Diffusion Imaging
Objectives
At the completion of this chapter, the student should be able to do the following:
Key Terms
Diffusion is one level closer to the cell than capillary perfusion. Diffusion is the random motion of molecules from a region of high concentration to one of low concentration.
Tissue Diffusion
Diffusion is also termed brownian motion for Robert Brown, the English scientist who first identified this process as thermal, or heat induced. At T = 0 K, absolute zero, there is no diffusion. As temperature increases, molecules in one medium vibrate, move, and diffuse into an adjoining medium more readily. The time required for molecules to diffuse from one medium to another is expressed by the diffusion coefficient, D.
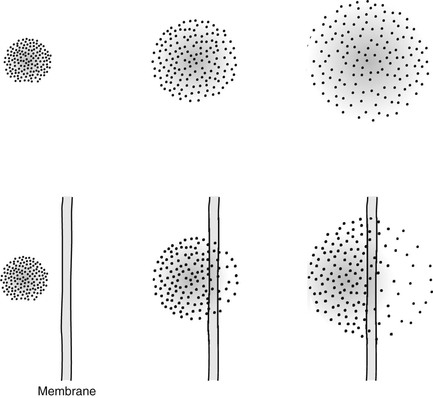
Consider the situation shown in Figure 25-1. If one could isolate two different molecular species into compartments, with time, the molecules would mix. The rate at which such mixing occurs is best described by D.
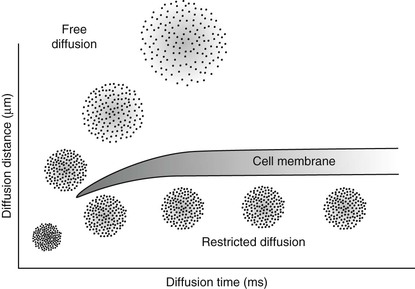
If the diffusion is restricted, as occurs across a cell membrane, the diffusion distance is limited (Figure 25-2). These relationships are described mathematically by Fick’s law and Einstein’s equation, from which one can derive D. The diffusion coefficient has units of mm2/s.
The diffusion coefficient, D, has been measured for many body fluids. The reciprocal of D, a value termed b
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree