Epilepsy
William B. Zucconi
Vivek Gupta
Richard A. Bronen
Recent advances in molecular biology, genomics, and neuroimaging have led to significant improvements in the understanding, evaluation, and management of epilepsy and seizure disorders. A seizure, or epileptic seizure, is the clinical manifestation of abnormal, excessive synchronous neuronal electrical activity. The pathophysiologic basis of a seizure is the loss of normal regulation of neuronal excitation and inhibition, resulting in a state of relative hyperexcitability. The word Epilepsy stems from “epilambanem,” the Greek verb meaning “to take hold of or to seize.” Epilepsy, as recently defined by Fisher et al. (1) is “a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures, and by the neurobiologic, cognitive, psychological and social consequences of the condition”. This is often a chronic condition characterized by recurrent seizures which are unprovoked by an acute systemic or neurologic insult; the term epilepsy itself does not indicate a specific underlying pathology. Although the treatment of an isolated seizure is directed toward the immediate underlying metabolic or neurologic derangement, epilepsy usually requires long-term pharmacotherapy or, in selected cases, neurosurgical intervention to eliminate or reduce recurrent seizures and to prevent progressive neurological and social sequelae. Neurosurgery is most often considered in medically refractory epilepsy when removal or isolation of the epileptogenic region is possible without an unacceptable neurologic deficit. A variety of brain lesions causing epilepsy are amenable to surgical treatment.
The clinical presentation of epileptic seizures is varied, and it is necessary to categorize them according to established classification schemes so that appropriate diagnostic workup, therapy, and prognoses can be assigned. The principal basis of current seizure classification is the distinction between focal seizures (formerly called partial seizures), and generalized seizures. A focal seizure originates in one brain area or network that is limited to its hemisphere. A generalized seizure is understood as beginning in one brain area and rapidly spreading through bilaterally distributed brain networks. Importantly, generalized seizures may still arise from a focal lesion that is amenable to surgical treatment. The most widely used classification of epileptic seizures is that put forth by the International League Against Epilepsy (ILAE) (Table 7.1, abbreviated). It was revised in 2010 and remains principally based on the clinical seizure type and interictal electroencephalography (EEG) findings (2). Notable changes from the prior scheme include replacing the term “partial” with “focal.” In addition, the terms “simple partial, complex partial, and secondarily generalized” were discarded in favor of the more straightforward descriptors: “seizure without impairment of consciousness,” “with impairment of consciousness or awareness, or dyscognitive,” and “evolving to a bilateral convulsive seizure,” respectively. Table 7.2 lists additional useful and well-established descriptors applied to focal seizures (3). There is an ongoing effort underway by the ILAE to allow greater flexibility and transparency in naming seizure types with a proposed 2016 “Operational Classification”, yet to be finalized at the time of this writing.
TABLE 7.1 International League Against Epilepsy Classification of Epileptic Seizures (2010) | ||||
|---|---|---|---|---|
|
The ILAE classification of epilepsy syndromes (Table 7.3) is organized by factors such as the age of onset and seizure type, by “distinctive constellations” (including mesial temporal lobe
epilepsy [TLE] with hippocampal sclerosis), and those epilepsy syndromes caused by structural–metabolic pathology (including malformations of cortical development [MCDs], tumors, etc.). This classification was similarly updated in 2010 to improve precision through descriptive nomenclature, adding several electroclinical syndromes which are impractical to list here (2). This categorization is essential for grouping patients with relatively predictable prognoses and indicating specific therapy, including the choice of antiepileptic drugs (AEDs). It also forms the basis of additional workup, including neuroimaging as well as treatment trials. For epilepsies associated with structural–metabolic conditions, the current scheme suggests that less emphasis be placed on seizure location than the underlying structural or metabolic cause.
epilepsy [TLE] with hippocampal sclerosis), and those epilepsy syndromes caused by structural–metabolic pathology (including malformations of cortical development [MCDs], tumors, etc.). This classification was similarly updated in 2010 to improve precision through descriptive nomenclature, adding several electroclinical syndromes which are impractical to list here (2). This categorization is essential for grouping patients with relatively predictable prognoses and indicating specific therapy, including the choice of antiepileptic drugs (AEDs). It also forms the basis of additional workup, including neuroimaging as well as treatment trials. For epilepsies associated with structural–metabolic conditions, the current scheme suggests that less emphasis be placed on seizure location than the underlying structural or metabolic cause.
TABLE 7.2 Terms Describing Focal Seizure Semiology | |||||
|---|---|---|---|---|---|
|
TABLE 7.3 International League Against Epilepsy Classification of Epilepsy Syndromes (2010, Abbreviated) | |||||||
|---|---|---|---|---|---|---|---|
|
The distinction between focal and generalized seizures has important implications, whereas the generalized seizures are usually well controlled on antiepileptic pharmacotherapy, 15% to 30% of patients with focal seizures continue to experience seizures (4,5). Surgical control of seizure activity in such patients with intractable epilepsy is an important consideration because psychosocial, medical, and financial implications are significant. Neuropathology series in patients undergoing surgical treatment of focal epilepsy show that hippocampal sclerosis (HS) is consistently one of the most common pathologic substrates (40% to 73%). MCDs (or cortical dysplasias—particularly the focal cortical dysplasias [FCDs] subtypes) are increasingly recognized in specimens in up to 45% of cases, at least in part due to improved neuropathologic analyses and more uniform adoption of MCD and FCD classification schemes. HS is commonly associated with other lesions, and is more commonly found in adult series, while MCDs, overall, are more common in pediatric populations (6,7,8). In one series of 243 patients with TLE, dual pathology was very common: HS was concurrently identified in 70% of MCD cases and 8% of tumors, and only in isolation in 14% of cases (6). Other common etiologies and approximate percentages include perinatal hypoxia or other insult (13% to 35%), tumors (15%), vascular malformations (3%), and traumatic gliosis (2%) (9). Table 7.4 categorizes the causes of epilepsy by the usual age of seizure onset. Even beyond the tremendous improvement in diagnosis and management of patients with epilepsy, magnetic resonance (MR) has had a great impact on the understanding of epilepsy syndromes by shifting emphasis purely from clinical and electrophysiologic diagnosis to structural abnormalities in brain responsible for the electrophysiologic and clinical features.
TABLE 7.4 Cause of Epilepsy Categorized by the Age at Seizure Onset | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Neuroimaging, in the form of computed tomography (CT) and MR, has become part of the standard evaluation of unexplained seizures in children and adults. CT is an appropriate choice in emergency setting for evaluation of new-onset seizure patients with symptomatic causes (i.e., focal deficits, persistent altered mental status, fever, trauma, persistent headaches, history of cancer, anticoagulation, ventriculoperitoneal shunts, or acquired immunodeficiency syndrome), and in the elderly, in whom acute stroke and tumors are the most likely. CT, however, has little or no role in preoperative evaluation of patients with intractable epilepsy. In a recent meta-analysis, emergent use of CT has been found to alter acute management in 3% to 9% of children and infants and up to 17% of adults. It was also found that up to 50% of infants less than 6 months old had clinically relevant CT abnormalities in the emergency setting (10,11). However, for a child with a first-time simple febrile seizure, the American Academy of Pediatrics recommends that neuroimaging should not be performed (12). In children with new-onset seizures who have no detectable symptomatic cause, MR imaging (MRI) is the neuroimaging study of choice (13,14). MR is two to three times more sensitive for detecting imaging abnormalities in patients with seizures as well as patients with intractable epilepsy (13,15).
TABLE 7.5 Investigative Modalities in Epilepsy Workup | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
MR has emerged as the most valuable tool for preoperative localization of the epileptogenic focus because of its excellent soft-tissue contrast, allowing for detailed depiction of anatomy, freedom from beam-hardening artifact in the basal brain that occurs with CT, and capacity for multiplanar and isovolumetric or three-dimensional (3D) imaging (13,14,16). MRI also provides the capability of functional imaging, white matter tractography, and spectroscopy. Other modalities for preoperative evaluation of surgical candidates with intractable epilepsy include single-photon emission computed tomography (SPECT), positron emission tomography (PET), magnetoencephalography, neuropsychological assessment, and lateralization of language and memory functions by intracarotid amytal procedure (IAP or Wada test). Because MR is currently the best structural neuroimaging modality, it has a unique role in management of epilepsy and epilepsy surgery. Table 7.5 summarizes the role of various investigative modalities in epilepsy workup.
MRI in Epilepsy and Epilepsy Surgery
Despite continued development of AEDs, uncontrolled seizures or undesirable side effects persist for 20% to 30% or more of the population with focal epilepsy. As such, it remains crucially important to identify structural lesions in these patients as many will be considered as candidates for surgery. The choice of surgery depends on seizure type and anatomic substrate, among other factors (Table 7.6). For surgical resection or disconnection to be offered, the seizure must be focal in origin, and accurate preoperative localization of the epileptogenic focus must be available. Localization of the epileptogenic focus is, therefore, the major task in preoperative evaluation of surgical candidates. In the past, EEG was essentially the only method of localizing the seizure focus. Accuracy of the conventional scalp EEG is limited, and alone it is neither sensitive nor specific enough for preoperative evaluation. Intracranial EEG is an expensive invasive procedure with potential risks and, therefore, cannot be universally employed in epilepsy patients. Moreover, it invariably requires some preliminary localization data to guide the electrode placement. Intracranial EEG is, therefore, usually reserved to confirm the epileptogenicity of indeterminate or widespread structural lesion(s) seen on MRI, validate the results of alternative methods of functional localization such as SPECT, PET, and magnetoencephalography in MR-negative epilepsy, or pinpoint eloquent brain tissue prior to resection. With regard to refractory focal seizures, anterior temporal lobectomy and selective amgdalohippocampectomy are the most commonly performed neurosurgical procedures. The surgical resection can only be performed unilaterally because of unacceptable neurologic consequences of bilateral temporal lobectomy. Therefore, preoperative localization and assessment of laterality of seizure focus must be carried out. The algorithm for localization of seizure focus and assessment of resectability varies according to institutional practice and resources (Fig. 7.1 and Table 7.7).
The general goals of neuroimaging in presurgical evaluation of epilepsy patients include (i) delineation of structural and, if possible, functional abnormality in the putative epileptogenic region, (ii) categorization into a specific epilepsy substrate, (iii) detection of additional abnormalities, and (iv) mapping of sensorimotor, language, and memory functions in the epileptogenic and adjacent regions of the brain. Although strategies for surgical treatment usually begin with identification of a structural abnormality, determining the epileptogenicity of the structural abnormality by electrophysiology is crucial for successful outcome. Over the last decades, it has become clear (from EEG, PET, MR connectivity, and other data) that epilepsy is a network disorder, affecting different well-defined networks within brain tissue. Effective anti-epileptic therapies must, therefore, address disruption or resection of the epileptogenic network. By itself,
MRI cannot determine the epileptogenicity of a lesion or the importance of a structural lesion in an epileptogenic network (i.e., are there several epileptogenic regions functioning as part of an epileptogenic network?). Patients with circumscribed epileptogenic lesions with convergent findings from analysis of semiology, MRI, scalp video-EEG, and neuropsychology undergo surgery without invasive recording. The standard initial strategy is to combine scalp EEG and MR, in addition to clinical semiology, and if the results are concordant, no further tests are usually necessary. In cases of discordant EEG and MR findings or when the epileptogenicity of the MR-identified lesion is indeterminate, intracranial EEG recordings using subdural or parenchymal depth electrodes are warranted. Invasive electrophysiologic studies may also be indicated in cases with more than one MR abnormality, when MR shows a large atrophic region, widespread noncircumscribed lesions or developmental abnormality, in cases of possible bitemporal epilepsy and when functional mapping of brain is warranted based on MR findings or other reasons. A minority of surgical candidates require such invasive evaluation with implantation of subdural electrodes.
MRI cannot determine the epileptogenicity of a lesion or the importance of a structural lesion in an epileptogenic network (i.e., are there several epileptogenic regions functioning as part of an epileptogenic network?). Patients with circumscribed epileptogenic lesions with convergent findings from analysis of semiology, MRI, scalp video-EEG, and neuropsychology undergo surgery without invasive recording. The standard initial strategy is to combine scalp EEG and MR, in addition to clinical semiology, and if the results are concordant, no further tests are usually necessary. In cases of discordant EEG and MR findings or when the epileptogenicity of the MR-identified lesion is indeterminate, intracranial EEG recordings using subdural or parenchymal depth electrodes are warranted. Invasive electrophysiologic studies may also be indicated in cases with more than one MR abnormality, when MR shows a large atrophic region, widespread noncircumscribed lesions or developmental abnormality, in cases of possible bitemporal epilepsy and when functional mapping of brain is warranted based on MR findings or other reasons. A minority of surgical candidates require such invasive evaluation with implantation of subdural electrodes.
TABLE 7.6 Procedures in Epilepsy Surgery | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
TABLE 7.7 Recommended Magnetic Resonance Seizure Protocol Based on Age at Presentation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
The sensitivity for detecting epileptogenic lesions varies with the clinical profile of patients evaluated. The positivity of MR is significantly lower in new-onset seizures than in intractable epilepsy. In the setting of new-onset seizures, epileptogenic abnormalities on MRI were found in 13% to 32% of patients, nearly all of whom suffered from focal seizures (15,17). In a more restricted group of subjects composed of adults with newly diagnosed focal seizures, MR abnormalities were found in 24% (10% HS and 14% other abnormalities), a higher percentage than in populations composed of all new-onset-seizure patients (18). As such, it is generally accepted that MR aids diagnosis and should be performed in all patients with new-onset seizures with the possible exception of simple febrile seizures, idiopathic generalized epilepsy, and benign partial epilepsy of childhood. In the intractable epilepsy group, patient characteristics such as age at onset and underlying brain abnormalities also influence the sensitivity of MR. An overall sensitivity of 82% to 86% has been reported for MRI of intractable epilepsy (13,19). For TLE (focal seizures), an MR sensitivity greater than 75% for HS and greater than 90% for other focal lesions, including tumors, has been reported based on histopathologic findings as the gold standard (20,21,22). The sensitivity of MR for detecting epileptogenic abnormalities in children undergoing epilepsy surgery has been found to be 75% or higher (23).
Coregistration of MRI with other functional imaging modalities, including PET and SPECT, has also proven valuable in localization of structural and functional alteration. Ictal SPECT, especially when quantitatively compared to interictal SPECT, has also become a valuable method for accurate localization of the epileptogenic focus. By early intraictal intravenous injection of a perfusion-dependent radiotracer, it is possible to detect local increases in cerebral blood flow caused by neuronal hyperactivity during the actual seizure. Focal blood-flow increase reflects seizure activity, either from cerebral cortex at the seizure-onset zone or from seizure spread to other areas. The accuracy of subtraction ictal SPECT coregistered to MRI (SISCOM) in the localization of the seizure focus has been assessed by several studies, comparing it with either invasive ictal EEG, site of surgery, or combined modalities (24). SISCOM can be valuable in guiding intracranial electrode placement. This application is very useful in patients with cortical dysplasia, when the onset is focally localized in the larger anatomic lesion or when the seizure-onset zone extends beyond the visible lesion on MRI, and in situations such as nonlesional epilepsy, when data from conventional methods do not provide enough localizing information to guide the electrode implantation. SISCOM findings can also result in re-evaluation of MRI in cases in which the MRI is initially considered normal (25). MRI, when reinterpreted in light of SISCOM data, may detect subtle abnormalities in nonlesional epilepsy. SISCOM localization, however, can be confounded by spreading patterns; a late injection could detect neuronal activity as a consequence of seizure spread and miss the epileptogenic region.
The role of MR in epilepsy surgery (Table 7.7), in addition to its principal value in identifying the epileptogenic focus, also lies in its ability to depict topographic relationships between the epileptogenic lesion and the eloquent regions of brain. Precise anatomic localization of PET and SPECT abnormalities by coregistration with MRI is key in assessing the appropriateness and type of surgery, as well as in minimizing postoperative neurologic deficits. It is critical, however, to correlate the static anatomic abnormalities on MR with clinical and electrophysiologic data to avoid false-positive localization (Fig. 7.1) (26). As noted earlier, concordance of noninvasive
tests including scalp EEG with MR findings may obviate the need for invasive monitoring. MR thus influences the need for invasive EEG and is also useful in planning the placement of subdural and parenchymal depth electrodes. Localization of intracranial electrodes by MR can be safely performed to verify the precise anatomic distribution of contacts and helps in the accurate determination of the extent of surgical resection (27). Postoperative MR may detect reasons for failure such as inadequate resection and can monitor tumor recurrence on follow-up imaging.
tests including scalp EEG with MR findings may obviate the need for invasive monitoring. MR thus influences the need for invasive EEG and is also useful in planning the placement of subdural and parenchymal depth electrodes. Localization of intracranial electrodes by MR can be safely performed to verify the precise anatomic distribution of contacts and helps in the accurate determination of the extent of surgical resection (27). Postoperative MR may detect reasons for failure such as inadequate resection and can monitor tumor recurrence on follow-up imaging.
MR is especially useful for prognosticating postoperative seizure control. Most patients undergoing MRI in surgical series are affected by focal or localization-related epilepsy. The convergence of anatomic MR abnormality and epileptogenic pathology in focal epilepsies has led to the development of the “substrate” concept of classification of focal epilepsies. Pathologic substrates for localization-related epilepsy can be categorized by characteristics such as developmental or acquired origin, histopathology, mechanism of epileptogenesis, and surgical outcome. The likelihood of postoperative seizure freedom is higher if an epilepsy substrate had been identified on MRI during the presurgical workup. A successful outcome after anterior temporal lobectomy is achieved in 70% to 95% patients with MR findings of HS, compared with 40% to 55% of patients in whom the MR is normal (14,22,28,29,30). Berkovic et al. (30) found the postoperative seizure-free state to depend on identification of a substrate by MR and the nature of MR abnormality—a seizure-free state in 80% with focal lesions other than HS, 62% in patients with HS, and only 36% with normal MR. In a study of 210 patients rendered seizure free after epilepsy surgery, those with normal preoperative MR had a higher rate of postoperative seizure recurrence after discontinuation of antiepileptic medication (31).
Magnetic Resonance Imaging of Epilepsy Substrates
The patients’ age at presentation influences the likelihood of presence of a particular epilepsy substrate (Table 7.4), clinical MR acquisition protocols must be accordingly optimized to maximize the diagnostic yield (Table 7.7). Surgical and pathologic data indicate that approximately four-fifths of patients undergoing epilepsy surgery have a definable substrate on MRI (20). The accuracy of MR in determining the substrate category in intractable epilepsy has been reported to be 88% (13).
Mesial Temporal Anatomy and Hippocampal Sclerosis
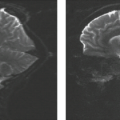
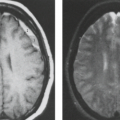
Mesial temporal or HS is a highly epileptogenic abnormality strongly associated with focal temporal lobe seizures and has been the most common pathologic substrate encountered in patients undergoing surgery. These patients often have a history of complicated childhood febrile seizures or febrile status epilepticus, and onset of recurrent medically intractable seizures during the first decades of life. However, MR evidence of HS has also been found in medically controlled patients with complex partial epilepsy. HS is defined histologically by pyramidal and granule cell neuronal loss that occurs in the cornu ammonis and dentate sections of the hippocampus (Fig. 7.2). The ILAE has classified HS into three types based on the predominant location of severe neuronal loss and gliosis: CA1 and CA4 regions for type 1, CA1 for type 2, and CA4 for type 3; no-HS type for reaction gliosis without neuronal loss. Hippocampal reorganization and changes in energy metabolism are also associated with HS and may be the result of a brain insult
occurring during brain maturation (32). Findings of reorganization include abnormal axonal sprouting and loss of interneurons, which is thought to change the balance of neuronal excitation and inhibition. The surgical treatment of HS is anterior temporal lobectomy, which, as noted earlier, has been the most rewarding and most commonly performed procedure in epilepsy surgery. Consequently, a great volume of data has been published on imaging, preoperative evaluation, and surgical outcome of HS.
occurring during brain maturation (32). Findings of reorganization include abnormal axonal sprouting and loss of interneurons, which is thought to change the balance of neuronal excitation and inhibition. The surgical treatment of HS is anterior temporal lobectomy, which, as noted earlier, has been the most rewarding and most commonly performed procedure in epilepsy surgery. Consequently, a great volume of data has been published on imaging, preoperative evaluation, and surgical outcome of HS.
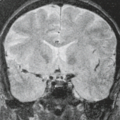
Understanding of the anatomy of the medial temporal lobe, namely the hippocampal formation and limbic system, is an important prerequisite for recognition and accurate interpretation of MR findings in HS (33,34,35,36). The anatomic terminology used by Duvernoy has become widely accepted and is utilized here (Table 7.8) (34,37). The limbic system can be thought of as four concentric arches. From the outermost inward, they are (1) the limbic lobe (i.e., parahippocampal, cingulate, and supracallosal areas), (2) a cerebrospinal fluid (CSF) space (i.e., callosal and hippocampal sulci), (3) Broca’s intralimbic gyrus (consisting mostly of the hippocampus), and (4) the fornix, which includes the fimbria (Table 7.8, Fig. 7.3) (33,34,37). Limbic structures, particularly the hippocampus and parahippocampus gyrus, are important sites for epilepsy development and propagation. This is due in part to the widespread connections between portions of the limbic arches and with extralimbic brain structures.
The hippocampus itself, is a 4- to 4.5-cm–long curved structure on the medial aspect of temporal lobe forming a curved elevation in the floor of the temporal horn of lateral ventricle. It is divided into three segments based on morphology and relationship to the midbrain. The hippocampal head, or pes hippocampus, lies anterior to the brainstem, is expansile in shape and has three to four characteristic digitations on its superior surface. The cylindrically shaped body extends posteriorly around the midbrain, and the terminal portion—the tail of hippocampus—rapidly narrows behind the brainstem. Internally, the hippocampus consists of two interlocking U-shaped layers: the dentate gyrus and cornu ammonis (Figs. 7.2 and 7.4). The cornu ammonis can be further segmented into four portions, CA1 through CA4. CA4 is normally completely enveloped by the dentate gyrus and is also known as the endfolium. On the other, inferolateral end, cornu ammonis blends into the subiculum inferiorly which forms the transition to the neocortex of parahippocampal gyrus below. Along the superior surface of the hippocampus, its primary efferent fibers called alveus pass medially, just beneath the ependymal lining of the ventricular floor. They converge to form the fimbria and continue as the
fornix. The amygdala is a gray matter structure located superomedial to the tip of the temporal horn of lateral ventricle (also known as the uncal recess), which separates it from hippocampal head (Fig. 7.5). When there is paucity of CSF in the uncal recess, the alveus may help delineate these structures.
fornix. The amygdala is a gray matter structure located superomedial to the tip of the temporal horn of lateral ventricle (also known as the uncal recess), which separates it from hippocampal head (Fig. 7.5). When there is paucity of CSF in the uncal recess, the alveus may help delineate these structures.
TABLE 7.8 Limbic System | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
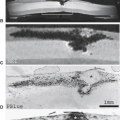
 FIGURE 7.4 Coronal diagram of the medial left temporal lobe. The hippocampus is composed of two U-shaped lamina of gray matter, the cornu ammonis (C) and the dentate gyrus (D). The stratum radiatum, lacunosum, and moleculare make up the white matter (asterisks) between the cornu ammonis and dentate gyrus. White matter tracks extend from the cornu ammonis to form the alveus (arrowheads), which converge medially to form the fimbria (F). Laterally, the hippocampus is surrounded by the temporal horn (TH), which also extends superiorly. More superior medially is the choroidal fissure (ChF). Medially, the ambient cistern (AC) and the brainstem (BS) are located. Inferiorly, the subiculum (S), parahippocampal gyrus (PHG) and subcortical white matter are found, as well as the collateral sulcus (CS). The fusiform gyrus (FG), which is also known as the lateral occipital temporal gyrus, and the inferior temporal gyrus (ITG) are found along the basal portion of the temporal lobe. (From Bronen RA. Epilepsy: the role of MR imaging. AJR Am J Roentgenol 1992;159:1165–1174, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|