Chromosomal
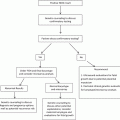
10.0 %
Single gene
3.0 %
Familial
14.5 %
Multifactorial
23.0 %
Teratogens
3.2 %
Uterine anomalies
2.5 %
Twinning
0.4 %
Unknown
43.2 %
Signaling Pathways Identified for Normal Embryo Development
Embryonic development in the first trimester is extensive, with a small group of totipotent (able to differentiate into any cell within the organism) stem cells located in the inner cell mass of the blastula responsible for cellular differentiation and subsequent organ formation. It is important to be aware that this complex formation appears to be controlled by cell signaling pathways, which guide normal development both by the location of their expression as well as the specific time at which they are active in the embryo and surrounding tissues. Detailed descriptions of all signaling pathways are beyond the scope of this chapter, but are well described in other texts [2–6].
Development of the Bilaminar Embryo (Weeks 1–2)
After successful fertilization, the resulting zygote quickly undergoes cleavage to rapidly progress through the blastula and morula stages. The morula will separate into an inner cell mass (the embryoblast, or future embryo) and an outer cell mass (the trophoblast component of the placenta).
The embryoblast differentiates into a bilaminar embryonic disc, consisting of dorsal epiblast and ventral hypoblast. This typically occurs around day 14 after fertilization, around the time of completion of implantation [6]. A new layer of cells is derived from the epiblast, the extraembryonic mesoderm, and proceeds to aid in the origins of extraembryonic components such as the amniotic cavity and the umbilical vesicle.
Embryonic Weeks 3–4
During this period, all the major organ systems of the embryo and fetus will begin to develop. During the third week of embryonic development (5 weeks after the last menstrual period), the most notable processes include the development of the primitive streak and notochord [7], and the creation of the three germ layers of the trilaminar embryonic disc (Fig. 4.1).


Fig. 4.1
The formation of the trilaminar embryonic disc. The arrows indicate invagination and migration of mesenchymal cells from the primitive streak. (c, e, g) Dorsal views of the trilaminar disc early in week 3, exposed by removal of the amnion. (a, b, d, f, h) Transverse sections through the embryonic disc, with the level of each section indicated in (c), (e), and (g). This figure was published in The Developing Human: Clinically Oriented Embryology, 9th ed., Moore KL, Persaud TVN, Torchia MG, Copyright Elsevier 2013
The process of gastrulation results in the development of the three critical germ layers of the human embryo—endoderm, ectoderm, and mesoderm [8]. From these three simple layers will develop all fetal tissues and organs (Fig. 4.2). In addition, gastrulation marks the beginning of morphogenesis, the shaping of an organism by the differentiation of cells, tissues, and organs and organ systems, according to its genetic direction [9].


Fig. 4.2
Schematic of the derivatives of the three germ layers of the trilaminar embryonic disc: ectoderm, endoderm, and mesoderm. This figure was published in The Developing Human: Clinically Oriented Embryology, 9th ed., Moore KL, Persaud TVN, Torchia MG, Copyright Elsevier 2013
During this time, the primitive streak develops (Fig. 4.3) and will give rise to the primitive node. This is critical to allow the development of mesenchyme, which will go on to serve as the progenitor for many supporting tissues of the fetus. Although the totipotent cells of the primitive streak typically regress by 4 weeks of development, remnants are believed to lead to the formation of a unique fetal tumor, the sacrococcygeal teratoma [10]. Newly formed mesenchyme will migrate through the streak and become a chord of tissue known as the notochord. The notochord determines the axis of the embryo and becomes a rod-like support for further axial development. Through signaling pathways that include sonic hedgehog (Shh) and bone morphogenetic proteins (BMPs), the notochord and overlying developing neural tube will orchestrate the establishment of the axial central nervous system and axial musculoskeletal system, as well as the segmentation of the nervous system [11]. Furthermore, the paraxial mesoderm (mesoderm located on each side of the developing neural tube) divides into intermediate and lateral mesoderm, which will give rise to components of the musculoskeletal system and the urinary tract [6].


Fig. 4.3
Development of the primitive streak and notochord. The embryo begins to length and change shape in the third embryonic week. The primitive streak lengthens by adding cells at its caudal end, while the notochord lengthens by migration of cells from the primitive node. The notochordal process and adjacent mesoderm induce overlying ectoderm to form the neural plate, which is the embryonic basis of the central nervous system. This figure was published in The Developing Human: Clinically Oriented Embryology, 9th ed., Moore KL, Persaud TVN, Torchia MG, Copyright Elsevier 2013
Formation of the Neural Tube
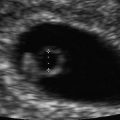
This critical component of embryonic development begins during the fourth embryologic week. As the notochord develops, signaling pathways induce the formation of the neural plate, which will give rise to the future brain and spinal cord. The neural plate becomes a groove and subsequently folds begin to form, a process known as neurulation [12]. Fusion of the neural groove into the neural tube occurs in a zipper-like fashion, beginning in the midline and progressing in both cranial and caudal directions. Non-fusion at any site is known as spina bifida, which can range from small defects that are functionally unimportant (spina bifida occulta) to severe defects, which are incompatible with life such as anencephaly (Fig. 4.4) [13].


Fig. 4.4
Ultrasound image at 12 week gestation, with anencephaly, a lethal abnormality in which a large portion of the fetal brain is absent, as is the superior portion of the fetal skull, due to incomplete closure of the rostral neuropore during spinal cord formation. Courtesy of Dr. Randall Kuhlmann, Division of Maternal Fetal Medicine, Medical College of Wisconsin, Milwaukee, WI
During the development of the neural tube, neural crest cells are differentiating as columns of cells along both sides of the neural tube. These cells are critical to normal embryonic development, as they migrate throughout the embryo to give rise to components of the heart, head and face, and to ganglia of the spine and autonomic nervous system, pigment cells, adrenal glands, and the medulla [14]. Abnormal development and or migration of the neural crest cells are believed to influence the development of such disorders as neurofibromatosis and CHARGE association [15].
Embryonic Weeks 5–8
After the formation of the neural tube, the embryo enters a period in which many major external and internal body structures are developed. This period extends from the fifth to eighth week of embryonic development (7–10 postmenstrual weeks) [6]. This critical phase of development is the time at which the conceptus is most vulnerable to teratogens potentially leading to abnormal development. Unfortunately, it is also a time at which many women might not yet be aware that they are pregnant, and thus exposure to environmental agents, which might alter embryonic development, may be increased. Individual organ systems and structures, as formed during the first trimester, are now addressed individually.
Division of the Embryonic Cavities and Diaphragm
The primordium of the body cavities, or intraembryonic coelom, is divided into cavities during the fourth and fifth week. These include the pericardial cavity, two pericardioperitoneal cavities and a single peritoneal cavity. As the fetus begins to fold cranially, the heart and pericardial cavity are located near the developing foregut, and remain in direct communication with the paired pericardioperitoneal cavities [6]. As development continues the peritoneal cavity will become isolated, and fusion and expansion of the remaining cavities will establish separate pleural and peritoneal cavities, and will contribute to the creation of the diaphragm. Development of the definitive diaphragm, which is also occurring at this time, is dependent on coordinated development of four separate components: the pleuroperitoneal membranes, the mesentery of the developing esophagus, muscular ingrowth from the lateral body wall, and the septum transversum, an outgrowth from the dorsal body wall (Fig. 4.5) [16]. Defects in any of these components can result in a congenital diaphragmatic hernia, which is most often caused by defective formation or fusion of the pleuroperitoneal membranes with the other three parts of the diaphragm, and occurs on the left side of the fetus in up to 90 % of cases [17].


Fig. 4.5
Development of the diaphragm. (a) Lateral view of the embryo at the end of the fifth embryonic week, indicating the level of the transverse sections in (b), (c), and (d). (b) Transverse section of the pleuroperitoneal membranes prior to fusion. (c) Similar section at the end of the sixth embryonic week. (d) Transverse section at 12 embryonic weeks. (e) Inferior view of the diaphragm in a neonate, identified the embryologic origin of its components. This figure was published in The Developing Human: Clinically Oriented Embryology, 9th ed., Moore KL, Persaud TVN, Torchia MG, Copyright Elsevier 2013
Development of the Fetal Face
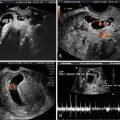
The development of the fetal face begins with embryonic primordial around the developing fetal mouth or stomodeum. Facial development is dependent on the formation of five structures: frontonasal prominences, maxillary prominences, and mandibular prominences. Appropriate migration and fusion are vital to allow for normal facial and palatal development (Fig. 4.6) [18]. Abnormalities in these processes can result in cleft lip and cleft palate or more severe major clefting of the fetal face. Clefting can also be associated with other midline anomalies such as holoprosencephaly, often due to inappropriate signaling to allow normal component migration and fusion. Such facial hypoplasia is often seen in trisomy 13 (Fig. 4.7a, b).



Fig. 4.6
Early development of the maxilla, palate and upper lip. (a) Facial view of a fifth embryonic week embryo. (b, c) Horizontal sections at the levels shown in (a). The arrows in (c) indicate growth of the maxillary and median nasal prominences. (d–f), Similar sections of older embryos identifying merging of the medial nasal prominences and maxillary prominences to form the upper lip. This figure was published in The Developing Human: Clinically Oriented Embryology, 9th ed., Moore KL, Persaud TVN, Torchia MG, Copyright Elsevier 2013

Fig. 4.7
Ultrasound scan of a fetal face at 12 weeks gestation with holoprosencephaly (a) and flattened facial profile (b) suggestive of severe facial clefting, which was identified in the second trimester. This fetus was ultimately diagnosed with trisomy 13 and midline facial clefting. Courtesy of Dr. Randall Kuhlmann, Division of Maternal Fetal Medicine, Medical College of Wisconsin, Milwaukee, WI
Development of the Respiratory System
The respiratory system also begins to develop during the fourth embryonic week, as the respiratory diverticulum buds from the primitive foregut. Subsequent migration of splanchnic mesoderm over the diverticulum results in the development of respiratory buds, which will further divide and differentiate over the course of fetal development. An important step in the respiratory system’s formation is the separation of the foregut and esophagus from the trachea through the development of the tracheoesophageal folds, which will fuse to form the tracheoesophageal septum [6]. Inappropriate or incomplete development of this septum can result in various types of tracheoesophageal fistulae (TEF). This abnormal passage is associated with incomplete formation of the esophagus (esophageal atresia) in 85 % of cases [6] and can lead to ultrasound findings of excess amniotic fluid, as the fetus is unable to swallow and assimilate appropriately during gestation [19].
Development of the Gastrointestinal Tract
The primordial gut tube begins to form in the fourth embryonic week as a portion of the yolk sac is incorporated into the embryo as it folds. Initially cell proliferation will obliterate the lumen of the tube, which will then recanalize and differentiate into foregut, midgut, and hindgut components [6]. Incomplete recanalization can result in subsequent areas of stenotic or atretic intestine [20]. The foregut is divided into the trachea and esophagus as addressed in the previous section. Additional components of the foregut include the stomach, which will dilate and rotate to its normal physiologic location in the left upper quadrant, as well as the liver and duodenum [21].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree