Fig. 1
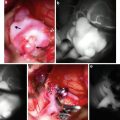
Fedor Serbinenko, the Russian neurosurgeon that conceived the idea of detachable balloons (a) and developed the technique of their use. Historical picture of latex detachable balloons (b). The mechanism of detachment of balloons (c)
The idea and the technique of detachable balloons became widespread and had a significant impact on intervention strategy for direct arteriovenous fistulas and brain aneurysms. Starting from there, therapeutic endovascular artery occlusion has become routine all over the world, in all cases of inoperable aneurysms [12, 13]. Even today, the choice of occluding the parent vessel remains a valuable and safe option in cases of extremely complex lesions, such as giant aneurysms of the internal carotid or dissected aneurysms (Fig. 2).
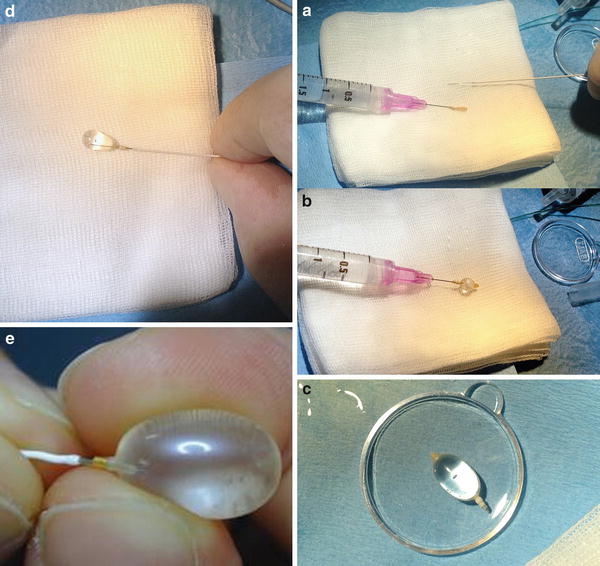

Fig. 2
Preparing the detachable balloon (Goldbal2; Balt) (a–e). A GBV2 mounted on the needle of a 2 cc syringe and a Magic 1.2 Fr catheter (Balt) (a). Inflation test of the balloon. The syringe is prepared with a solution of ½ saline and ½ contrast medium (b). Testing the continence of the GBV (c). The GBV is inflated and mounted on the tip of the Magic catheter with the help of a Stylet wire (d, e)
Procedure (Tips and Tricks)
The procedure of vessel occlusion has to be previously confirmed by an occlusion test. The test allows the interventionist to know whether it is possible to occlude the target vessel. A rough test could be done with a manual compression at the neck of the vessel one plans to occlude while carrying out angiographic runs of the other vessels. To obtain more complete information on collaterals and safety, it is necessary to perform a balloon test occlusion (BTO).
The BTO is done with the patient awake and under systemic anticoagulation with heparin (80 IU/kg). Two femoral sheaths must be placed: one for the guiding catheter and the other one for angiographic control from the other vessels during the occlusion test. A complete angiographic study of the cerebral circulation must always be obtained before starting the procedure. The angiographer has to carefully evaluate the function of the communicating arteries and the presence of anastomosis, particularly among the branches of the external carotid artery and internal carotid or vertebral artery. The second step is to perform BTO. The BTO is realized by placing an inflatable balloon into the target vessel under fluoroscopic guidance at the point where one wants to occlude the artery. The point of occlusion should be chosen very carefully, in light of the previous angiographic study, to avoid occluding the artery in the wrong location, i.e., a location where the occlusion may leave a persistent filling of the aneurysm sac through anastomosis. The balloon is inflated up to the point of occluding the lumen of the vessel; the occlusion is maintained for at least 15–30 min during which time, through angiographic runs; the intra- and extracranial collateralization is evaluated. Neuropsychological tests are performed to assess the possible occurrence of a neurological deficit. It is always possible to perform stress tests, provoking a drug-controlled hypotension, in such a way as to highlight subliminal neurological deficit.
The balloon test occlusion has two results: an angiographic result and a clinical one. The angiographic test is passed only when the vein opacification of the territory downstream from the occluded artery is contemporary to the rest of the brain up to, but not more than, two seconds later. The clinical test is passed if the occlusion does not provoke any neurological deficits. If the occlusion test is not tolerated, the balloon is deflated, the procedure is discontinued, and one may consider the realization of a surgical bypass. If the test occlusion is instead tolerated, one can finally occlude the artery, by detaching the balloon (in case of a detachable balloon) or by substituting the balloon with coils or a different occlusion device.
Arterial occlusion is simple and very effective, but it is certainly not riskless [14, 15]. Detachable balloons are not available anymore, mostly due to commercial reasons. For this reason in recent years, therapeutic occlusion is almost always performed using coils (Figs. 3, 4, and 5).
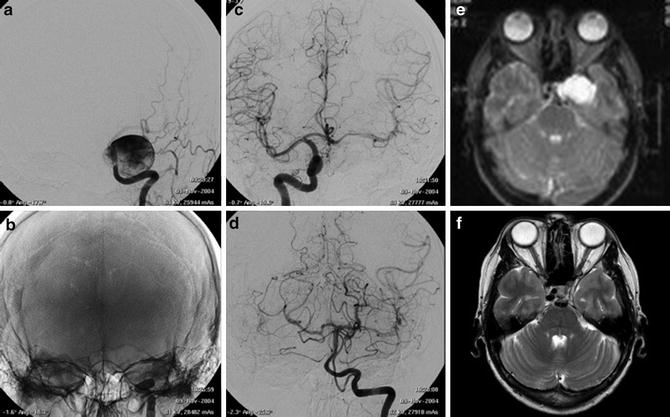
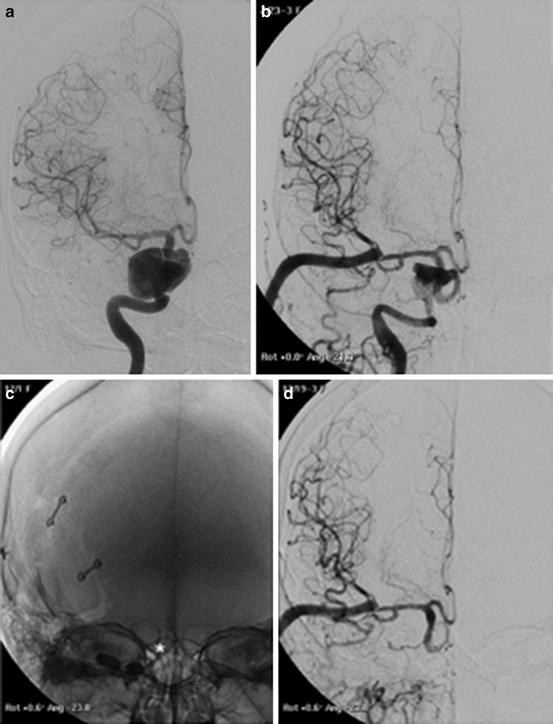
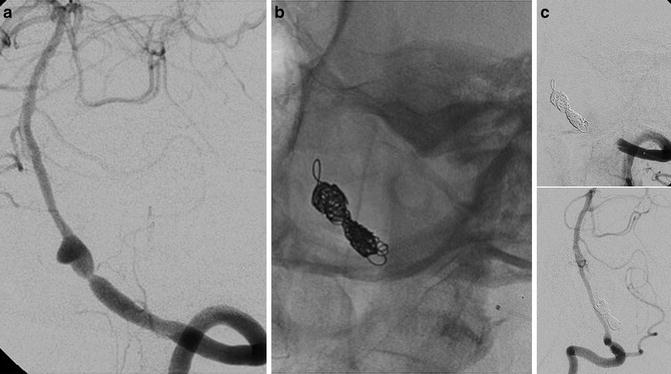

Fig. 3
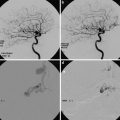
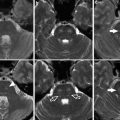
Images obtained in a 50-year-old with a giant cavernous aneurysm symptomatic for headache. Endovascular treatment by vessel occlusion. Frontal view of the left internal carotid injection (ICA) (a). The occlusion of left ICA by 2 gold valve balloon (Balt) (b). Frontal views of the right ICA and the left vertebral (VA) injections showing the collateralizations through communicating arteries and through pial collaterals (c, d). T2-weighted brain MR images of the aneurysm before (e) and after 2 years from the treatment (f). Note the complete shrinking of the aneurysm

Fig. 4
Images obtained in a 68-year-old man suffering from right CN III palsy caused by a partially thrombosed cavernous aneurysm. A combined surgical and endovascular treatment: bypass and artery occlusion. Frontal view of right ICA injection before the treatments (a). Frontal view of right common carotid (CCA) injection after the creation of a high-flow bypass between right CCA and ipsilateral MCA (b). Note the initial slow downing of the ICA. Occlusion of right ICA with balloons (*, c). Frontal view of bypass injection with excellent revascularization of the right hemisphere and reflow in the supraclinoid ICA and in the ophthalmic artery (d)

Fig. 5
Endovascular treatment by artery occlusion with coils after repeated subarachnoid bleedings in a 63-year-old lady. Oblique view of the left VA injection (a) showing an intracranial dissection of the VA. Note the absence of branches rising from the dissected tract. Oblique views of left and right VAs showing the complete occlusion of the left artery by coils and good supply of the posterior circulation by the right one (b, c)
Iron Age (Coiling and Balloon Remodeling)
In the early 1990s a new device changed forever the treatment of cerebral aneurysms: the electrical detachable coil invented by Guido Guglielmi (Guglielmi detachable coil). Coils (metallic filaments that would curl up on themselves) were already being used for filling aneurysms, but the problem was that by pushing them through a microcatheter it was almost impossible to control their correct final position, with the risk of losing them in distal arteries (Fig. 6).
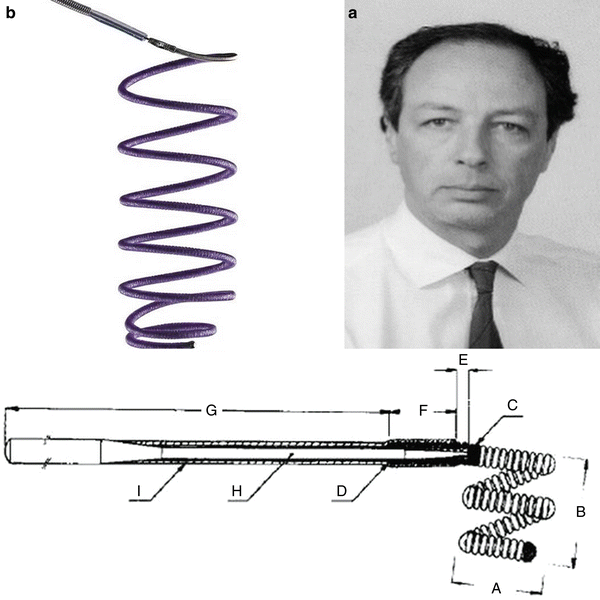

Fig. 6
Guido Guglielmi (a, b) and his revolutionary coils
Guglielmi achieved the goal of controlling the detachment of the coils from the pusher wire. One could finally detach the coil only once there was the evidence of a correct positioning. The detachment is realized with an electrolytic mechanism. The aneurysm is then filled through the release of several consecutive coils, obtaining a small ball of thread. Initially the idea had been to cause the thrombosis of the aneurysm sac by coagulating the blood through an electric current necessary to detach the coil inside the aneurysm. It was soon evident that that approach would not work and that it was necessary to fill the sac with filaments rather than causing thrombosis [16, 17].
The first GDCs made their appearance on the world stage in 1991 and very quickly demonstrated their effectiveness. The first coils had a filament diameter of 0.010 in., and subsequently coils of 0.015 in. were produced, making more rapid filling of large aneurysms possible; the 0.015 in. versions were later replaced by those measuring 0.018 in. Platinum was chosen because it is a metal that does not undergo electrolysis and because it is spontaneously radiopaque, as well as being thrombogenic and inert.
The detachment of the coil takes place by the closing of an electric circuit powered by a battery, in which the positive pole is coupled to the tail of the coil, placed outside the microcatheter, and the negative pole is coupled to a needle inserted under the skin of the patient. The passage of a low-voltage current causes the electrolysis of the micro-welding causing the detachment of the platinum filament. The newest detachment systems do not need needle insertion anymore.
GDCs were initially used for the treatment of ruptured aneurysms which neurosurgeons considered technically impossible – or at least very difficult – to address. It could be because of their location (e.g., the tip of the basilar) or of their characteristics (Fig. 7). The selected patients were mostly elderly or in poor clinical condition [18]. The range of measurements and spatial conformations of the initially available GDCs, defined as the diameter of a single loop of the coil and the length of the entire filament, was very limited. Nevertheless, the idea had an immediate extraordinary success. Endovascular treatment with coils showed clear advantages compared to neurosurgical clipping: in many “difficult” cases the treatment was relatively easy, fast and, especially, less aggressive than a neurosurgical intervention, at the same time ensuring a more-than-satisfactory result in excluding the aneurysm. At the beginning of the coiling experience, there was no scientific proof of the truth of these observations, but the efficacy of the method was simply evident [19–22]. Over time different companies provided a more comprehensive range of sizes of coils with different degrees of stiffness (normal, soft, ultrasoft, etc.) and with different spatial conformations of the loops (2D, 3D, complex, etc.).
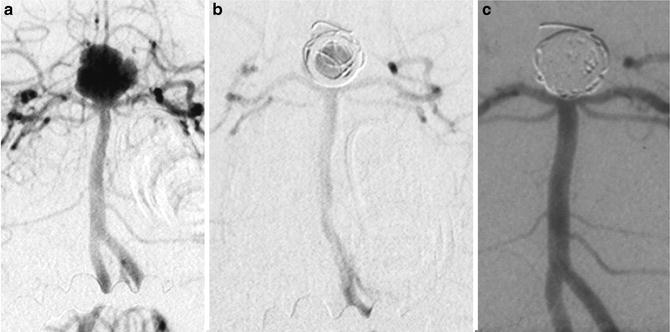

Fig. 7
Images from one of the oldest cases (first in Italy) of endovascular treatment with GDC coils in 1993. A 34-year-old lady with a basilar tip ruptured aneurysm. Frontal views of left vertebral injection (a–c). Before the treatment, after the detachment of the first coil, and in the 5-year follow-up
Procedure (Tips and Tricks)
The procedure is performed under general anesthesia to ensure immobility of the patient and to allow the catheterization to be as safe as possible. Systemic anticoagulation is usually required, and some authors would consider also antiplatelet therapy. A good practice in neurointerventions is that all catheters – such as the guiding catheter and the microcatheter – must be constantly perfused using heparinized saline.
It is highly recommended to start the procedure by performing an accurate angiographic study with 3D reconstructions of the aneurysm, in order to define the working projections that should be used during the intervention. The projections must ensure the best possible view of the aneurysmal neck, namely, the plane passing between the aneurysm and parent vessel. The working projection should also take account of the presence of other vessels, especially if they originate in the vicinity of the sac. The accurate study of 3D and 2D images is crucial in allowing the operator to build a mental image of the aneurysm and its surroundings. In addition, the 3D helps in choosing both the measures of the first coil and the curve that the tip of the microcatheter should have. The tip of the microcatheter might come pre-curved in the package or it might be prepared using steam, depending on the characteristics of each single aneurysm. It is also very important to bend the tip of the microwire.
The choice of the size and shape of the coils for the treatment is entirely operator dependent: the goal being to place coils in order to obtain a complete and compact filling of the aneurysm. The common practice is to start with a coil of a diameter corresponding to the maximal diameter of the aneurysm and then fill the interstices with progressively smaller coils. It is advisable to use a Y connector with rotating hemostatic valve for both the guiding catheter and the microcatheter to ensure maximum stability of the system. The microcatheter with the microwire is tracked inside the guiding catheter and under fluoroscopic vision pushed close to the aneurysmal neck.
The entrance of the wire and entrance of the catheter into the aneurysm are two key moments, especially in the case of ruptured aneurysms. The maneuver should therefore be performed with great care, avoiding touching the walls of the aneurysm if possible. Once the aneurysm is catheterized, the microwire should be withdrawn and replaced with the first coil. The release of the coil must be very gentle, a smooth movement, continuous, progressive, and slow. In order to allow the adaptation of the coil to the walls of the aneurysm, creating a so-called basket. The release of the first coil is crucial: if well realized, it allows easier filling of the basket by the subsequent coils. The loop protrusion outside the aneurysmal neck in the parent artery is an occurrence that should obviously be avoided due to the chance that this will stimulate platelet aggregation with subsequent embolism risk. After the release of the first coil, coils with a progressively smaller diameter are usually chosen. The filling of the sac should be continued until the coil release becomes too difficult.
Along with the advantages of this technique, its limitations have also quickly emerged. The wider the aneurysmal neck is, the more difficult the treatment of an aneurysm with coils is. The most frequent issues with coiling have been ischemic complications determined by the formation of blood clots, along catheters or on the coils placed at the level of the aneurysmal neck. The risk of thromboembolic events is directly related to the length of the procedure. Simple and fast procedures have major advantages in terms of reducing complication rates. The ischemic complications are best taken care of with a good technical approach, clean sheets and catheters, and heparin injections. Only rarely one has to try to dissolve some major clot in cerebral vessels. The use of fibrinolytics is forbidden, because of high chances of having a rebleed from the aneurysm. Glycoprotein IIb/IIIa inhibitors may be of help in some cases, but often the clot especially if limited in size will dissolve spontaneously.
Hemorrhages caused by the rupture of aneurysms during catheterization of the sac or during the release of coils are less frequent. In this unlikely event, the only thing to do is to deploy coils inside the aneurysm as quickly as possible until the bleeding stops (Figs. 8 and 9)
.


Fig. 8
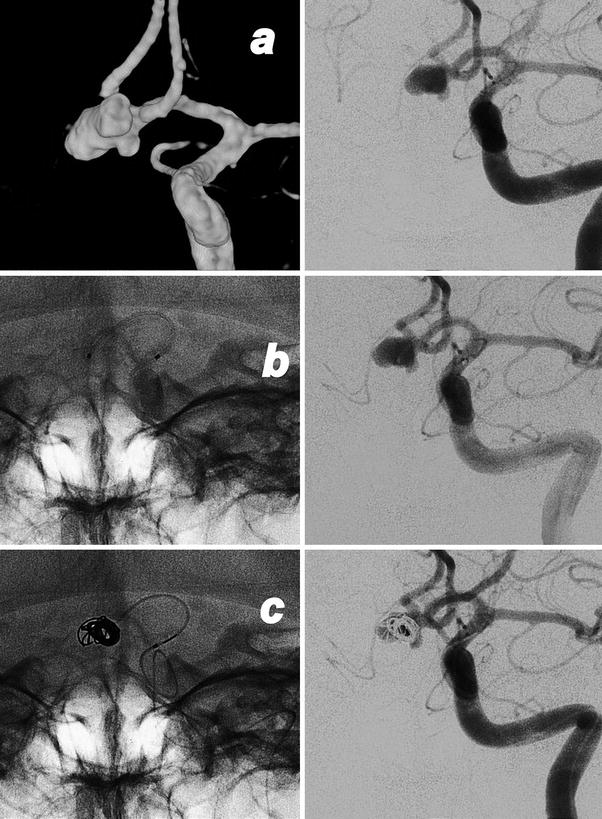
Endovascular treatment of a ruptured aneurysm of the AComm. Images obtained in a 52-year-old lady who suffers of a subarachnoid hemorrhage. Preliminary 3D rotational angiography (a) and frontal view of the left internal carotid injection showing the work projection. AP views subtracted and un-subtracted showing the consecutive steps of the procedure (b–e). The positioning of the microcatheter in the aneurysmal sac (b). Progressive filling of the lesion with consecutive deployment and detachment of four coils (c, d). Final result after removal of the catheter (e)

Fig. 9
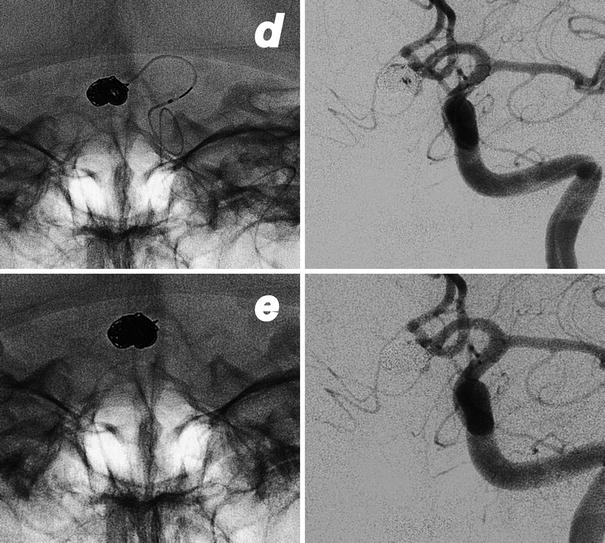
Intraprocedural rupture of an AComm aneurysm in a 49-year-old lady. The tip of catheter punches the aneurysm at the level of the neck (a, b). Immediate leakage of contrast medium (b, c) in the subarachnoid spaces during the left ICA injection. Fast, consecutive deployment of three coils with the arrest of bleeding (d, e) and good exclusion of the aneurysm
Another complication of this type of treatment is stretching of the coil: by pushing and pulling repeatedly the platinum filament may stretch to the point of rupture. At that point placing the coil into the aneurysm or withdrawing it could be impossible. Coil stretching was more frequent in the past. Now, with the introduction of the stretch-resistant mechanism, constituted by a further filament which links the first and the last loop of the coil, stretching has become a more rare phenomenon. Stretching a coil is still possible though, particularly in cases of long and difficult positioning with continuous advances and withdrawals of the coil. If the event occurs stretched coils may be retrieved using a retrieval system (goose neck or similar). If unsuccessful one can stretch even the proximal part of the coil down into the aorta, so that the downward blood stream will keep it in place.
Angiographic follow-up of the tens of thousands of aneurysms treated with coils all over the world has highlighted another important limitation of this type of treatment: the tendency of coiled aneurysms toward recanalization. Recanalization is variable from aneurysm to aneurysm, partly depending on the coil density at the end of treatment. Recanalization is more frequent in large and giant aneurysms and in wide-neck aneurysmal necks, but it often occurs even in those aneurysms which appear to be perfectly filled at the end of treatment. The first cause of the phenomenon has to be found in a spontaneous settling and compaction of the coils toward the bottom of the sac, under the effects of blood flow and blood pressure. The degree of recanalization of the sac, as we have said, is variable and is usually highlighted in angiographic controls already at 6–12 months after treatment, and then, in most cases, it stabilizes over a longer period of 2–5 years. The main consequence of this observation has been that a high proportion of treated aneurysms have undergone a second, a third, or additional endovascular treatments in pursuit of the best possible compaction of the coils inside the sac, increasing, for each treatment, the overall risk of clinical and technical complications [22].
In 1994, a French neuroradiologist, Jacques Moret, devised and put into practice a strategy for limiting the problem of wide necks. The idea was to place a catheter with an undetachable compliant balloon in the parent vessel in front of the aneurysmal neck and inflate it during the release of the coils through a second catheter previously positioned in the aneurysmal sac. The inflated balloon allows an easier deployment of coils preventing coil prolapse in the parent vessel. Moreover, the presence of the inflated balloon allows to place more coils in the sac, but most importantly, in cases of a rupture of the aneurysm, the balloon can immediately stop the bleeding.
Moret named this advance in endovascular techniques the remodeling technique. This improvement quickly proved its effectiveness and allowed the treatment of aneurysms until then considered extremely difficult [23].
Nowadays single-lumen balloon and double-lumen balloon catheters are available. The balloon is inflated and deflated by a small (1–2 ml) syringe injection. The balloon is maintained inflated for the time necessary for the deployment of each coil, but usually for no longer than 5 min, in order to avoid ischemic complications. The procedure is obviously best performed in a condition of systemic heparinization in the majority of cases.
Endovascular treatment of aneurysms of the bifurcation became possible thanks to the contemporary positioning of two balloons: one balloon for each of the branches of the bifurcation, in order to protect both of them.
Thanks to the remodeling technique the treatment of aneurysms with coils had a further extension of its indications and, during the 1990s, became quikly the treatment of choice for all aneurysm [24, 25]. All over the world surgery was progressively replaced by the endovascular approach, and in some countries neurovascular surgery has progressively declined almost to the point of disappearance.
In 2002, The Lancet published the results of the ISAT (International Subarachnoid Aneurysm Trial) which compared the outcomes of the two types of intervention in a population of over 2000 patients with subarachnoid hemorrhage from ruptured aneurysm suitable for both endovascular treatment or surgical clipping. ISAT compared the rate of mortality and the degree of disability at 2 months and at 1 year after treatment. The final results showed a reduction of 7.4 % in the absolute risk of death or dependency at 1 year from the endovascular treatment [26, 27]. The trial results certified what experience and history had already shown: endovascular treatment with coils is a safe and effective way to treat aneurysms, and in most cases it is safer than neurosurgical clipping. For ruptured aneurysms it is the treatment of choice, thanks to its simplicity and to its low degree of invasiveness (Figs. 10 and 11).
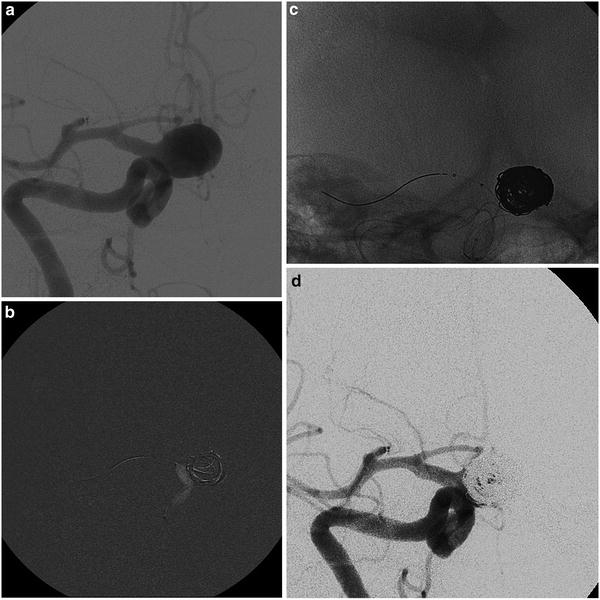
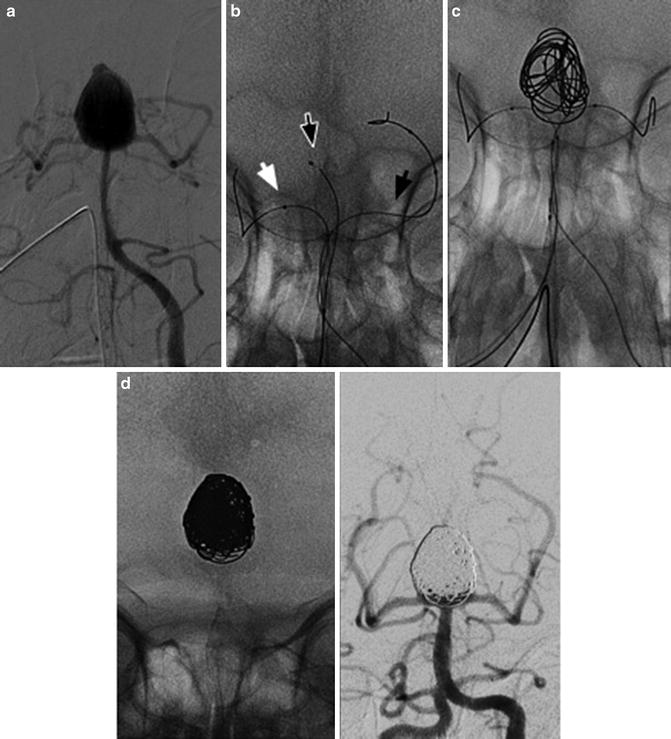

Fig. 10
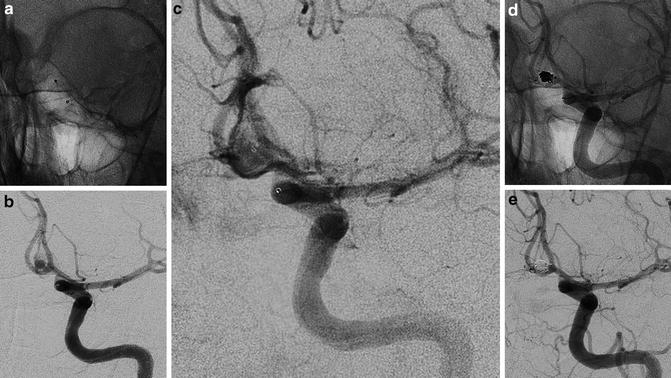
The “balloon remodeling technique” of a ruptured aneurysm in an 81-year-old lady. Frontal view of the right internal carotid injection before the treatment of a wide-neck supraclinoid aneurysm (a). Road mapping image of the first coil deployment protected by the balloon inflated (Scepter; MicroVention). Note the little adjustment (tiny white stripes) of the loops caused by the inflation of the balloon (b). The cast of coils and the position of both catheter and balloon at the end of the filling (c). Final angiogram of the right internal carotid (d)

Fig. 11
Images obtained in a 65-year-old lady with a ruptured basilar tip aneurysm. The endovascular treatment with coils and double balloon. Frontal views of left vertebral injections (a–d). The arrowheads indicate the catheter (Echelon 10, Covidien-Ev3) and two balloons (Transform, Striker), one in every PCA
Renaissance (Stent-Assisted Coiling)
Treatment with coils and the balloon remodeling technique aroused great enthusiasm and interest, and many interventionists tried new ways to advance the techniques. From the mid-1990s onward, some of them experimented sporadically with intracranial stenting procedures – the idea being that a stent might produce the cure of an aneurysm by changing the flow inside the sac and by being a scaffold for endothelial regrowth. They used coronary balloon-mounted stents, but the maneuvers were extremely difficult and cumbersome due to the high rigidity of the systems [28, 29]. The idea of an intracranial stent subsequently considered the possibility to better fill the difficult wide-neck aneurysms, being a support to the coils, in an even more effective way than did the remodeling technique. The need of a stent dedicated to the specificity of intracranial vessels became apparent: the new stent should be softer and easily trackable along curves and tortuosities and should be self-expandable, in order to avoid possible ruptures of vessels by balloon inflation (Fig. 12).
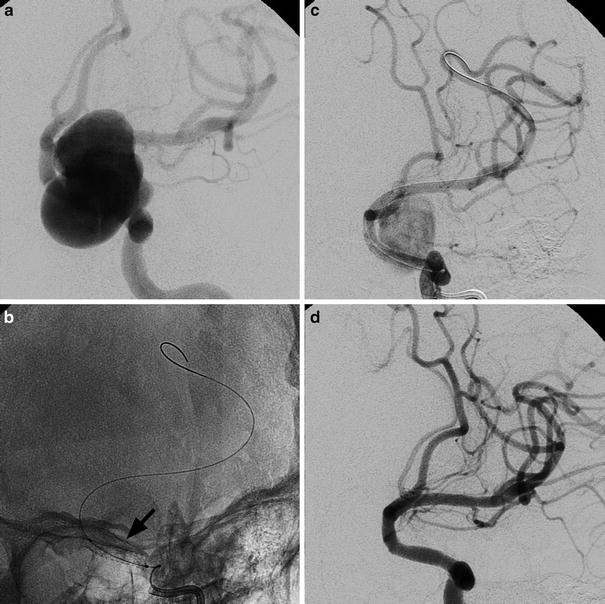

Fig. 12
Oblique view of left internal carotid angiograms in a 53-year-old lady suffering from headache caused by a large supraclinoid aneurysm (a). An un-subtracted image immediately after the deployment of a stent graft (arrowhead); the balloon is still inside (b). Left ICA angiogram showing the initial exclusion of the aneurysm (c). Note the positions of the guiding catheter and the tip of the exchange wire, respectively, in the petrosal tract of ICA and in a distal branch of MCA to guarantee enough support. Final left ICA angiogram (d)
The first prototype of a self-expandable intracranial stent, the Smart, appeared in 2001: it was developed by Dr Peter Kim Nelson and his team at Smart Therapeutics (San Leandro, California), and renamed Neuroform in 2002 when the patent was acquired by Boston Scientific and approved by the US FDA [30, 31]. The Smart system was composed of three parts, a self-expandable stent, a microcatheter, and a stabilizer over the wire. The stent, preloaded in the microcatheter, was an open-cell stent, formed by radiolucent filaments made of nitinol. The diameters of the devices ranged from 3 to 4.5 mm, while the lengths ran from 15 to 20 mm. At the two extremities of the stent, four radiopaque markers ensured visibility and controllability. The delivery catheter was 3 Fr in outer diameter. The stabilizer was a second microcatheter of smaller diameter (0.25 in.) with a radiopaque marker at the distal end. The whole system was navigated by an exchange 0.014″ wire, needed to reach the landing zone. Both the catheter and the stabilizer were equipped with “Y” valve connectors. The system was assembled on the sterile surgical field by inserting the stabilizer in the proximal end of the microcatheter containing a preloaded stent. At the time of the release of the stent, the distal end of the stabilizer was placed next to the proximal end of the stent. Upon reaching the area of release, the microcatheter was withdrawn, keeping the stent in position thanks to the stabilizer; so doing, the stent was released at the target point, across the neck of the lesion. The Neuroform (Smart) was followed by other competing devices: Leo (Balt, Montmorency, France), Enterprise (Cordis, Neurovascular J&J), Solitaire (Ev3, Covidien, Paris, France), and LVIS (MicroVention, Tustin, California, USA), characterized by different design philosophies [32–35] (Fig. 13).
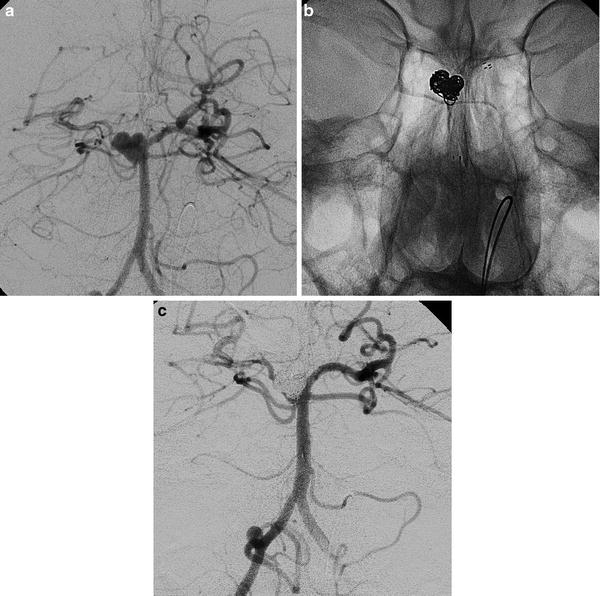

Fig. 13
Stent-assisted coiling of an aneurysm of the right superior cerebellar artery (SCA) (a–c). Images obtained in a 36-year-old man suffering from headache. The right vertebral angiogram showing the origins of the right SCA and the ipsilateral PCA, respectively, from the bottom of the aneurysm and from the basilar artery, behind the sac (a). The presence of the stent (Neuroform, Boston S.) avoids the protrusion of loops in the basilar artery and in both PCAs. Coils have been placed away from the bottom of the aneurysm in order to keep regularly patent the SCA (b, c)
Intracranial stents used for assisted coiling are tubular structures in cells, made of a metal alloy with shape memory, typically nitinol or cobalt-chromium. The stents are released into the parent vessel of an aneurysm, across the aneurysm sac, usually before filling the aneurysm with coils. The self-expandable stents do not need a balloon to open, and hence need an intrinsic radial force, produced in part by the material of which they consist and in part by the geometry with which the cells that constitute them are joined. The memory metal alloys, at a predetermined temperature (usually 37 °C), tend to shape themselves, expanding and bending in space, allowing the stents to achieve a preprogrammed diameter once unconstrained by the catheter. It is mainly the different types of construction – open cell (Smart, Neuroform I, II, and III), braided stent (Leo and LVIS), closed cell (Enterprise), or folded sheet (Solitaire) – which influence the characteristics of different stents: their radial force, their capacity in adapting to vessel tortuosity, their opening and closing resistances, and their ability to retrieve the stent. Every stent has its unique combination of these characteristics. The Solitaire adds the specificity of being fully retrievable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree