SPECT
PET
Presynaptic
AADC activity and vesicular storage of 18F-dopamine
18F-dopa
DAT
123I-β-CIT
11C-CFT
123I-FP-CIT
18F-CFT
123I-altropane
18F-FP-CIT
99mTc-TRODAT-1
11C-methylphenidate
11C-RTI-32
VMAT-2
11C-DTBZ
Postsynaptic
D2 receptor
123I-IBZM
11C-raclopride
11C-NMSP
18F-6-fluorodopa (18F-dopa) is a PET tracer that is taken up by dopaminergic neurons, where it is converted by AADC into 18F-Dopamine. This is stored in the synaptic vesicles and transported to pre-synaptic terminals before being released. The influx constants (Ki) of 18F-dopa therefore reflects DOPA transport into the dopaminergic terminals, AADC activity and dopamine storage capacity [5]. Striatal 18F-dopa influx constants (Ki) correlated with nigral dopaminergic cell density in a human post-mortem study [6].
Several PET ligands (11C-CFT, 18F-CFT, 18F-FP-CIT, 11C-methylphenidate and 11C-RTI-32) and SPECT tracers (such as 123I-β-CIT, 123I-FP-CIT, 123I-altropane and 99mTc-TRODAT-1) are now available to measure DAT availability as an indicator of pre-synaptic dopaminergic function. 11C-dihydrotetrabenazine (11C-DTBZ) is a PET tracer that binds to VMAT2. SPECT (123I-IBZM) and PET (11C-raclopride, 11C-NMSP) tracers that bind to dopamine D2 receptors are also available to image post-synaptic dopaminergic functions.
Due to its expense, PET is generally available only as a research tool. By contrast, several DAT SPECT tracers such as 123I-β-CIT and 123I-FP-CIT are now commercially available for diagnostic use in clinical settings.
Imaging Pre-synaptic Dopaminergic Function in Parkinson Disease
In iPD, the loss of nigrostriatal dopaminergic projection is consistently reflected by the above PET and SPECT pre-synaptic dopaminergic markers as asymmetric reduction of tracer uptake or binding in the putamen, greatest in the side contralateral to the clinically more affected limbs [7–9]. There is also a caudal–rostral gradient loss of uptake/binding, starting from caudal putamen, and eventually progressing to rostral putamen (Fig. 8.1), reflecting the regional selectivity of iPD pathology in the SNc as described above. The caudate nucleus is also affected in the later stages. The nigrostriatal dopaminergic dysfunction as measured by PET or SPECT correlated with the clinical severity of iPD as measured with Unified Parkinson’s Disease Rating Scale (UPDRS) . More specifically, it correlated with the degree of rigidity and bradykinesia , but not tremor [9–13]. This indicates that tremor is either mediated by non-nigrostriatal and/or non-dopaminergic pathways. The latter would be in keeping with the clinical findings that bradykinesia and rigidity are more responsive than tremor to levodopa treatment [14].
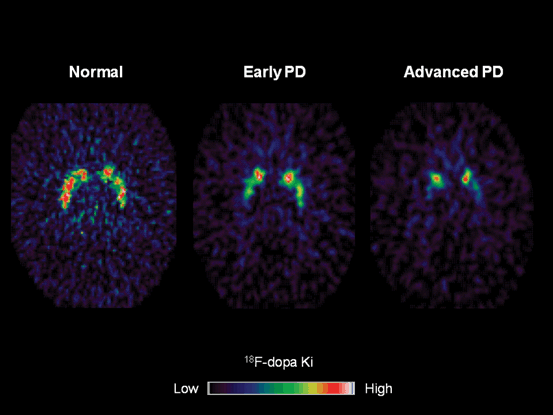
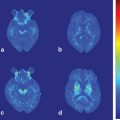
Fig. 8.1
18F-dopa PET parametric images showing progressive and asymmetric reduction in striatal 18F-dopa influx constants (Ki) in idiopathic Parkinson disease (iPD). There is a caudal–rostral gradient in nigrostriatal dysfunction, with the caudal putamen being most affected, followed by rostral putamen and caudate nucleus
18F-dopa PET has detected extrastriatal reductions in 18F-dopa uptake in iPD, such as in hypothalamus, globus pallidus and subthalamic nucleus, reflecting advancing iPD pathology [15]. However, studies have also identified regions of increased 18F-dopa uptake in dorsolateral prefrontal cortex, anterior cingulate, and globus pallidus interna (GPi) of patients with early iPD compared with both normal controls and patients with more advanced disease [16–18]. It is likely that these increases in extrastriatal 18F-dopa uptake in early PD reflect a compensatory upregulation of AADC activity to maintain dopaminergic output. For example, Whone et al. [18] reported a 40 % increase in GPi 18F-dopa uptake in early iPD when there is relative clinical stability. This is likely to arise from the upregulation of nigropallidal projection to normalise basal ganglia output to the ventral thalamus and motor cortex. The loss of the upregulation of GPi signal coincided with the onset of more rapid clinical progression and development of motor complications.
Lee et al. [19] studied 35 iPD patients using three pre-synaptic dopaminergic PET tracers, 11C-DTBZ, 18F-Dopa and 11C-methylphenidate. They showed that in iPD, the striatal terminal AADC activity appears to be relatively upregulated and DAT binding relatively downregulated compared with VMAT2 density. These are interpreted by the authors as evidence of compensatory mechanisms of the surviving dopaminergic terminals to enhance dopamine transmission in the denervated striatum. As the disease progresses, these compensatory mechanisms are lost [15, 20].
Dopamine Imaging as a Diagnostic Tool in Parkinsonism
When a patient presents with the classical signs of iPD, the diagnosis is usually fairly straightforward. However, distinguishing iPD from other degenerative and non-degenerative forms of parkinsonism can be difficult especially in the initial stages. One post-mortem study reported that 24 % of patients diagnosed with iPD in life turned out to have an alternative diagnosis [21]. In an epidemiological study, around 20 % of patients with iPD in the community were misdiagnosed [22]. Making the correct diagnosis would enable the appropriate treatment of the underlying condition and avoid exposing the patients unnecessarily to medications that might not be effective or have significant side effects. It is also important for correct prognostication.
Numerous studies have been performed to validate the diagnostic accuracy of SPECT or PET in parkinsonism and its mimics, but most are slightly limited by the fact that they use clinical diagnosis as the gold standard.
One multi-centre study looked at the accuracy of visual assessment of 123I-FP-CIT SPECT imaging in differentiating patients with degenerative parkinsonism from essential tremor (ET). Scans with normal DAT binding were classified as ET whilst abnormal scans were classified as having degenerative parkinsonism, and clinical diagnosis is the gold standard. Visual assessment of DAT SPECT achieved a sensitivity of 97 % for the diagnosis of parkinsonism, and a specificity of 100 % for ET [23]. In a prospective 123I-FP-CIT SPECT study, patients in whom there was initial diagnostic uncertainty as to whether they have degenerative or non-degenerative parkinsonism received SPECT at baseline and serial clinical assessments over 3 years, with the eventual clinical diagnosis being used as the gold standard. Baseline 123I-FP-CIT SPECT diagnosed degenerative parkinsonism with a sensitivity of 78 % and specificity of 97 %, versus baseline clinical diagnosis which has sensitivity and specificity of 93 and 46 %, respectively [24]. The authors concluded that degenerative parkinsonism is over diagnosed clinically at baseline and that DAT SPECT can help improve the diagnostic accuracy.
Approximately 10–15 % of patients diagnosed with iPD and recruited into clinical trials have scans without evidence of dopaminergic deficit (SWEDDs) [25]. These patients respond poorly to dopaminergic medications and do not display progression on serial DAT SPECT. Some of these patients have signs of dystonia or components of their arm tremor that are compatible with dystonic tremor [26].
Differing patterns of pre-synaptic dopaminergic involvement have been reported in patients with atypical neurodegenerative parkinsonism [27, 28]. Diffuse loss of nigrostriatal dopaminergic projection is seen in multiple system atrophy (MSA) and progressive supranuclear palsy (PSP), as reflected by the symmetrical loss of 18F-dopa uptake in the entire striatum. Patients with corticobasal degeneration (CBD) show equivalent reduction in 18F-dopa uptake of caudate and putamen, but the striatum opposite the most affected limbs has the greatest reduction. However, accuracy of 18F-Dopa PET and DAT SPECT in distinguishing the various forms of degenerative parkinsonism on an individual patient basis is rather low [29, 30].
Post-synaptic D2 imaging with 11C-raclopride PET may help further distinguish iPD from the atypical parkinsonisms . Striatal D2 receptors binding is normal or slightly upregulated in patients with early iPD, but it is significantly reduced in patients with atypical parkinsonism. However, in more advanced cases of iPD especially following long-term treatment with dopaminergic medications, the D2 receptors can become downregulated [31, 32].
Cognitive impairment is a significant cause of morbidity in iPD and the average prevalence of dementia in iPD patients is up to 40 % [33, 34]. For practical purpose, patients who develop dementia within 1 year of onset of iPD motor symptoms are classified as dementia with Lewy Bodies (DLB), whereas those who develop dementia more than 1 year after onset of motor symptoms are classified as PD with dementia (PDD). Many authors, however, consider these two conditions as representing different spectrum of the same underlying pathological process, i.e., Lewy Body disorders [35]. Distinguishing patients with DLB and Alzheimer’s disease (AD) can be difficult in some patients. Both of these conditions respond to cholinesterase inhibitors but patients with DLB have marked sensitivity to neuroleptics and this group of medications should be avoided if possible. One study compared the accuracy of clinicians and 123I-FP-CIT SPECT in diagnosing DLB, using post-mortem findings as the gold standard. The sensitivity and specificity of an initial clinical diagnosis of DLB was 75 and 42 %, respectively. Using 123I-FP-CIT SPECT, the sensitivity and specificity improved to 88 and 100 %, respectively [36]. In a phase III multi-centre study, 123I-FP-CIT SPECT was able to diagnose clinically probable DLB with a sensitivity of 77.7 % and a specificity of 90.4 % when measured against clinical diagnosis established by a consensus clinical panel [37]. The International Consensus Criteria for DLB has now recommended reduced striatal pre-synaptic dopaminergic markers, including DAT, on SPECT/PET as a suggestive feature for the diagnosis of DLB [38]. Dopaminergic imaging is not able to distinguish between PDD and DLB as they both exhibit nigrostriatal dopaminergic dysfunction.
Dopaminergic imaging has also been used to differentiate neurodegenerative from non-neurodegenerative forms of parkinsonism. Drug-induced parkinsonism is usually caused by the use of anti-dopaminergic agents such as neuroleptics or anti-emetics. It can be difficult to distinguish it clinically from iPD. Pre-synaptic dopamine imaging is usually normal in these patients unless they have concomitant iPD or degenerative parkinsonism where the symptoms have been unmasked or worsened by the use of anti-dopaminergic agents [39, 40]. Most patients with vascular parkinsonism have normal striatal pre-synaptic dopamine binding [41] though some may have reduced binding [42, 43]. In those that display a reduction, the pattern is usually more symmetrical and diffuse than that of iPD. Dopamine imaging is also helpful in evaluating patients with suspected psychogenic parkinsonisms. An abnormal pre-synaptic dopamine PET/SPECT is highly suggestive of an organic aetiology [44].
The United States Food and Drug Administration (FDA) has recently approved the use of 123I-FP-CIT SPECT (DaTscan™) for the detection of dopamine transporters in patients with suspected parkinsonism syndromes, and the decision was largely based on two prospective phase III diagnostic studies discussed above [23, 24]. Re-analyzing data from these two studies, however, one recent report concluded that the clinical diagnostic accuracy of PD is very similar to that achieved by DAT SPECT [45]. The author of the study cautioned against routine use of DAT SPECT for the diagnosis of iPD.
In patients with classical signs of iPD and good levodopa responsiveness, DAT SPECT probably has little to add to clinical diagnosis. We feel that DAT SPECT is of most value clinically when used (1) in patients with indeterminate tremor (e.g., to distinguish atypical ET or SWEDDs from tremor-dominant iPD); (2) to differentiate degenerative forms of parkinsonism from the non-degenerative forms (particularly drug-induced and vascular parkinsonism); and (3) to identify patients with mixed pathology, e.g., AD and DLB, or drug-induced parkinsonism and iPD.
Detecting Subclinical Dopaminergic Dysfunction in High-Risk Patients
PET and SPECT have been able to detect subclinical dopaminergic dysfunction in subjects at high risk for iPD. Studying these subjects can provide important insight into the preclinical phase of iPD, and enable trials of putative neuroprotective agents to delay or even prevent disease onset. One longitudinal PET study investigated the role of inheritance in sporadic iPD by investigating twin pairs who were clinically discordant for iPD at baseline. Subclinical nigrostriatal dopaminergic dysfunction was found in 55 % of monozygotic twin pairs, versus only 18 % in dizygotic pairs. All the 18 asymptomatic monozygotic co-twins showed deterioration in striatal 18F-Dopa uptake over 7 years, and four of them developed clinical iPD [46].
Interestingly, two PET studies have demonstrated reduced striatal 18F-Dopa uptake in asymptomatic carriers of a single parkin mutation, which causes autosomal recessive parkinsonism[47, 48]. Longitudinal studies are required to establish whether these subjects will progress to clinical iPD.
123I-β-CIT SPECT has been used to evaluate dopamine terminal integrity in asymptomatic relatives of iPD patients. Among those with idiopathic hyposmia, a known risk factor for PD, 17.5 % had reduced striatal 123I-β-CIT binding, and half of these 17.5 % converted to clinical iPD over a 2-year follow-up [49].
Asymptomatic carriers of the Leucine-rich repeat kinase 2 (LRRK2) mutation, an autosomal dominant form of parkinsonism, have been found to display compensatory changes similar to those seen in early iPD patients, with downregulation of DAT binding and upregulation of AADC activity, probably as an attempt to delay the onset of parkinsonian symptoms [50]. Some of these subjects showed progressive reduction in pre-synaptic dopaminergic function, with DAT being the most sensitive indicator. Reduction in AADC activity as evidenced by abnormal 18F-dopa uptake is associated with the emergence of clinical symptoms [51].
Using Dopamine Imaging to Measure Disease Progression and Effects of Putative Neuroprotective Agents in Parkinson Disease
PET and SPECT have been employed to monitor the progression of nigrostriatal degeneration in iPD. Using serial 18F-dopa PET, the mean annual rate of reduction in striatal uptake in iPD is reported to be 8–12 % in the putamen and 4–6 % in the caudate nucleus, compared with 0.5 and 0.7 % in the putamen and caudate respectively in healthy volunteers [15, 52–54]. Extrastriatal involvement is detected only at a later stage of iPD and the annual rates of reduction in 18F-dopa uptake in these regions are slower than that seen in the putamen, with 7.7 % in the GPi, 6.1 % in the hypothalamus, 4.9 % in the ventral striatum, and 2.4 % in the globus pallidus externa. The authors found no correlation between the rates of reduction of 18F-dopa uptake in the putamen and extrastriatal regions, suggesting that the degenerative processes affecting nigrostriatal and extrastriatal structures may occur independently [15]. One of the longitudinal PET studies also looked at the absolute, rather than the percentage, decline in striatal 18F-Dopa Ki in iPD and found that the rate of decline is the same in all striatal subregions [54].
In a multi-tracer longitudinal PET study, 11C-DTBZ, 18F-Dopa and 11C-methylphenidate PET were used to investigate the rate of progression of nigrostriatal dopaminergic dysfunction in iPD. The authors also found that the decline in nigrostriatal dysfunction affects all striatal subregions at a similar rate and the anteroposterior gradient of reduced tracer uptake/binding in the putamen is preserved as the disease progresses [55]. Findings from this paper and another study [56] also reveal a negative exponential progression in nigrostriatal dopaminergic dysfunction, with a higher rate of cell loss in the earlier stage of the disease.
Dopamine imaging with PET/SPECT has been used as a marker of disease outcome in many disease modifying trials in iPD. Parkinson disease patients taking dopamine agonists such as ropinirole [57] or pramipexole [58] showed a significantly slower decline in nigrostriatal function, as measured with 18F-Dopa PET and 123I-FP-CIT SPECT, respectively, compared with patients assigned to levodopa. However, the latter group of patients have better clinical scores as measured using UPDRS compared with patients taking dopamine agonists. In another study that assessed the effects of levodopa on iPD disease progression, although levodopa improved the clinical status of de novo iPD patients, these patients showed a greater reduction in striatal 123I-FP-CIT SPECT compared with patients taking placebo even after a 2-week washout period [59]. Numerous theories have been proposed to explain this apparent discordance between clinical and imaging outcomes. If the imaging markers indeed reflect the true biological state of the nigrostriatal dopaminergic neurons, it is possible that dopamine agonists are neuroprotective and levodopa is neurotoxic to dopaminergic neurons, and the better clinical outcome seen in patients taking levodopa is due to the greater symptomatic effect of the medication. However, levodopa and dopamine agonists may have direct and differential effects on the imaging markers, with the former acting to depress DAT binding and AADC activity. Further studies are required to discover and validate a truly objective biological marker. Nevertheless, current PET/SPECT markers still have a role to play in demonstrating dopaminergic neuron survival in neurorestorative studies such as fetal neural transplantation [60] and infusion of neurotrophic factors [61].
Assessment of Synaptic Dopamine Release with 11C-Raclopride PET
11C-raclopride PET, which reflects post-synaptic D2/D3 status, is sensitive to competition with endogenous dopamine and has been used to measure changes in extracellular dopamine levels induced by pharmacological challenges, motor and behavioural tasks. Animal microdialysis studies have confirmed that a 10 % reduction in striatal 11C-raclopride binding reflects a fivefold increase in synaptic dopamine levels [62]. This method provides an innovative approach to study the motor and behavioural manifestations of iPD, including dopamine dysregulation syndrome (DDS) and impulse control disorder which are thought to be related to sensitisation of the dopamine pathways mediating reward.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree