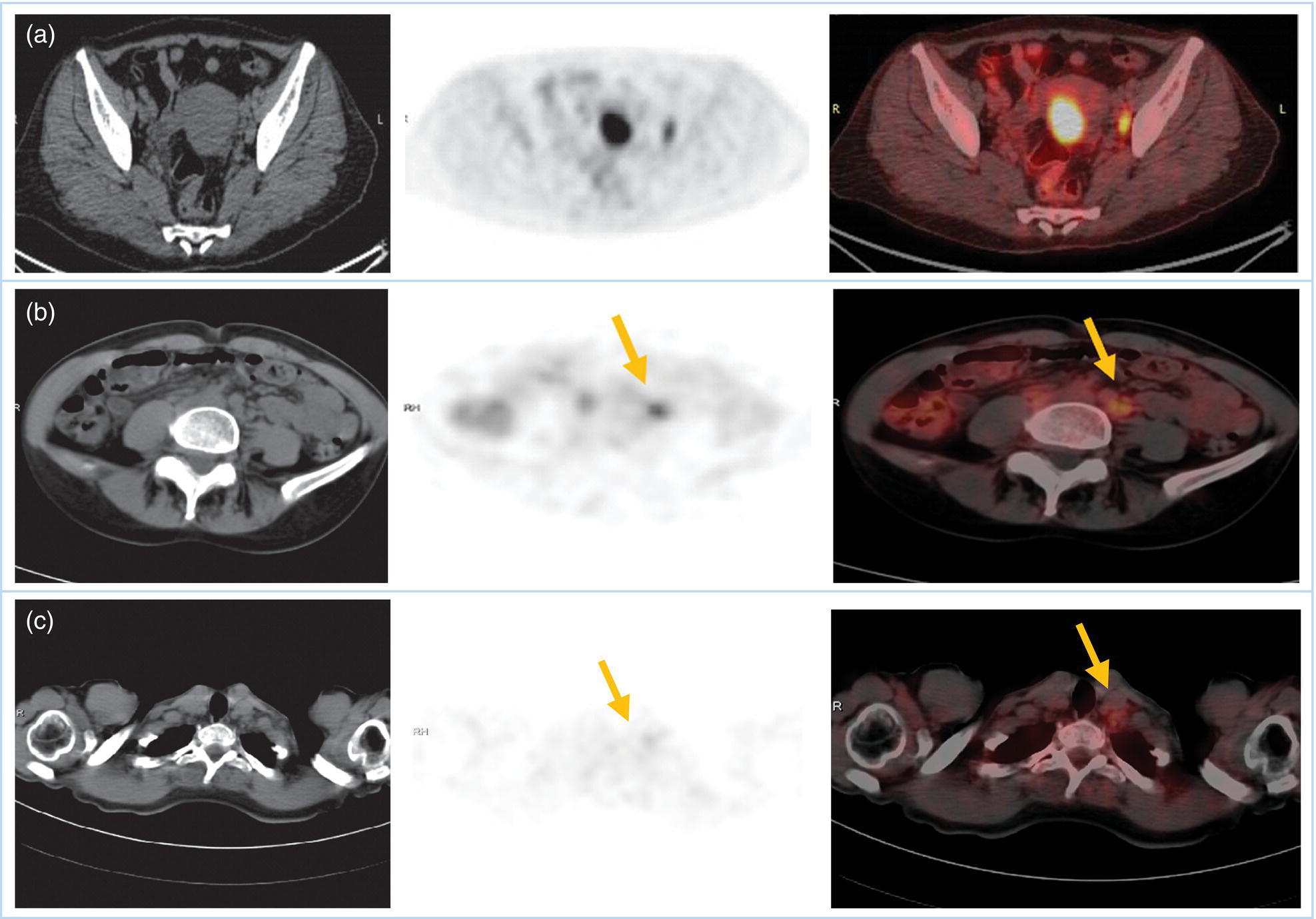
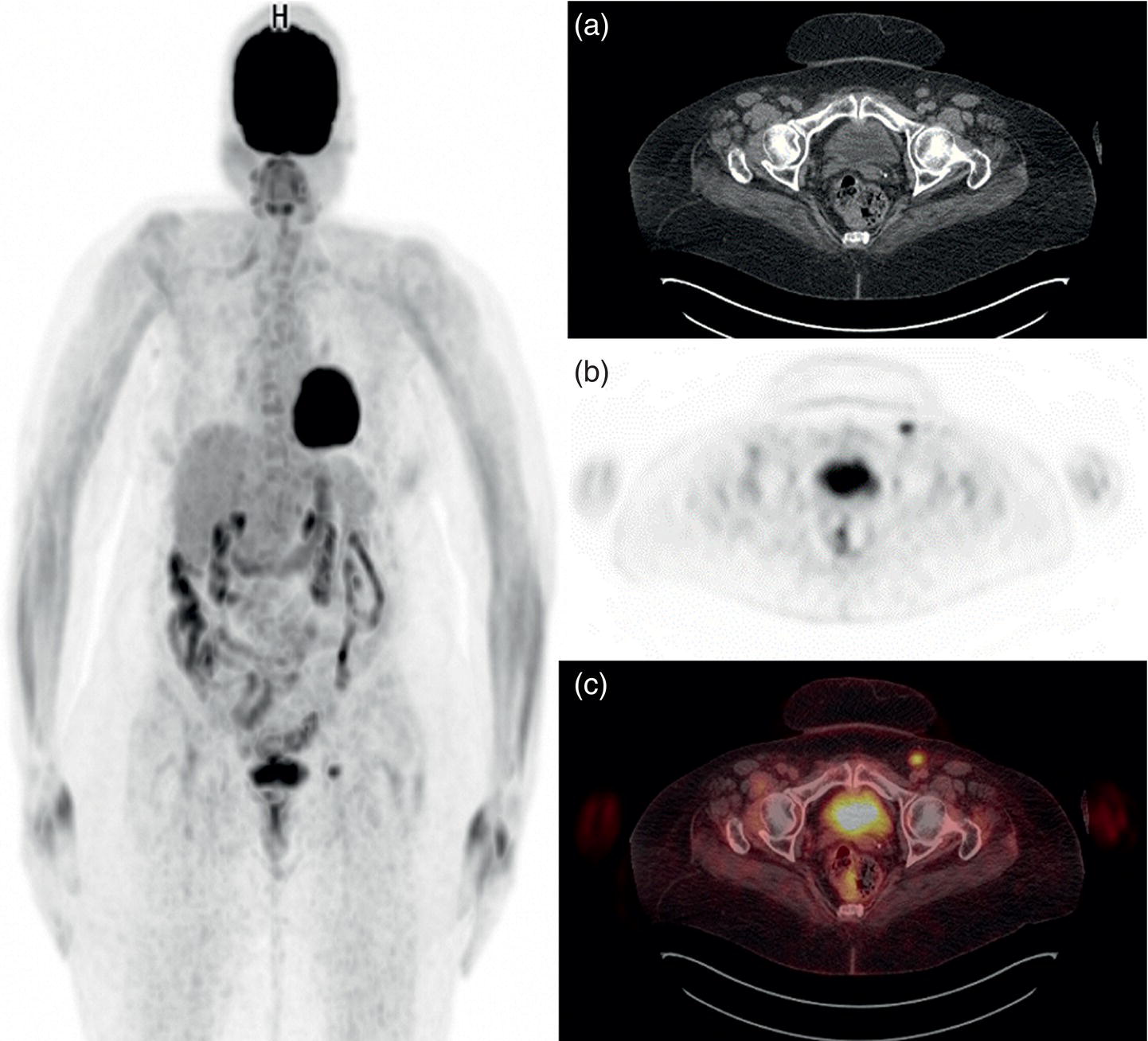
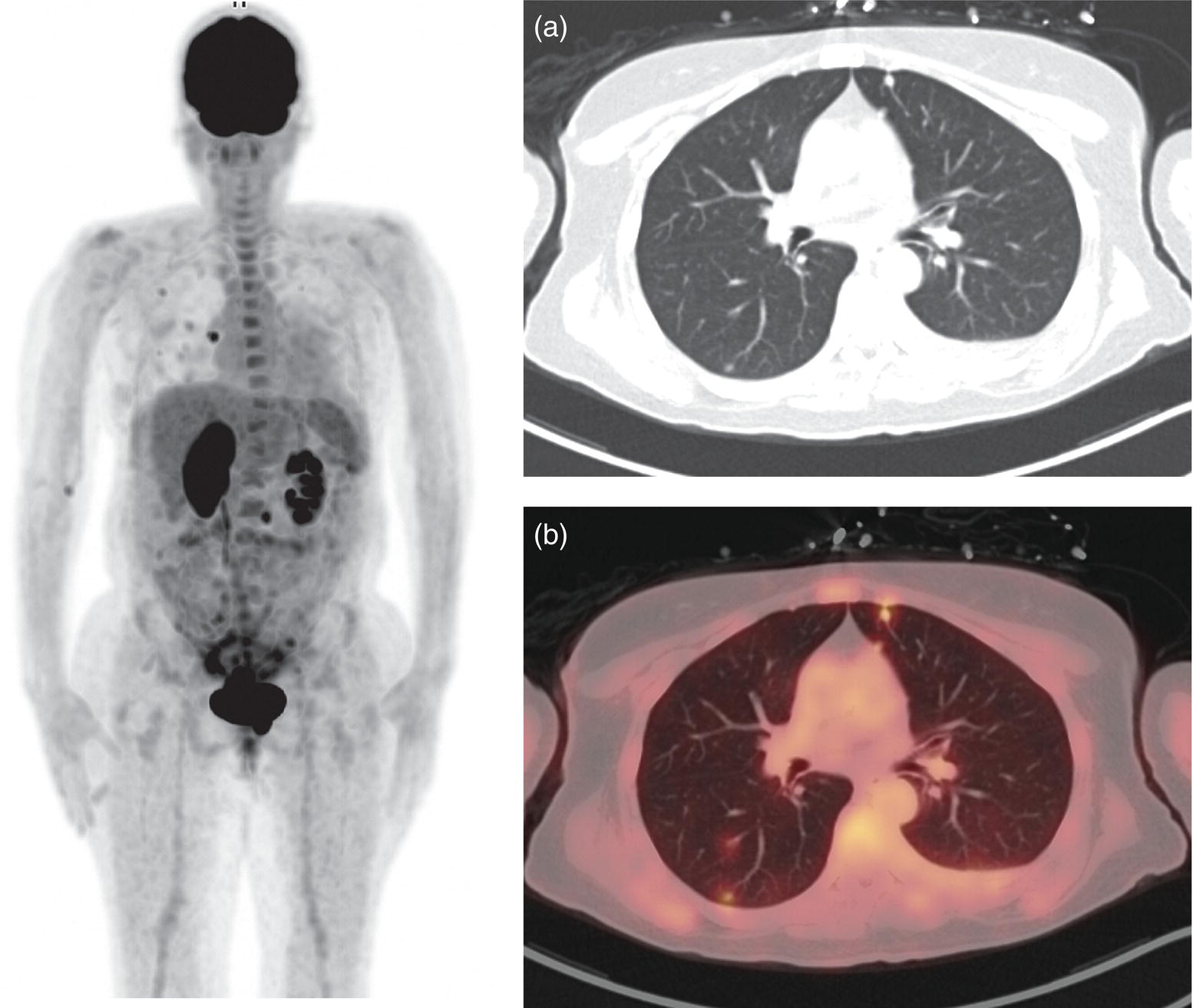
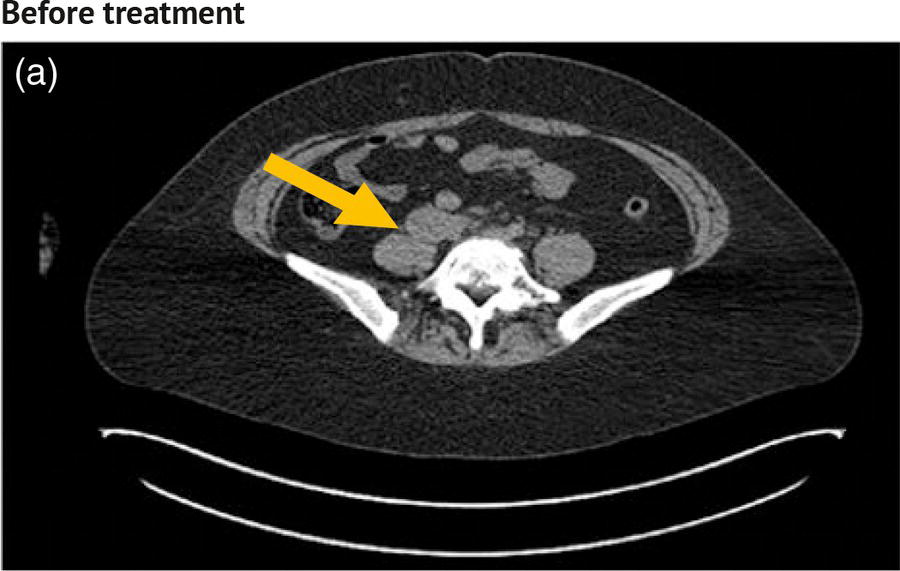
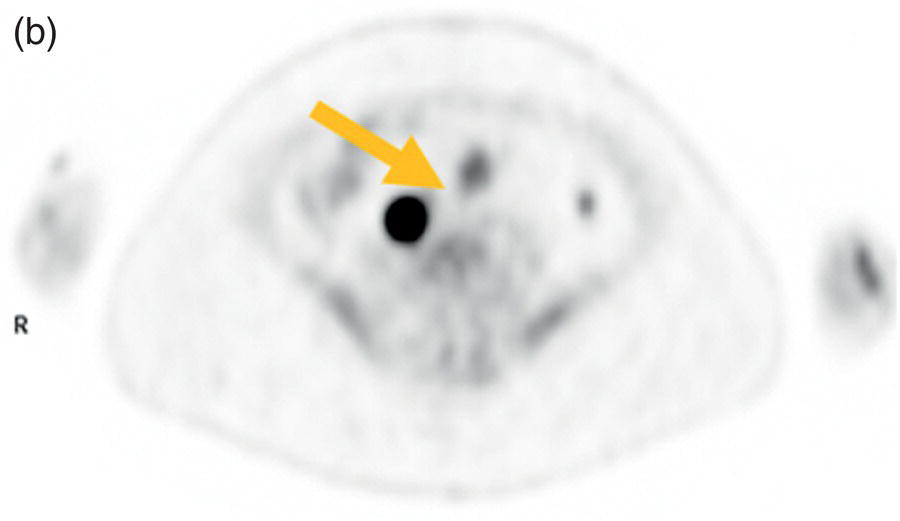
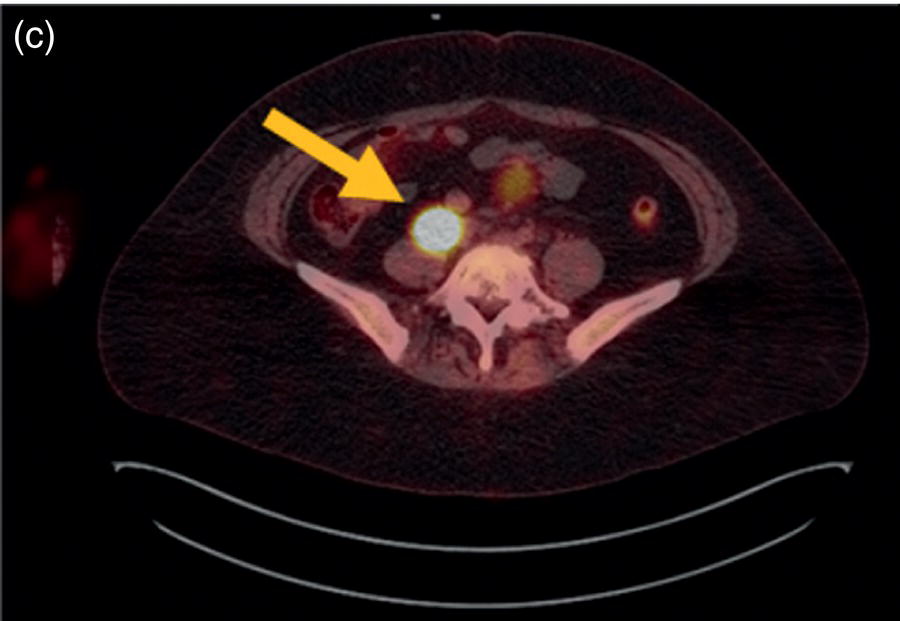
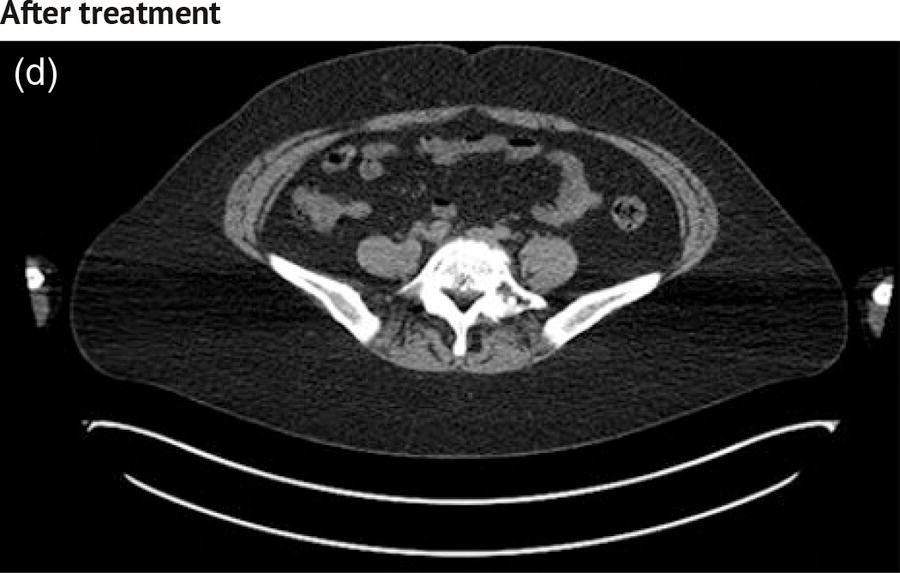
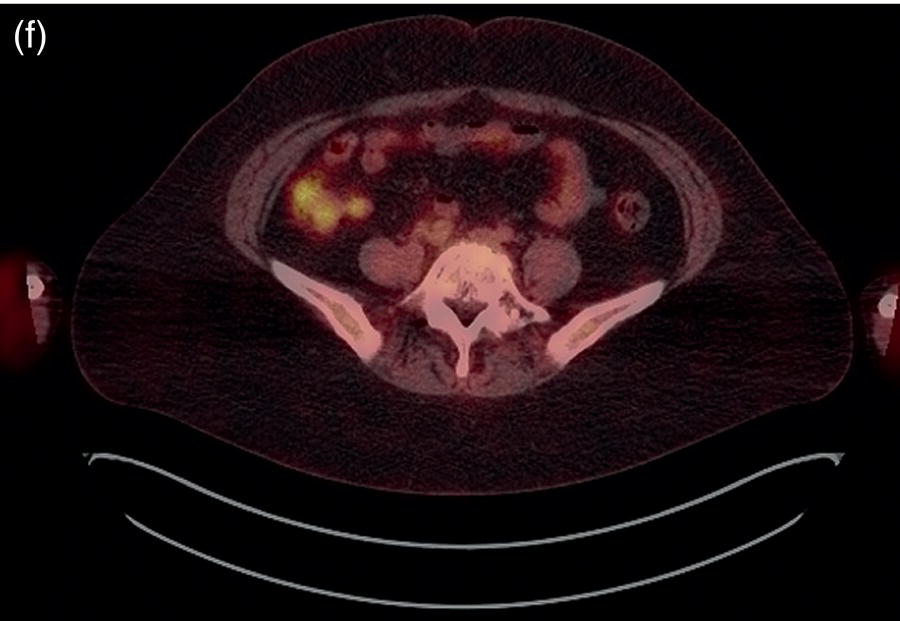
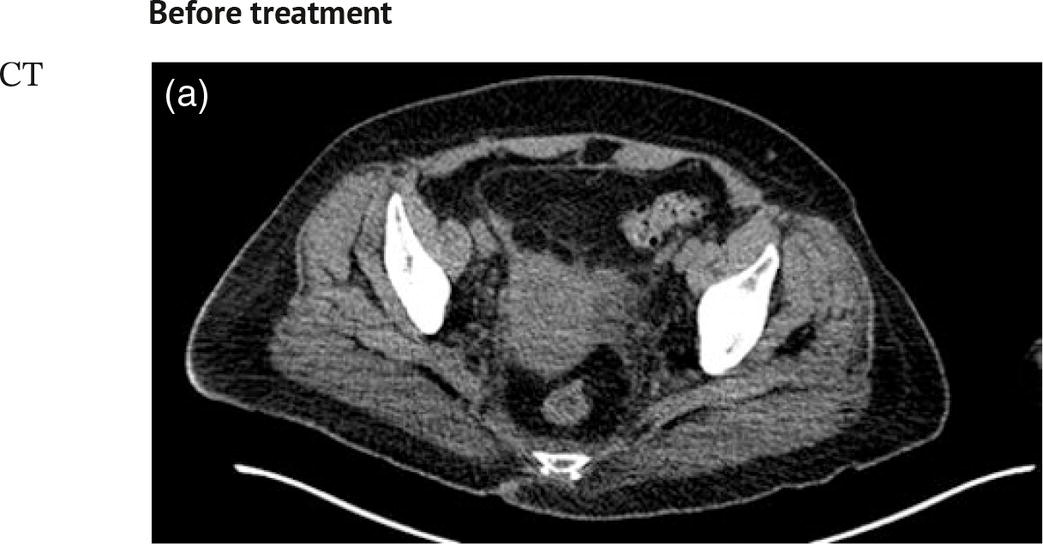
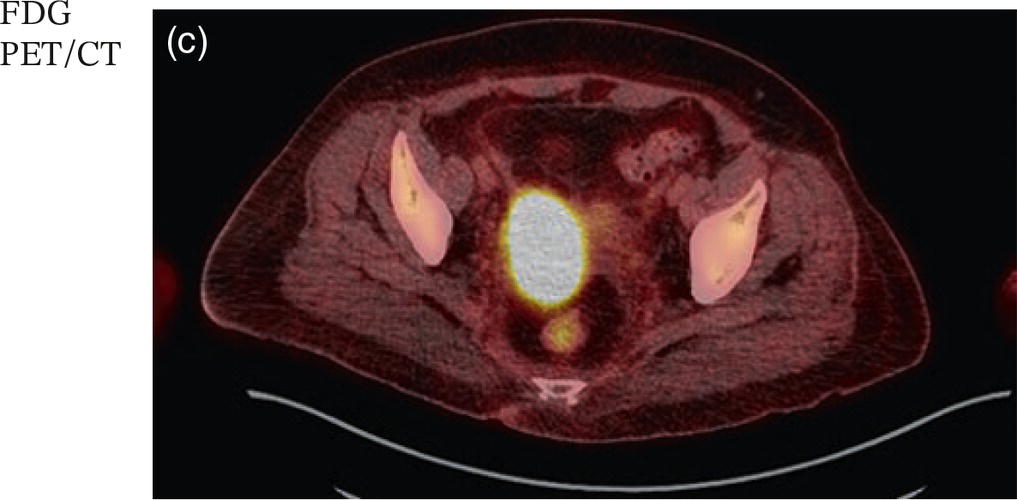
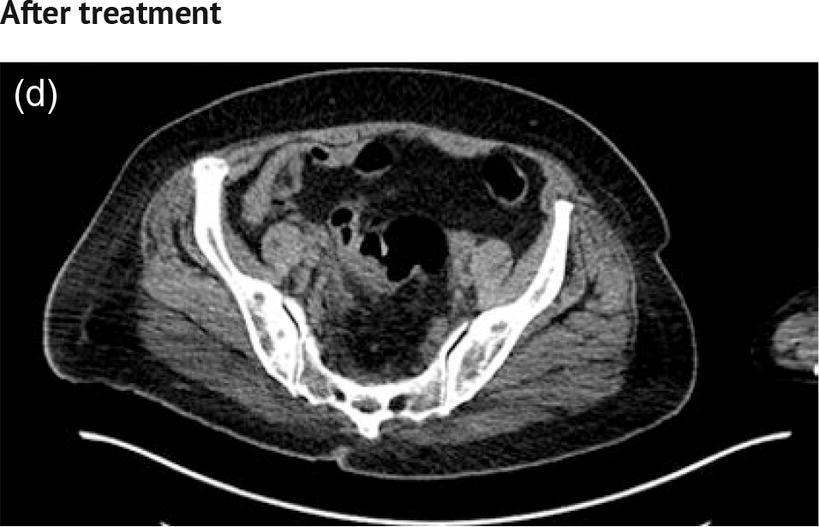
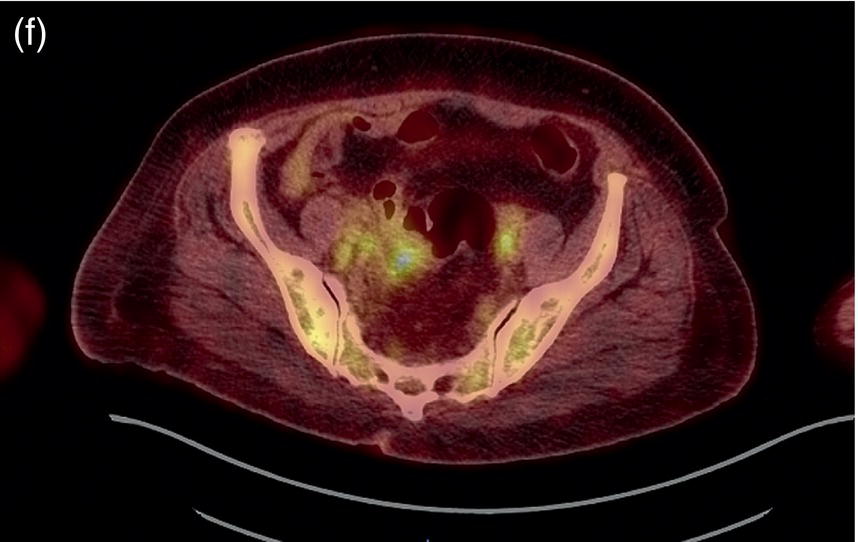
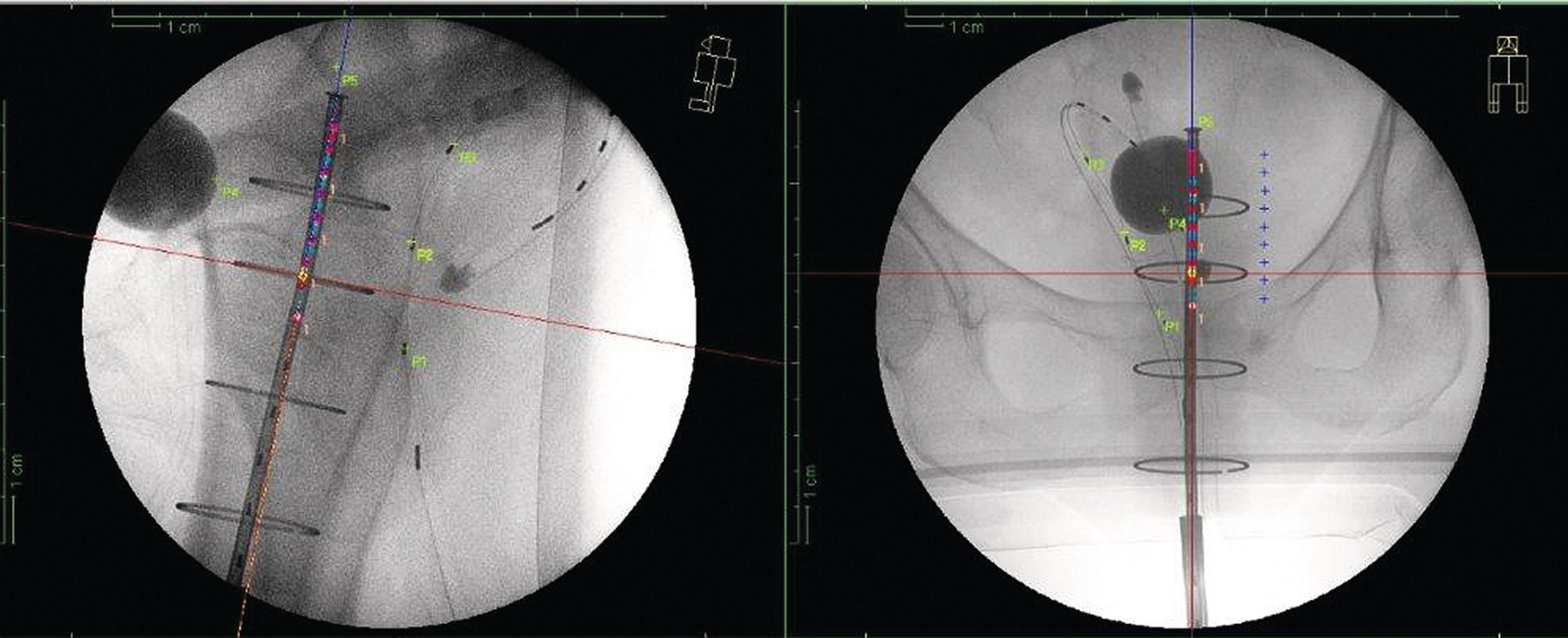
Sanaz Katal1, Akram Al‐Ibraheem2, Fawzi Abuhijla3, Ahmad Abdlkadir2, Liesl Eibschutz4, and Ali Gholamrezanezhad4 1 Nuclear Medicine Fellow, Medical Imaging Department, St Vincent’s Hospital Melbourne, Australia 2 Department of Nuclear Medicine, King Hussein Cancer Center, Amman, Jordan 3 Department of Radiation Oncology, King Hussein Cancer Center, Amman, Jordan 4 Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA Gynecologic malignancies involve the female reproductive organs and are an important cause of morbidity and mortality worldwide. There are five main types of gynecologic cancer: vaginal, vulvar, cervical, endometrial, and ovarian. This chapter will primarily discuss the latter two malignancies, endometrial and ovarian. Endometrial cancer is the most prevalent gynecologic cancer, primarily affecting women over 50 years old [1]. The majority of endometrial cancer cases are detected early, with a history of abnormal vaginal bleeding reported as the most common symptom [2–5]. While ovarian cancer is the second most common gynecologic cancer, accounting for roughly 4% of all cancers among women, it is the leading cause of gynecologic cancer‐related death [6]. The high mortality rate associated with ovarian cancer can be attributed to delayed detection, as most ovarian cancer patients present in a later stage. The first section of this chapter will discuss endometrial cancer, a malignancy that begins in the endometrial lining of the uterus. Conditions that promote higher estrogen exposure are known to increase the risk of endometrial cancer development, including hormonal replacement therapy, obesity, tamoxifen usage, early menarche, late menopause, nulliparity, and polycystic ovarian syndrome history [7–9]. The main prognostic risk factors for endometrial cancer include histological grade, depth of myometrial invasion, cervical invasion, and lymph node status [10]. Histologically, endometrial cancer is subdivided into two subtypes: type I (80–85%) and type II (10–15%). Type I is estrogen‐dependent, affects younger women (pre‐menopausal or peri‐menopausal women), and is typically detected early with presentation of abnormal vaginal bleeding. Type I endometrial cancer is a grade 1–2 endometrioid adenocarcinoma with a favorable prognosis (5‐year survival rate of 80%). Type II endometrial cancer, on the other hand, affects older women (post‐menopausal), is frequently identified at an advanced stage (60%), and can proceed to peritoneal carcinomatosis. On a histopathological level, type II endometrial cancer encompasses grade 3 endometrioid adenocarcinomas, as well as other uncommon etiologies such as clear cell carcinoma, undifferentiated serous carcinoma, and carcinosarcoma. Type II endometrial carcinoma is known for its aggressive behavior, with an overall 5‐year survival rate of 40% [3, 5, 7]. Recent studies have introduced a variety of imaging modalities such as ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose (FDG) positron emission tomography/CT (PET/CT) to play a complementary role in the pre‐treatment assessment of endometrial cancers. This pre‐operative imaging not only plays a crucial role in the pre‐treatment evaluation of endometrial malignancies but also provides vital information post‐intervention. For instance, pelvic MRI is often employed to evaluate disease recurrence, and in certain scenarios FDG PET/CT can be utilized in post‐therapy surveillance due to its high diagnostic performance in identifying recurrent or residual disease. By elucidating the role of correlative imaging (using US, CT, MRI, and FDG PET/CT) in women with endometrial cancers, clinicians can optimize decision‐making in these patients and better understand how and when diagnostic imaging modalities should be performed. Currently, surgical staging of endometrial cancer is performed according to the International Federation of Gynecology and Obstetrics (FIGO) guidelines, along with the World Health Organization (WHO) criteria for histological classification [11]. The FIGO recommendation for standard surgical staging of endometrial cancer includes abdominal exploration, peritoneal cytology washing, hysterectomy, bilateral salpingo‐oophorectomy, pelvic and paraaortic lymphadenectomy, and biopsy of any suspicious lesions found during surgery. As accurate determination of the tumoral extent and lymph node status impacts the type and extent of surgical intervention, pre‐operative staging with noninvasive diagnostic methods is vital for identification of optimal treatment. In addition, patients with a medical contradiction for surgical staging, or those with an equivocal pelvic examination, will greatly benefit from these noninvasive techniques. In an effort to identify high‐risk patients, and pre‐operatively predict extrauterine extension, a multitude of imaging techniques have been evaluated, including conventional morphologic and novel molecular imaging tools. With the emergence of less invasive surgical techniques over recent decades, pre‐treatment imaging is now included in the updated FIGO staging system [12]. Although surgical staging is primarily utilized to evaluate endometrial cancer prognosis [13, 14], it is highly invasive and may only be advantageous in select patient populations. While full lymphadenectomy is oftentimes utilized in the surgical staging of uterine cancers, this technique remains controversial, particularly in patients with early‐stage disease [15]. In addition, surgical lymphadenectomy increases the likelihood of immediate and delayed complications. Thus, noninvasive detection of lymph node metastasis would enable us to tailor the extent of surgery and prevent unnecessary lymphadenectomy. Furthermore, pre‐surgical tools that accurately reveal distant metastases can assist in the development of adjuvant therapies. As post‐menopausal bleeding (PMB) is the most commonly presenting symptom of endometrial cancer, a thorough investigation is essential in women presenting with this finding. The initial diagnostic step when working up PMB is transvaginal sonography (TVS), the modality of choice when evaluating endometrial thickness. This technique can reliably rule out endometrial cancer, particularly if a normal‐appearing endometrium with a thickness of less than 3 mm is visualized [16]. While the upper threshold value suggested for endometrial thickness in post‐menopausal women is 4 mm or 5 mm, cut‐off values in pre‐menopausal women might depend on many factors, such as the menstrual cycle [17]. Therefore, no standard specific values have been defined in this group yet. In cases where the endometrial thickness is greater than 5 mm, the sonogram should be considered abnormal. A meta‐analysis reported that an endometrial thickness of 5 mm in post‐menopausal women could detect endometrial cancers with 96% sensitivity and acceptable specificity [18]. Moreover, a focal endometrial abnormality or indistinct endometrial margins in TVS of women with PMB should be considered abnormal, warranting tissue sampling or hysteroscopy with dilation and curettage (D&C) [19]. When TVS is unable to definitively characterize endometrial abnormalities, additional techniques such as transabdominal sonography and hysterosalpingography can be useful. Three‐dimensional (3D) sonography and color‐Doppler sonography have been suggested for evaluation of endometrial volume and tumoral vascular structures studies, predictors of endometrial cancer. However, there is no agreement on a certain cut‐off value for the time being [20]. Various scoring models have been proposed to predict the risk of endometrial cancer using TVS results [21]. TVS parameters of endometrial thickness, Doppler score, interrupted endo‐myometrial junction, and irregular surface on gel infusion sonography are among the most important features for predicting endometrial cancer. Ultimately, increased endometrial thickness and abnormal morphologic findings of the endometrium on sonography should raise the possibility of uterine malignancies in women presenting with PMB. Another advantage of TVS is its ability to assess deep myometrial invasion with a moderate to high diagnostic accuracy in endometrial cancer [22, 23]. Some studies have reported that when performed by expert practitioners, TVS provides a diagnostic accuracy comparable to that of MRI in the pre‐operative local staging of endometrial cancers. Thus, while MRI is the gold standard technique for this purpose, TVS may be a feasible first‐line imaging modality in the assessment of women with suspected or proved endometrial cancer. While TVS remains the preferred modality for screening and primary detection of endometrial cancer, pelvic MRI has emerged as an effective method for assessing disease status and treatment planning. Due to its excellent soft‐tissue resolution, MRI is considered the most accurate imaging tool in the pre‐operative evaluation of endometrial cancers [24]. For years, FDG PET/CT techniques have been successfully utilized in the evaluation of gynecologic malignancies as the combination of anatomic and metabolic information using hybrid FDG PET/CT allows this technique to overcome the limitations of morphological imaging alone. This technique can be used to establish a therapeutic strategy based on the diagnosis of distant metastases, as well as to diagnose recurrence after therapy, treatment response, and lymph node metastases. As a result, FDG PET/CT is being employed to enhance traditional diagnostic imaging modalities [31]. Figure 20.1 FDG PET MIP image of a 61‐year‐old female patient presenting with a suspected uterine mass (left). FDG PET/CT scan showed intensely hypermetabolic uterine mass lesion suggestive of primary tumor (as shown in image a below) as well as hypermetabolic borderline sized left para‐iliac and retroperitoneal lymph nodes (arrows in image b) and left supraclavicular lymph node (arrows in image c), which were confirmed to be metastasis. Figure 20.2 A 70‐year‐old woman who proved to have endometrial cancer after total abdominal hysterectomy and bilateral salpingo‐oophorectomy. While her staging CT scan only showed a small nonspecific left inguinal lymph node by the CT criteria (as shown in a), the patient had an FDG PET/CT which revealed a hypermetabolic left inguinal lymph node (as shown in b and c), suggesting a metastasis, which was confirmed by histopathology. Figure 20.3 A 53‐year‐old female patient who proved to have endometrial cancer after total abdominal hysterectomy. Her staging work‐up showed multiple small bilateral pulmonary nodules of indeterminant significance on CT scan (as shown in a). FDG PET/CT depicted the hypermetabolic status of these lung nodules despite their small volume and correctly denoted them as metastases (as shown in b). In addition, FDG PET/CT detected para‐iliac and retroperitoneal hypermetabolic lymph node metastases. Figure 20.4 A 53‐year‐old female endometrial cancer patient who had a hypermetabolic enlarged right common iliac lymph node on FDG PET/CT (as shown in a, b, c). After receiving radiotherapy, this lymph node completely resolved (as shown in d, e, f). This is an example of FDG PET/CT utilization in a post‐therapy setting. Figure 20.5 A 66‐year‐old female patient with endometrial cancer as shown in axial CT, PET, and FDG PET/CT images before treatment (as shown in a, b, c). After undergoing an abdominal hysterectomy and bilateral salpingo‐oophorectomy, follow up CT images showed a questionable soft tissue thickening in the right side of the pelvis with no definite metastasis (as shown in d). In addition, the patient had an FDG PET/CT that demonstrated a lesion with abnormal FDG uptake in the ride side of the pelvis, suggesting local recurrence along with hypermetabolic pelvic lymph nodes confirming the presence of regional metastatic process (as shown in e, f). This clinical picture reflects the vital role of FDG PET/CT in detecting disease recurrence postoperatively when anatomical landmarks are distorted. Current research is focused on improving the overall quality of PET scanning, primarily through the utilization of novel agents such as methionine and choline, as well as tracers other than 18F (11C, 13N, 15P) [43]. Combining tracers may ultimately help elucidate the molecular basis behind malignancies, allowing for more accurate differential diagnosis and personalized treatment [31]. A recent study by Tsujikawa et al. focused on conducting PET scans using 16‐[18F] fluoro‐17‐estradiol (FES) and FDG to evaluate estrogen receptor (ER) expression and FDG uptake [43]. As FES is an 18F‐labeled estradiol compound with the highest physiological activity among estrogens, it is anticipated to be beneficial for diagnosing estrogen‐dependent disorders and assessing the effectiveness of hormonal therapy. These authors ultimately identified a direct correlation between the FDG to FES accumulation ratio and the aggressiveness of the endometrial cancer [43]. Other studies have noted similar findings, with semi‐quantification of tumor FDG and FES uptake as standardized uptake values (SUV) revealing that an FDG to FES uptake ratio of ≥2 has a 91.3% accuracy in detecting uterine sarcoma [44]. When combined with glucose metabolism evaluation by FDG PET, FES PET techniques can identify estrogen‐dependent activity and differentiate benign and malignant uterine tumors. This is due to the fact that gynecological malignancies like endometrial cancer and uterine sarcomas have high glucose metabolism and low ER expression, whereas benign tumors like endometrial hyperplasia and uterine leiomyomas have low glucose metabolism and high ER expression [43]. Although limited data exists regarding this technique, PET/MRI has potential diagnostic utility in endometrial cancer patients when compared to the traditional modalities of CT, MRI or FDG PET/CT. A study conducted by Tsuyoshi et al. [45] discussed the high diagnostic value of this technique, which is comparable to MRI for assessing the primary tumor, and akin to CT for assessing nodal and distant metastasis. In addition, PET/MRI imaging systems can yield high resolution soft tissue images as well as improved anatomic assessment of cancers [46]. Thus, PET/MRI could be implemented as an alternative to traditional imaging modalities for pre‐operative endometrial cancer staging [45]. Further advancements in PET/MRI technology, as well as the development of additional positron tracers and expanded availability, are expected to make PET/MRI an increasingly significant tool in the pre‐operative staging and surveillance of patients with endometrial cancer. A recent study found that fusing PET and MRI enhances the individual benefits of MRI and PET, and aids in the assessment of the primary endometrial carcinoma and overall lymph node staging [47]. By combining the advantages of PET in staging nodal and distant metastatic disease with the advantages of MRI in local staging, endometrial cancer patients can receive more accurate staging results. However, this novel technology was just recently launched into the clinical arena, thus the diagnostic value of PET/MRI for recurrent endometrial cancer and ability to assess treatment response is still unclear. Prior to beginning radiation therapy, imaging is necessary to make informed treatment decisions. Radiation therapy is currently used for the management of endometrial cancer, as neoadjuvant or adjuvant therapy in relation to surgery [48]. Postoperatively, imaging plays a primary role in post‐treatment evaluation. Prior to initiating radiation therapy for endometrial cancer, CT scanning is often utilized to generate an overall treatment plan. This includes 3D model generation of the abdomino‐pelvic region for target definition and dose calculation. The organs at risk within the radiated area are also contoured to ensure the dose limit is not exceeded for these structures. For endometrial cancer, the radiation volume usually includes regional nodal chains in addition to the tumor bed and upper vagina. In addition to CT scanning, other imaging modalities as MRI and or FDG PET/CT can also be used for target guidance when fused with CT simulation scans [49]. Since EBRT is primarily given over 25–30 fractions for endometrial cancer, routine images are obtained to monitor regional anatomical shifts and ensure adequate patient positioning. In addition, kilovoltage and megavoltage images aid visualization of the pelvic bony landmarks for endometrial radiation therapy. More advanced imaging forms, such as cone‐beam CT (CBCT), aid visualization of pelvic soft tissue structures and assessment of vaginal vault motion due to changes in bladder and rectal filling during therapy, particularly when sophisticated radiation techniques are used as inverse modulated radiation therapy (IMRT) [50]. Based on pathological findings, brachytherapy can be given as monotherapy or in combination with EBRT after surgery for endometrial cancer. Imaging modalities are used for ICBT planning (as shown in Figure 20.6) using C‐arm X‐ray for two‐dimensional planning. In unresectable or recurrent endometrial cancer, US imaging oftentimes guides brachytherapy applicator insertion, and CT and MRI are utilized for three‐dimensional planning and proper dose adjustment for recurrent disease (as shown in Figure 20.7) [51, 52]. Figure 20.6 Lateral and anterior–posterior (AP
20
Correlative Imaging of the Female Reproductive System
Introduction
Background: Endometrial Cancer
Staging/Pre‐operative Assessment
The Role of Imaging
Transvaginal Sonography
MRI
FDG PET/CT


PET/MRI
The Role of Imaging in Radiation Therapy
CT Simulation
Image Guidance
Imaging in Brachytherapy

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree