Modality
Pro
Con
Relative expense
Radiography
Universally available
Small radiation dose
$
Fluoroscopy
Physiologic, dynamic visualization of anatomy
Higher radiation dose
$$
Ultrasound
No radiation, dynamic visualization of anatomy
Operator dependent
$$$
CT
Anatomy easily understood
High radiation dose
$$$
MRI
High level of anatomic detail; exam can be protocolled to target-specific questions; comprehensive evaluation of multiple systems including CNS
Poor imaging of bone, calcifications; general anesthesia required for young children/long exams (more detailed protocols), not universally available
$$$$
Nuclear imaging
Small radiation dose; physiologic, dynamic imaging
Not universally available
$$$$ (particularly when radiotracer is not made at institution)
Latex sensitization and latex allergy are concerns for all patients undergoing many diagnostic tests and surgical procedures due to increased exposure to the latex antigen. The patients described in this chapter fit these criteria. All patients in this category should be treated with full latex precautions from the beginning of their care [1].
Genital ambiguity is present in some patients discussed in this chapter, particularly those in the exstrophy-epispadias complex (EEC). Radiologists are often called upon to assist in establishing gender in the fragile neonate through the use of portable ultrasound to detect pelvic organs (e.g., uterus) and characterize the gonads. Early gender assignment helps parents cope with their child’s diagnosis and bond with the child.
Exstrophy-Epispadias Complex
Clinical Overview
EEC comprises a spectrum of congenital genitourinary abnormalities ranging in severity from epispadias to CBE to CE. These conditions all affect the genitalia, infraumbilical abdomen, and pelvic ring. EEC is a multisystem birth defect involving the genitourinary tract, musculoskeletal system, pelvic floor, bony pelvis, and anus.
Epidemiology of EEC
CBE is the most common form of EEC. The incidence is estimated to be between 1 in 10,000–50,000 live births [2], while the International Clearinghouse for Birth Defects estimates 3.3 per 100,000 live births [3]. CE is the rarest manifestation of EEC with incidence approximated at 1 per 200,000 live births. EEC is most common in Caucasians, and the male to female ratio ranges from 2.3:1 to 6:1 [3]. It is also more common in infants born to younger mothers and multiparous women. Maternal tobacco exposure is associated with more severe forms (e.g., CE). There is evidence to suggest an increased incidence of this complex of abnormalities in pregnancies utilizing assisted reproductive technologies [4].
Epispadias
Epispadias affects the genitalia and pubic symphysis. The pubic symphysis is diastatic with divergent distal rectus abdominis muscles. In males, the phallus is short and broad with a dorsal chordee. The glans is open along the dorsal surface and shaped like a spade. The dorsal foreskin is absent. The urethral meatus is located along the dorsal penile shaft, between the peno-pubic angle and the proximal glans. In females, the clitoris is bifid with superiorly divergent labia, and the distal dorsal aspect of the urethra is open with a patulous bladder neck (Figs. 19.1 and 19.2). Prolapse of bladder mucosa can sometimes occur.
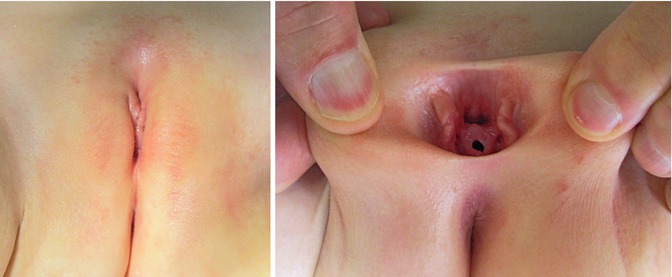

Figs. 19.1 and 19.2
Epispadias in a 2-year-old female patient. Note bifid clitoris and superiorly divergent labia
Classic Bladder Exstrophy
CBE presents with an open bladder exposed through a triangular-shaped fascial defect on the infraumbilical abdomen (Fig. 19.3). The umbilicus is low set and located on the upper edge of the bladder plate. In both sexes, the distance between the umbilicus and anus is shortened. The anatomy of the levator ani and puborectalis muscles is distorted, leading to varying degrees of fecal incontinence and rectal prolapse. The pubic symphysis is widely diastatic with distally divergent rectus abdominis muscles, which is the result of outward rotation of the innominate bones. There is outward and downward rotation of the anterior pelvic ring ultimately resulting in the classic waddling gait [5–7]. Indirect inguinal hernias are common secondary to wide inguinal rings and short inguinal canals [8, 9].
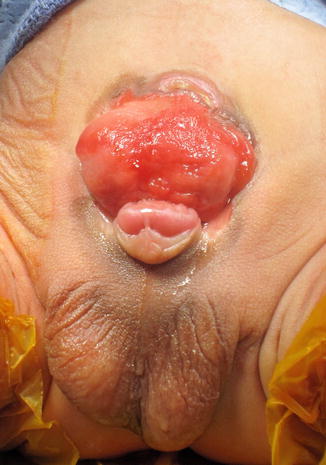

Fig. 19.3
Classic bladder exstrophy in a 1-week-old male patient. Note features of epispadias including a shortened phallus with dorsal chordee, spade-shaped glans, and dorsal meatal opening
In males, genital features of epispadias are also present as described above. The urethral and bladder plates are in continuity along the lower abdominal wall and onto the dorsal surface of the penis. The verumontanum and prostatic ducts are present and visible along the prostatic urethral plate. Females also have the same genital features of epispadias, and the open urethral plate is in continuity with the bladder plate. There is anterior displacement of the vagina, which is short.
Cloacal Exstrophy
As in CBE, the bladder is widely open on the infraumbilical abdominal wall but separated into two distinct halves in CE. Cecum is present in between both halves of the bladder. Two appendices may be seen within the cecal plate, and the terminal ileum may prolapse between the hemi-bladders resulting in the so-called “elephant trunk sign”. Most patients with CE have an associated omphalocele.
The pelvic ring has a similar configuration to CBE. 65 % of these patients can have major deformities of the lower extremity, such as clubfoot. Up to 75 % have vertebral anomalies and neural tube defects [10, 11]. In males, the phallus is small and bifid, with a hemi-glans caudal to each hemi-bladder. Females have a bifid clitoris and two hemi-vaginas. The uterus is most commonly bicornuate, but there are varying degrees of Müllerian duplication [12, 13].
Embryology of EEC
A definite cause of EEC has not yet been elucidated. It is hypothesized that early in fetal development (approximately during the fourth gestational week) there is an abnormal overdevelopment of the cloacal membrane, which results in failure of the medial migration of mesenchyme between the ectodermal and endodermal layers of the lower abdominal wall, thus preventing the normal development of the lower abdominal wall [14]. This occurrence makes the cloacal membrane unstable and prone to early rupture (before it migrates into its normal caudal location during gestational weeks 6–8). The stage of development when this premature rupture occurs will determine the severity along the EEC spectrum. When rupture occurs after complete separation of the genitourinary and gastrointestinal tracts via descent of the urorectal septum, CBE results. When rupture occurs prior to this event, externalization of the lower urinary tract and distal gastrointestinal tract occurs, resulting in CE [15].
Prenatal Imaging of EEC
Transabdominal ultrasonography is the mainstay of prenatal imaging. Although not utilized routinely, there is a role for fetal MRI, which provides more anatomic detail, particularly when anomalies are detected but not fully elucidated on ultrasonography.
CBE: Prenatal Imaging
Prenatal ultrasonographic findings suggestive of CBE include the repeated failure to visualize the filled bladder, a lower abdominal wall echogenic mass that increases in size with an increase of the intra-abdominal viscera, a low-set umbilicus, abnormal or diminutive genitalia, an increased pelvic diameter, and widening of the pubic symphysis [16–18].
CE: Prenatal Imaging
Prenatal ultrasonographic findings compatible with CE include the above characteristics of CBE, as well as a lower abdominal wall defect, herniation of bowel between bladder plates (“elephant trunk sign”), limb abnormalities, neural tube defects, and vertebral anomalies.
Postnatal Imaging Modalities for EEC
The diagnosis of EEC is clinical, yet radiographic imaging modalities can help detect associated anomalies and better define the internal anatomy of the patient.
Pelvic Radiography
Pubic diastasis is noted in patients with EEC, as well as outward rotation of the innominate bones and squaring of the iliac notch (Figs. 19.4 and 19.5). Hip dysplasia is not typically seen as part of this complex.
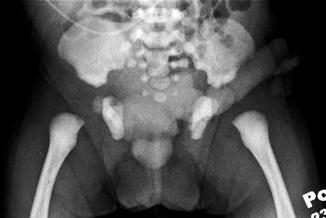
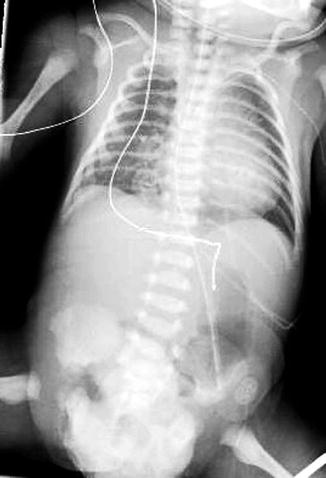

Fig. 19.4
AP plain radiograph of the pelvis in a newborn male with classic bladder exstrophy. Note diastasis of the symphysis pubis. Soft tissue density over the pelvis represents the bladder plate and the scrotum. The umbilical cord stump passes to the patient’s left

Fig. 19.5
AP plain radiograph of the abdomen and pelvis in a newborn male patient with cloacal exstrophy. Soft tissue density overlies the lower abdomen and pelvis, representing omphalocele. Splayed pelvic bones and dysplastic sacrum are present, but nearly obscured, as is often the case
Cross-Sectional Imaging
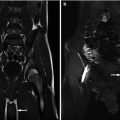
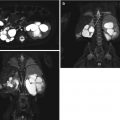
Postnatal MRI has been increasingly used to understand the pelvic anatomy in patients with EEC. These patients have a wider pelvic diameter with pubic diastasis, externally rotated sacroiliac joints, a flatter pelvic floor with a more laterally placed levator ani complex, an anteriorly displaced anus, and shorter anterior corporal bodies [19]. Given its myriad of protocol possibilities, imaging of the spine can also be included in the same examination (Figs. 19.6, 19.7, and 19.8). MRI, however, has the constraint of requiring general anesthesia for obtaining quality images, as well as being time consuming (especially with more complex protocols).
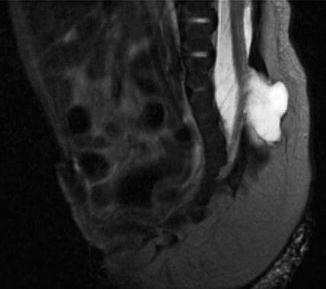
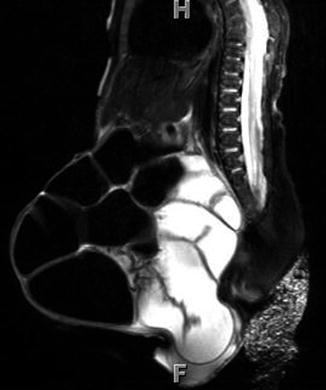
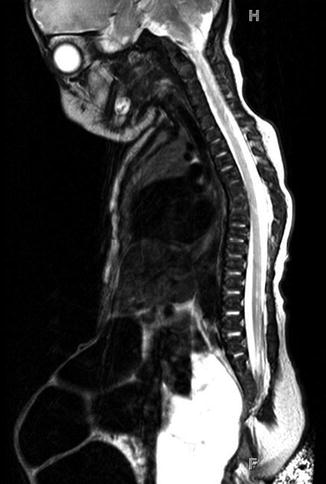

Fig. 19.6
T2-weighted midline sagittal MRI of the pelvis and spinal cord in a 5-day-old male with cloacal exstrophy. Soft tissue plate on the anterior abdominal wall represents bladder; lipomeningocele is seen at L5/S1

Fig. 19.7
Midline sagittal T2-weighted abdomen and pelvis MR of a 4-day-old male with cloacal exstrophy demonstrates large omphalocele. Included spine suggests anomalies, but these are better demonstrated on dedicated spine MR obtained in the same session

Fig. 19.8
Sagittal T2-weighted spine MR in same patient shows mid-thoracic dyssegmentation and kyphosis, short sacrum, and low cord with spinal canal lipoma
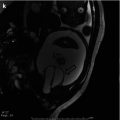
Bony and soft tissue pelvic anatomy can also be studied utilizing CT (Fig. 19.9).
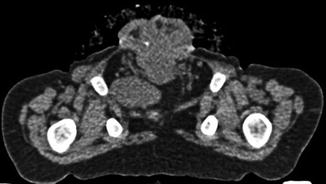

Fig. 19.9
Axial non-contrast CT image through the pelvis in a 19-month-old male with CE. The pubic bones are outwardly rotated and the open anterior bladder plate is shown. The cartilaginous tip of the coccyx is displaced anteriorly and to the right. The left slip of the pubococcygeus muscle is visible; the right is displaced by a loop of pelvic bowel
Cystourethrography
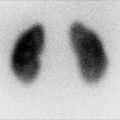
Post-closure gravity cystography is performed in the operating suite under general anesthesia in patients with CBE and CE to look for vesicoureteral reflux (VUR), which is present in nearly all patients after closure, due to the anatomy of the ureterovesical junction (Figs. 19.10 and 19.11). This imaging modality is also used to assess bladder capacity in preparation for bladder neck reconstruction and continence surgery.
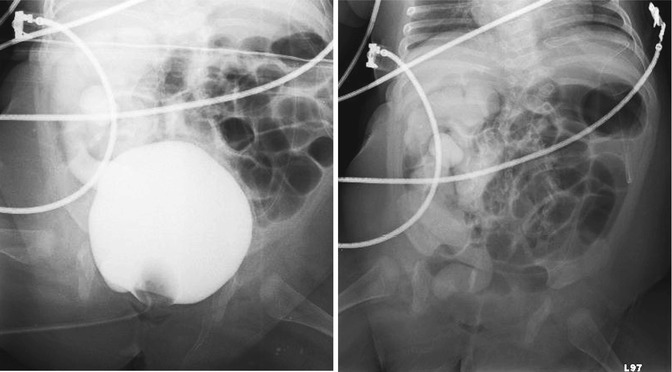

Figs. 19.10 and 19.11
Cystogram in a 4-month-old male patient with skin covered exstrophy variant. Fig. 19.10: AP pelvis radiograph shows Grade V vesicoureteral reflux into the right collecting system. Fig. 19.11: Post-emptying AP abdominal radiograph demonstrates persistence of reflux in the right collecting system and ureter
Additionally, voiding cystourethrography (VCUG) using fluoroscopy in the radiology suite is useful to evaluate the bladder neck and proximal urethra in patients with epispadias.
Renal Ultrasonography
Baseline renal ultrasonography should be obtained when the clinical diagnosis along the spectrum of EEC has been made. Associated congenital anomalies of the upper urinary tract are rare with epispadias and CBE but are more common in patients with CE. These can include renal agenesis, renal ptosis, and multicystic dysplastic kidney.
Virtually all patients will develop VUR after bladder closure. Increased intravesical pressure after closure can lead to hydroureteronephrosis with upper urinary tract pressure transmission and subsequent upper tract deterioration. Therefore, baseline knowledge of existing hydronephrosis is helpful in following these patients postoperatively.
Imaging of the Spine
Spinal ultrasonography should be performed in all neonates with cloacal exstrophy, as spinal dysraphism must be diagnosed when present.
Differential Diagnosis of EEC
The differential diagnosis of EEC includes patent urachus, omphalocele, gastroschisis, persistent cloaca and cloacal malformation, as well as the common variants of EEC. These include superior vesical fissure, pseudoexstrophy, duplicate exstrophy, covered exstrophy, and the omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex.
Exstrophy variants all have a widely diastatic pubic symphysis with distally divergent rectus abdominis muscles. A low-set umbilicus is common. There may be a small bladder opening or patch of isolated bladder mucosa as seen in superior vesical fissure.
The OEIS complex is the most severe form of EEC and is characterized by an omphalocele, imperforate anus or anal atresia, spinal dysraphism with incomplete development of the lumbosacral vertebrae, and exstrophy of a common cloaca that receives ureters, ileum, and a rudimentary hindgut. There is also failure of fusion of the genital tubercles and pubic rami, epispadias in males and Müllerian duct anomalies in females. There is a wide spectrum of urinary tract malformations, including supernumerary kidney [20].
Treatment of EEC
Patients undergo surgical correction, urinary diversion, or urinary augmentation as treatment of CBE. Planning for surgical correction can be complex and is tailored to the individual patient.
Surgical correction can be performed by the modern staged repair, which involves primary bladder closure with or without pelvic osteotomy within 72 h of birth, epispadias repair between 6 and 12 months of age, and bladder neck reconstruction at 4–5 years of age. Epispadias repair allows for increase in bladder outlet resistance and subsequent increase in bladder capacity. Ureteral reimplantation for VUR is almost universally indicated at the time of bladder neck reconstruction.
Complete primary repair, an alternative approach, encompasses primary bladder closure and genital reconstruction in one operation [21–23]. Because of the advantage of early bladder cycling, some patients have not required additional bladder neck reconstruction to achieve early continence, yet some evidence suggests an increased number of surgical procedures and complications in these patients [24, 25].
Follow-Up Imaging in EEC
All EEC patients that have been reconstructed or diverted should be monitored with periodic renal and bladder ultrasonography to assess for evidence of obstruction, upper tract deterioration, or calculus formation.
Prune-Belly Syndrome
Prune-belly syndrome (PBS) is characterized by deficient or absent abdominal wall musculature, dilation of the urinary tract, and intra-abdominal testes [27]. It has a number of other names, including Eagle-Barrett syndrome, triad syndrome, mesenchymal dysplasia syndrome, and abdominal musculature deficiency syndrome, to mention a few. PBS is the most commonly used name, given its evocative nature.
These patients have a wrinkled abdomen (like a prune), secondary to the lack of abdominal musculature, and males have an empty scrotum due to undescended testes (Fig. 19.12). The incidence ranges from 1:35,000 to 50,000 live births [28]. This is increased in twins, blacks, and patients born to younger mothers. PBS rarely occurs in females [29], but its clinical manifestation in these patients includes the abdominal wall deficiency and urinary tract dysmorphism without any gonadal abnormality [30, 31].
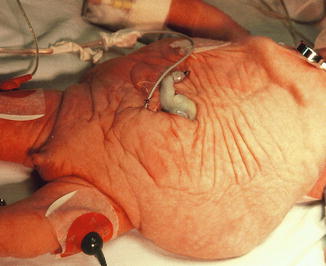

Fig. 19.12
Newborn male with prune-belly syndrome
There are two dominant theories regarding the pathogenesis of PBS yet the exact mechanism is unknown. The obstructive theory suggests that urethral obstruction—either atresia or transient obstruction during a critical period in fetal development [29, 32, 33]—results in bladder distension, hydroureteronephrosis, and atrophy of the abdominal musculature. The mesodermal defect theory proposes a defect in lateral plate mesoderm development early in embryogenesis and is supported by related mesenchymal defects (e.g., prostatic hypoplasia in PBS) [34, 35].
Similar to EEC discussed earlier, there is a spectrum of severity associated with PBS. PBS can be categorized into three main groupings, first classified by Woodard in 1978 [36]. Category I patients have urethral obstruction, pulmonary hypoplasia, oligohydramnios, renal dysplasia, and Potter’s facies. These patients are among the most seriously affected group and usually suffer an early death from pulmonary hypoplasia. Category II patients have mild renal dysfunction or dysplasia and hydroureteronephrosis. These patients may go on to develop progressive renal failure [37]. Category III patients have normal renal function and a mild degree of uropathy. The single most predictive factor in survival of the patient with PBS is the severity of renal dysplasia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree