Fig. 1.1
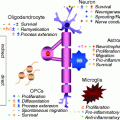
In cell cultures, oligodendrocyte precursors (OPCs) develop through a series of stages that can be identified by cell-type-specific markers. (a) Immature oligodendrocyte precursors are largely bipolar cells that are highly migratory and label on their surface with the monoclonal antibody A2B5 that recognizes a series of gangliosides. (b) A largely overlapping but somewhat distinct population of OPCs can be identified through the expression of the NG2 epitope. NG2 cells are similar to A2B5+ OPCs; however, NG2 is also expressed on a population of pericytes and neural stem cells. Immunopanning with mAb A2B5 or NG2 antibodies generates a relatively pure population of OPCs that can be utilized for multiple experiments. (c) When OPCs cease division, they differentiate into oligodendrocytes, which express galactocerebroside on their surface. Galactocerebroside can be identified by labeling with monoclonal antibody 01. Upon differentiation, oligodendrocytes develop multiple processes, cease to be highly migratory, and ultimately generate cells capable of myelination. Bar = 20 mm in a and b and 10 mm in c
1.2.2 OPCs Arise in Distinct Locations in the Developing CNS
Oligodendrocytes are relatively ubiquitously distributed in the adult CNS. While the highest density of oligodendrocytes and myelin is in white matter axon tracts, there is substantial myelination in gray matter and a large population of morphologically identified and phenotypically characterized oligodendrocytes. Classical studies suggested that in any specific region of the CNS, the three major cell types of the CNS—neurons, astrocyte, and oligodendrocytes—were derived from cells of neural tube as a result of periods of coordinated cell induction, proliferation, and differentiation. In general, birth-dating studies indicate that neuronal populations arise first followed by astrocytes and then oligodendrocytes (Jacobson 1978). Remarkably, the timing of the relative appearance of different CNS cell types is largely recapitulated in cultures of neonatal neural cells (Abney et al. 1981) and even neural stem cells. Using an approach pioneered by Reynolds and Weiss (1992), culturing dissociated neural cells on a nonadherent substrate results in the death of the vast majority of cells. Some of the residual cells, however, form neurospheres that can be passaged repeatedly and when replated give rise to neurons, astrocytes, and oligodendrocytes. Such preparations have been extensively studied, and in general, neurospheres derived from virtually all regions of the developing and adult CNS have the capability to give rise to cells of the oligodendrocyte lineage, although the number of OPCs is relatively small compared to the numbers of neurons or astrocytes and they are often the last cell type to arise. It seems likely that this reproducible timing of cell development is a reflection of the interactions occurring between cells in the neurosphere culture rather than a cell intrinsic property of neural stem cells. While useful for defining the properties of neural stem cells and potential molecular regulators of early oligodendrocyte development, neurosphere studies provide few insights into the localization of initial appearance of OPCs during normal development.
The application of specific markers and growth factor receptors expressed by OPCs to in vivo preparation combined with appropriate in vitro assays allowed a detailed description of the timing and localization of OPC’s initial appearance in different regions of the CNS. In the spinal cord, for example, separation of dorsal and ventral regions along the sulcus limitans early in embryogenesis suggested that OPCs originated selectively from ventral tissues (Warf et al. 1991), an unexpected result given the wide spread distribution of oligodendrocytes in the adult CNS. Subsequent studies identified an initial source of OPCs at the ventral midline directly above the floor plate. In mouse, the localized expression of PDGF alpha receptor (PDGFαR)-expressing cells revealed the earliest OPCs around embryonic day 12 (Pringle and Richardson 1993), while in chick embryos, OPCs first appear in the ventral ventricular zone around stage 25 (Ono et al. 1995; Orentas and Miller 1996). In both species, there is a close correlation between the appearance of OPCs with the development of motor neurons from the same embryonic domain that reflects the potential shared ancestry of the two cell types (Rowitch 2004). This localized ventral origin of OPCs is a consequence of signaling between neighboring tissues. For example, in chick embryos, transplantation of an additional notochord adjacent to lateral or dorsal regions of the neural tube results in the local induction of an additional floor plate, motor neuron, and OPCs (Jessell 2000; Ono et al. 1995; Orentas and Miller 1996). The accurate spatial and temporal characterization of the initial appearance of OPCs in the spinal cord facilitated the identification of major regulators of OPC induction, including environmental cues such as Sonic hedgehog (Shh) and intracellular cues such as the expression of distinct cohorts of transcription factors including olig1 and olig2 that orchestrate oligodendrocyte development (Rowitch 2004). The ventral midline is not the only source of OPCs in the developing spinal cord. A smaller source of OPCs arises from a discrete location in the dorsal spinal cord later in development (Cai et al. 2005), while intermediate regions of the spinal cord germinal zone do not appear to give rise to oligodendrocytes although they generate both neurons and astrocytes.
In more rostral regions of the CNS, multiple discrete sources of OPCs have been identified (Spassky et al. 1998). These include the floor of the third ventricle, which generates a subset of OPCs that migrate down the optic nerve (Gao and Miller 2006) and the medial ganglion eminence (Perez Villages et al. 1999; Spassky et al. 1998; Timsit et al. 1995) that contributes oligodendrocytes to specific domains of the CNS. Not all these regions are adjacent to an obvious source of an inductive signal such as the floor plate or notochord, and at least in the optic system, the source of inductive signals appears to be retinal ganglion cell axons (Gao and Miller 2006) that express Shh (Dakubo et al. 2008) and are the ultimate target of these OPCs. Our current understanding of the origins of OPCs in the developing CNS remains incomplete, and it seems likely based on cell tracing studies that many regions of the neural tube generate OPCs (Kessaris et al. 2006) but do so in response to specific local cues in a temporal and spatially regulated manner.
1.2.3 Environmental Factors That Dictate the Location of OPCs
The localized appearance of OPCs at defined stages in the developing CNS provided insights into environmental cues mediating their initial appearance. The ability to induce oligodendrocytes in dorsal and lateral regions of the developing spinal cord through notochord transplantation (Orentas and Miller 1996, 1998; Pringle et al. 1996) demonstrated the importance of the cellular environment and implicated the morphogen Sonic hedgehog in their initial appearance.
Sonic hedgehog is a member of the hedgehog family that contains three different members termed Sonic, Indian, and Desert hedgehogs (Ingham and McMahon 2001). The hedgehog pathway is critical in a wide range of morphogenetic events including patterning of the digits, lungs, and neural axis. Each member of the hedgehog family is functionally similar but has a distinct distribution in the embryo (Ingham and McMahon 2001). In the nervous system, the most highly expressed member of the hedgehog family is Sonic hedgehog (Shh) that is responsible for contributing to the patterning of the neural tube in both rostral–caudal and dorsoventral axis (Ericson et al. 1997; Jessell 2000). In caudal regions of the embryo during early development, Shh is expressed in the notochord where it contributes to the induction of ventral midline derived neural cells including the floor plate and motor neurons (Jessell 2000). In many cases, there is a connection between the types of cells induced by Shh and their subsequent expression of Shh. For example, the floor plate is induced by high concentrations of Shh generated by the adjacent notochord but then contributes to the further patterning of adjacent ventral spinal cord cell populations through the release of Shh (Jessell 2000). At high concentrations, Shh released by the notochord and floor plate induces adjacent ventral midline cells of the neural tube to develop into motor neurons and subsequently OPCs (Jessell 2000; Rowitch 2004). It has been proposed that the graded signaling of Shh from the notochord and overlying floor plate establishes a series of ventral to dorsal domains in the ventricular zone in which precursor cells become specified to distinct cell fates depending on the specific repertoire of transcription factors that are induced (Jessell 2000; Rowitch 2004). The downstream signaling of the hedgehog pathway has been extensively studied (Jiang and Hui 2008). In general, in the absence of hedgehog, ligands signaling through the smoothened receptor are inhibited by a surface molecule termed patched. The binding of hedgehog to patched releases this inhibition, and smoothened signaling is initiated. Downstream of this interaction, in the absence of hedgehog, the Gli proteins are cleaved to generate a Gli repressor that blocks the transcription of hedgehog-responsive genes, while in the presence of hedgehog, the activation of smoothened leads to a switch from a repressor to an activator (Jiang and Hui 2008). The activation of the Gli signaling pathway has been described in neural stem cells and OPCs, and although the details of the hedgehog signaling pathway in the oligodendrocyte lineage have yet to be fully resolved (Hu et al. 2009), it seems likely that they are similar to those described in other mammalian cell systems.
The inductive effects of hedgehog signaling are countered by inhibitory cues that suppress the initial appearance of OPCs. Several factors have been implicated in inhibiting OPC appearance including members of the bone morphogenetic protein family (BMPs) and the Wnts. The appearance and development of OPCs in dorsal spinal cord are significantly delayed compared to ventral regions. One mechanism that inhibits OPC development in dorsal regions is the localized expression of BMPs (Gomes et al. 2003; Grinspan et al. 2000). Bone morphogenetic proteins are members of the transforming growth factor beta superfamily and signal through SMAD intracellular pathways (Massague 1998). In the spinal cord, BMPs are primarily expressed in dorsal midline regions of the spinal cord until mid-embryogenesis and in vitro studies have shown that exposure of developing neural cells to BMP4 or 7 inhibits the appearance of oligodendrocytes and enhances the appearance of astrocytes (Grinspan et al. 2000; Miller et al. 2004). Signaling through the BMP pathway may not simply inhibit the induction of oligodendrocytes. Since in vitro OPCs are bipotential cells capable of giving rise to type 2 astrocytes or oligodendrocytes, one possibility is that BMPs influence OPCs to give rise to type 2 astrocytes rather than oligodendrocytes and this has been clearly shown in a series of in vitro studies (Grinspan et al. 2000; Miller et al. 2004). Consistent with this hypothesis, perturbation of the BMP receptor 1 in OPCs in vivo results in a transient reduction in the number of immature oligodendrocytes and an enhancement in the number of astrocytes (Gomes et al. 2003). Remarkably, this cellular imbalance is rapidly corrected during subsequent development (Samanta et al. 2007). Direct evidence for an antagonism between BMP and Shh signaling comes from in vitro studies in which the induction of oligodendrocytes by Shh can be directly inhibited by BMPs (Miller et al. 2004). It seems likely that in normal development the inhibition of oligodendrocyte differentiation by BMPs effectively outcompetes the inductive effects of Shh, and in regions where Shh is operative, additional local inhibitors of BMP signaling such as noggin are also present. The induction of OPCs in dorsal regions of the spinal cord may also reflect localized blockade of BMP signaling. The generation of OPCs in dorsal spinal cord is independent of Shh but stimulated by fibroblast growth factor (FGF) (Cai et al. 2005). In vitro studies suggest that in neural precursor cells treatment with FGF blocks BMP-stimulated nuclear translocation of phosphorylated Smads and subsequent development of OPCs (Bilican et al. 2008). Not only are BMPs important during development for oligodendrogenesis, it is becoming clear they regulate neural cell responses and fate after demyelination or spinal cord injury (Cheng et al. 2007; Fuller et al. 2007).
Additional signaling pathways may regulate OPC development. One such pathway that has received considerable attention is the Wnt signaling system that may contribute to the initial patterning of the spinal cord. Multiple studies implicated Wnts in the control of dorsal cell proliferation and more recently in neural tube patterning (Ulloa and Marti 2010). Explant studies using the chick spinal cord demonstrated expression of Wnt in dorsal regions of the developing spinal cord at the time that OPCs were being generated ventrally and that inhibition of Wnt signaling in the ex vivo model resulted in a dramatic increase in the number of OPC cells (Shimizu et al. 2005) suggesting that Wnt had an inhibitory effect on OPC development. Subsequent studies suggest that Wnt may have a more profound effect on OPC maturation than induction. Cell culture studies implicated Wnt signaling through β-catenin in the regulation of OPC maturation that was independent of the induction of OPCs or the control of their proliferation or cell fate determination (Feigenson et al. 2009). These functional characteristics distinguish Wnt signaling from other inhibitors of OPC development such as BMPs. Genetic manipulations resulting in constitutive activation of Wnt/β-catenin signaling specifically in cells of the oligodendrocyte lineage resulted in similar numbers of OPC compared to wild-type littermate controls but delayed the development of mature oligodendrocytes and myelination. Somewhat unexpectedly, these effects were transient and the histology of the adult animals was relatively normal (Feigenson et al. 2009). Confirmation of a critical role for Wnt in the timing of OPC development and identification of a persistent role for Wnt/β-catenin in the adult followed the identification of the transcription factor Tcf4 as a major mediator of oligodendrocyte Wnt signaling (Fancy et al. 2009). Conditional activation of the β-catenin pathway or perturbation of APC, an inhibitor of Wnt, resulted in a significant delay in the timing of oligodendrocyte maturation during development and a delay in remyelination in the adult CNS (Fancy et al. 2009). Thus far all the data suggest Wnt signaling in oligodendrocytes or OPCs is mediated through the canonical pathway, and whether there is a role for the noncanonical pathway in mediating the effects of Wnt on OPCs and oligodendrocytes remains to be determined.
Multiple factors may initiate the induction of OPCs in the developing CNS. While Shh is clearly important in early OPC development, OPCs also develop in cultures derived from Shh null animals indicating the existence of alternative pathways for their induction. The best defined of these is through FGF signaling pathway. As discussed above, FGF signaling may induce a blockade of BMP signaling (Bilican et al. 2008) and promote OPC induction. Later in development, OPCs express multiple FGF receptors that initiate a variety of cellular responses (Fortin et al. 2005). The relative roles of FGF in OPC induction are likely to be more pronounced in rostral regions of the CNS that contain multiple anatomically distinct areas of OPC appearance (Kessaris et al. 2006; Spassky et al. 1998). Axonal signals such as the neuregulins have been implicated in OPC induction; however, their role remains somewhat controversial. While in vitro the absence of NRG-1 significantly inhibited the appearance of OPCs (Vartanian et al. 1999) and suggested involvement of distinct ErbB receptors (Park et al. 2001; Sussman et al. 2005), subsequent studies using genetic manipulations in vivo have failed to provide any evidence of a role for neuregulins or their receptors in the development of CNS myelination (Brinkmann et al. 2008). It seems likely that while not essential in vivo for OPC development and CNS myelination, distinct isoforms of NRG and their receptors contribute significantly to normal OPC development and further studies are needed to clarify their precise roles.
1.3 Control of Oligodendrocyte Precursor Differentiation in the CNS
1.3.1 Regulation of Neonatal OPC Differentiation
The number of OPCs that are generated in germinal zones of the developing animal is small compared to the number of oligodendrocytes in the adult CNS. This difference is a consequence of the extensive proliferation of OPCs during subsequent development. A large number of studies have been focused on understanding the molecular control of OPC proliferation. While the identification of mitogenic signals is critical for understanding how the appropriate cohort of oligodendrocytes is generated, equally important is an understanding of the cellular and molecular cues that lead to the cessation of proliferation and differentiation of OPCs.
A wide range of growth factors have been shown to promote the proliferation of OPCs. The best characterized of these is platelet-derived growth factor (Richardson et al. 1988). Overexpression of PDGF results in increased numbers of OPCs during development, while conditional knockout of the PDGFαR in OPCs results in a dramatic reduction in their numbers (Calver et al. 1998). Furthermore, the proliferative response of OPCs to PDGF can be significantly modulated by signaling through the CXCR2 chemokine receptor (Robinson et al. 1998). Many other growth factors including FGF and IGF-1 promote OPC proliferation in vitro, as do several neurotrophins such as NT3 and BDNF. Whether all the growth factors that have been shown to promote OPC proliferation in vitro have specific or overlapping roles in vivo is not currently clear.
A critical step in the generation of the appropriate number of oligodendrocytes during development is the conversion of proliferative OPCs to differentiated, nonmigratory oligodendrocytes. How this transition is accomplished is not well understood. One attractive hypothesis is that intrinsic timers or clocks in OPCs control the timing at the level of individual cells and that this is clonally related (Temple and Raff 1986). Classical in vitro studies using clonal analysis of rat optic nerve OPCs in which sister cells were separated and grown in isolation revealed that they generated clones of similar sizes and the cells within a clone differentiated synchronously (Temple and Raff 1986). These studies raised the possibility that OPC differentiation might be regulated by cell intrinsic timers. How such timers might operate is uncertain but may be related the number of cell divisions a cell undergoes (Raff et al. 1988; Temple and Raff 1986). What sets such a clock is also unknown, and it seems likely that this intrinsic control is modulated by local environmental cues. For example, in high-density mixed cell cultures of developing rat spinal cord, clonally derived OPCs do not differentiate synchronously, but rather virtually every clone contains both OPCs and differentiated oligodendrocytes (Zhang and Miller 1995), which may reflect the immediate environment of individual OPCs. Subsequent studies in the optic nerve have, however, provided molecular substrates for inductive timers of OPC differentiation including regulators of the cell cycle, such as p21 and p57. How such an intrinsic mechanism actually controls cell differentiation is uncertain. It has been proposed that mitogenic signaling cascades may be diminished with each cycle such that, ultimately, the progenitor cell drops out of the cell cycle and differentiates constitutively. Support for this hypothesis comes from studies in which removal of growth factors drives precocious differentiation of OPCs, and promoting their continued proliferation through stimulation with both PDGF and FGF inhibits their differentiation (Bogler et al. 1990).
Recent studies provide insights into the molecular mechanism regulating neonatal OPC differentiation. Analyses of the expression of small noncoding microRNAs (miRNA) demonstrated differentiation-associated changes in expression levels for several miRNAs in the oligodendrocyte lineage. Single miRNAs can regulate the expression of multiple genes, and perturbation of their processing in OPCs has been shown to block differentiation into oligodendrocytes (Dugas et al. 2010; Zhao et al. 2010). Specifically, a distinct miRNA (miR-219) appears to be a critical regulator of oligodendrocyte differentiation and is both necessary and sufficient to promote OPC differentiation (Dugas et al. 2010; Zhao et al. 2010). One of the mechanisms by which miR-219 may operate is through the repression of genes that are required to maintain OPC proliferation such as PDGFαR. The inhibition of proliferation coupled with the upregulation of specific transcription factors essential for the onset of oligodendrocyte differentiation and myelination (Emery et al. 2009; Howng et al. 2010) is likely the central regulator of intrinsic control of OPC differentiation. The coordinated control of the expression of multiple genes can be affected by epigenetic regulation, and there is strong evidence for a role of epigenetic factors in controlling oligodendrocyte differentiation. Culture studies have suggested that HDAC1 is a critical modulator of genes that regulate oligodendrocyte differentiation. Manipulation of HDAC1 results in altered timing of oligodendrocyte appearance both in culture and in vivo (Li and Richardson 2009; Marin-Husstege et al. 2002). The precise signaling cascades that regulate this phenomenon are currently being resolved.
1.3.2 The Adult Oligodendrocyte Precursor
During development, not all OPCs differentiate, and the adult CNS contains a significant number of cells that have the morphological phenotype of OPCs and express OPC markers including PDFGαR and NG2. These adult progenitors were originally described in cell cultures where they had a longer cell cycle and reduced migratory capacity (Wolswijk and Noble 1989). The development of animals with reporters driven from promoters that are largely restricted to OPC such as NG2 or PDGFαR has provided remarkable insights into this interesting cell type (Rivers et al. 2008; Tripathi et al. 2010). For example, there are unexpectedly high numbers of adult OPCs in the CNS, and they are located in both gray and white matter. The cells have a unique morphology and appear to have a distinct physiological profile. Several studies have indicated that adult OPCs are electrically active (De Biase et al. 2010; Tripathi et al. 2011) and suggest that they may contribute to monitoring or regulating electrical activity particularly in white matter. Not only are adult OPCs numerous, but they are also capable of continued proliferation as might be anticipated for precursors cells. It was generally thought that in the absence of injury or disease there was relatively little turnover or de novo generation of oligodendrocytes in the adult CNS. The use of inducible reporters has, however, indicated that a substantial fraction of the oligodendrocytes in the adult corpus callosum are generated from precursor cells after the major period of cell division. Whether these newly formed oligodendrocytes represent replacement or additional cells is currently unclear. While a primary function of the adult OPCs may be to provide a resource to replenish cells lost to injury or that are required during the normal life span of the animal, there appears to be too many of these cells for that to be their only function. Likewise, the ability of these cells to sense electrical activity argues against a purely precursor function. Somewhat surprisingly, deletion of the majority of adult OPCs and blockade of their replacement do not appear to result in a major functional deficit, so the ultimate function of these cells remains unclear.
1.4 Control of OPC Migration in the Developing CNS
1.4.1 Signals Regulating OPC Migration
The restricted location of OPC’s origins in the embryonic CNS combined with the widespread distribution of oligodendrocytes in the adult CNS reflects the extensive migratory capacity of OPCs. Unlike the exquisite guidance of distinct neuronal cells or axonal growth cones to their targets, the migration of OPCs is regulated in a more general way even though in some cases similar molecular mechanisms appear to be operative. Culture studies demonstrate that PDGF is a strong chemoattractant for OPCs and is implicated in facilitating the widespread dispersal of these cells. It seems likely, however, that PDGF is not directing OPCs to particular locations. It is relatively ubiquitously distributed throughout the CNS and consequently appears to act as a general dispersal cue or simply to promote motility (Frost et al. 2009). Several other signals have been implicated in regulating OPC migration. These encompass a wide range of different classes of molecules, and it seems likely they all converge on a common target that modulates basic cell motility mechanisms at the level of the cytoskeleton. Such signals include neurotransmitter receptors such as AMPA and kainate receptors for glutamate (Karadottir and Attwell 2007). These receptors associate with alpha (v) b-3 integrin receptors (Gudz et al. 2006) as part of a complex that also contains proteolipid protein (PLP) (Gudz et al. 2006). Functionally, PLP is important in the signaling pathway since OPCs with mutations in PLP fail to show AMPA-stimulated response. In addition, OPCs express the chemokine receptors CXCR2 and CXCR4. Stimulation of CXCR4 with the ligand CXCL12 promotes OPC migration that is blocked by CXCR4 antagonists (Dziembowska et al. 2005). A common linkage between these different stimuli is the modulation of intracellular calcium, suggesting that it is an important modulator of OPC motility. The downstream targets of Ca2+ activation and the mechanisms by which Ca2+ is activated appear to be important in determining OPC responses since activation of the chemokine receptor CXCR2, another G-protein-coupled receptor that fluxes Ca2+, inhibits rather than enhances OPC migration.
1.4.2 Guidance of OPC Migration
The ability to specifically identify OPCs in culture and in vivo has allowed their dispersal and migration to be studied in culture as well as in fixed and living tissue (Jarjour and Kennedy 2004; Tsai and Miller 2002; Tsai et al. 2003, 2009). These studies indicate that while the dispersal of OPCs is not totally random, highly defined preexisting pathways of OPC migration do not appear to exist. Rather the migration of OPCs is guided by more general chemoattractants and chemorepellents (reviewed in Jarjour and Kennedy 2004) that direct OPCs to presumptive white matter regions.
In the spinal cord, OPCs initially arise in the ventral midline and subsequently migrate widely to the peripheral white matter. This dispersal of OPCs is largely the result of the localized expression of the chemorepellent netrin-1. Netrin-1 is expressed at the ventral midline at the time that OPCs initially arise and in vitro OPCs respond to a gradient of netrin-1 by orienting away from high concentrations and migrating toward lower concentrations (Jarjour et al. 2003; Tsai et al. 2003). In animals lacking netrin-1, the dispersal of spinal cord OPCs is inhibited (Jarjour et al. 2003; Tsai et al. 2003), and the cells remain concentrated at their source. The response of OPCs to netrin-1 depends on the receptors DCC and UNC5H1 (Jarjour et al. 2003; Spassky et al. 2002; Tsai et al. 2003), and blocking DCC signaling negates netrin-1 directional cues. The dispersal of OPCs from the ventral midline appears to be important to position cells to receive appropriate proliferative and differentiated cues (Tsai et al. 2009) that are localized in the developing white matter. What roles netrin-1 plays in OPC guidance in other regions of the CNS is less clear. Localization studies suggest that it acts as a chemorepellent to promote migration of OPCs that arise in the brain along the optic nerve toward the retina, and this is consistent with some in vitro studies (Sugimoto et al. 2001) but not others (Spassky et al. 2002). Whether these differences reflect the responses of OPCs at different stages of development or whether there are distinct subpopulations of OPCs who have distinct responses to dispersal cues is unclear.
Other guidance cues such as the semaphorins have been shown to influence OPC migration. The semaphorins are a large family of guidance molecules that signal through receptors composed of neuropilins and plexins (Raper 2000). Oligodendrocyte precursors express neuropilins (Spassky et al. 2002; Sugimoto et al. 2001) and are either attracted or repelled by semaphorins depending on the specific semaphoring family member. For example, Sema 3A appears to be a chemorepellent for OPCs, while Sema 3F is a chemoattractant (Spassky et al. 2002). The importance of semaphorin-guided migration during development is currently unclear although it has been implicated in myelin repair (Williams et al. 2007).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree