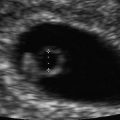
Fig. 18.1
Complete hydatidiform mole with classic diffuse vesicular changes. Figure provided by Dr. Carol B. Benson, Director of Ultrasound, Department of Radiology, Brigham and Women’s Hospital and Professor of Radiology, Harvard Medical School, Boston, MA

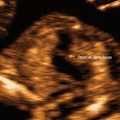
Fig. 18.2
Partial hydatidiform mole with focal vesicular changes and nonviable fetus. Figure provided by Dr. Carol B. Benson, Director of Ultrasound, Department of Radiology, Brigham and Women’s Hospital and Professor of Radiology, Harvard Medical School, Boston, MA
Ultrasound Diagnosis of Early Molar Pregnancy
The classic sonographic appearance of CHM described above may be lacking in patients who present with bleeding early in the first trimester, making the sonographic diagnosis more difficult. The hydropic villi are smaller at earlier gestational ages and the molar tissue may appear as a complex echogenic intrauterine mass with several anechoic or cystic spaces [10, 13] (Fig. 18.3). In early gestations it may also be more difficult to differentiate PHM from CHM. One characteristic that may help to differentiate the two types of molar pregnancy is the presence of a gestational sac in PHM that is either empty or contains small fetal echoes surrounded by a large rim of placental echoes with cystic spaces [14]. Additionally, the earlier the presentation the more difficult it is to differentiate molar pregnancy from a hydropic nonmolar abortion. Ultrasonic descriptions of early histologically confirmed molar pregnancies include: an empty gestational sac or intrauterine anechoic fluid collection, fluid collection in association with an echogenic mass, thickened endometrium and echogenic fluid-filled levels within the endometrium [15, 16].
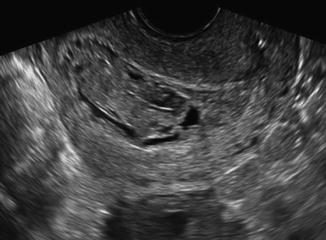

Fig. 18.3
First-trimester complete hydatidiform molar pregnancy with limited vesicular changes that are less prominent and best seen on transvaginal ultrasound images. Figure provided by Dr. Carol B. Benson, Director of Ultrasound, Department of Radiology, Brigham and Women’s Hospital and Professor of Radiology, Harvard Medical School, Boston, MA
Ultrasound can also be used to assess the volume of molar tissue present in the uterus which has been shown to be a risk factor for GTN [17–20]. A three-dimensional assessment of volume is felt by some experts to be superior to a single measurement. However, in one study the size of the lesion measured sonographically was not predictive of need for chemotherapy [18]. Although myometrial invasion can be assessed with ultrasound, MRI is often a better modality to demonstrate the presence of invasion of molar tissues into the uterine wall [19, 20].
Sensitivity of Ultrasound in Detecting Molar Pregnancy
Several investigators have examined the accuracy of ultrasound in diagnosis of molar pregnancy. Fowler et al. [21] published the largest study evaluating the role of ultrasound in detection of molar pregnancy. The authors reviewed 1053 consecutive cases of molar pregnancy with early ultrasound evaluation and found that the sensitivity for detecting either a CHM or PHM was 44 %. The mean gestational age at diagnosis was 10 weeks. They found that ultrasound was better at detecting CHM versus PHM (79 % vs. 29 %, p < 0.0001) and there was a false-positive rate of 10 % whose final pathology demonstrated hydropic degeneration of nonmolar pregnancies. Other smaller reviews have shown sensitivity of ultrasound in detecting molar pregnancy to range from 34 to 57 % [15, 16, 21, 22]. Correlation with hCG is critical to the diagnosis. It has been shown that the ultrasonographic diagnosis of complete molar pregnancy is facilitated by clinical factors such as hCG [23]. When analyzing ultrasound sensitivity by type of molar pregnancy, ultrasound is consistently more sensitive in detecting CHM (sensitivity range 58–95 %) [13, 16, 21–24] as compared to PHM (sensitivity range 17–29 %) [16, 21, 22]. The sensitivity of ultrasound improves with increasing gestational age as the hydropic villi grow in size with advancing gestational age and are more easily seen by ultrasound.
Mimics of Molar Pregnancy
There are other conditions in early pregnancy that mimic the sonographic appearance of molar pregnancy such as hydropic degeneration of the placenta, missed abortion, blighted ovum and retained products of conception. False-positive rates of ultrasound in molar pregnancy have been estimated anywhere from 4 to 10 % [21, 25]. Hydropic changes of the placenta in other gestations can appear similar to the hydropic villi of molar disease but tend to be less homogeneously distributed [21, 26]. Although it can be difficult to differentiate an early partial molar pregnancy from other abnormalities of early pregnancy, the presence of an echogenic rim around the sac may be more indicative of a missed abortion or blighted ovum [14].
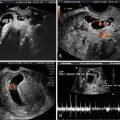
Color Doppler Ultrasound in Diagnosis of Molar Pregnancy
Uterine Artery Doppler Measurement in Molar Pregnancy and Development of GTN
Several studies have shown that uterine artery resistive indices correlate well with hCG levels and may therefore be helpful in diagnosis and monitoring of treatment [28–33]. Yalcin et al. followed 21 patients with molar pregnancy with hCG and uterine artery Dopplers and found significant negative correlations with hCG and Doppler indices, i.e., as the hCG declined demonstrating resolution of disease, the resistive indices rose [31]. In this study, the patients who ultimately developed postmolar GTN had significantly lower Doppler indices than those whose disease regressed spontaneously. Others have also demonstrated this inverse relationship of lower resistive indices indicating a greater risk of developing postmolar GTN [29, 30, 34–36]. Finally, one study of 25 patients with molar pregnancy undergoing surveillance with hCG and transvaginal ultrasound (TVUS) with Doppler also noted that surveillance with Doppler predicted GTN 1–3 weeks before routine hCG monitoring and that ultrasound findings tended to resolve about 8 weeks earlier than hCG normalization [32].
Ultrasound in the Diagnosis and Management of Ovarian Theca Lutein Cysts
Ovarian theca lutein cysts develop in 25–65 % of complete molar pregnancies in association with markedly elevated hCG levels >100,000 mIU/ml. In a review of 386 patients with untreated hydatidiform mole, 102 patients (26.4 %) had concurrent theca lutein cysts [37]. Theca lutein cysts appear sonographically to be anechoic, multi-loculated ovarian cysts [38] (Fig. 18.4). Mean cyst diameters are reported around 7 cm but can range in size from 3 to 20 cm [26, 38]. Typically these cysts will be accompanied with the uterine sonographic findings detailed previously. Of note, theca lutein cysts are less likely to be seen in the first trimester complete or partial moles pregnancies where the hCG level is generally <100,000 mIU/ml [15]. The size of the theca lutein cysts has not been shown to correlate with persistent disease, although the presence of bilateral cysts is associated with an increased risk of GTN [37]. Serial ultrasound examination is useful to monitor the regression of theca lutein cysts which tend to regress slowly over 2–4 months following molar evacuation as the hCG level declines [39]. Although theca lutein cysts rarely rupture spontaneously or undergo torsion, prompt laparoscopic intervention can be used effectively. The use of ultrasound guidance during percutaneous drainage of massively enlarged theca lutein cysts may also provide considerable relief of abdominal discomfort.
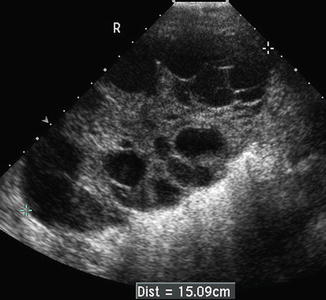

Fig. 18.4
Theca lutein cysts filling an ovary and appear as anechoic multi-loculated cystic structures. Figure provided by Dr. Carol B. Benson, Director of Ultrasound, Department of Radiology, Brigham and Women’s Hospital and Professor of Radiology, Harvard Medical School, Boston, MA
Ultrasound in the Diagnosis of Molar Pregnancies with a Coexistent Twin
Concurrent twin pregnancy with a hydatidiform mole and coexisting fetus is estimated to occur 1 in 22,000–100,000 pregnancies [40]. The diagnosis of a molar pregnancy with coexisting fetus is almost always made based on ultrasound findings and tends to be diagnosed at later gestational age than a singleton CHM [41]. Ultrasound findings show a live fetus with either a single enlarged placenta with the classic cystic changes and increased echoes or there may be two placentas, one normal and one molar [42] (Fig. 18.5). Ultrasound is also critical in the ongoing management of these pregnancies to help ensure the well-being of the coexisting fetus. Of those pregnancies described in the literature that were continued after diagnosis of a twin molar gestation, more than half continued beyond the 28th week of gestation with almost 70 % of children surviving [40]. The rate of GTN was not significantly different between the group who chose to continue the pregnancy and those that interrupted the pregnancy at diagnosis. However, these twin molar pregnancies are more likely to develop GTN as compared to singleton molar pregnancies [41].


Fig. 18.5
Twin gestation with one normal placenta anteriorly and one complete molar pregnancy posteriorly demonstrating classic diffuse vesicular changes. Figure provided by Dr. Carol B. Benson, Director of Ultrasound, Department of Radiology, Brigham and Women’s Hospital and Professor of Radiology, Harvard Medical School, Boston, MA
Ultrasound in the Evacuation of Molar Pregnancy
The first step in management of a molar pregnancy is uterine evacuation, typically with suction curettage [43]. The technique for uterine evacuation is similar to that used for spontaneous and induced abortions. However, there is a greater concern for blood loss due to the increased vascularity of molar gestations. Intraoperative ultrasound may be a very useful tool during a suction curettage for molar pregnancy, particularly in cases with a large volume of intrauterine disease. When available, ultrasound should be used at the start of the procedure to examine the intrauterine disease and assess for pelvic extension. Ultrasound can be utilized to help prevent uterine perforation during the serial dilation of the cervix and placement of the suction curette. Finally, at the conclusion of the procedure, ultrasound allows the clinician to visualize the uterine cavity and confirm complete evacuation of molar tissues [43].
Ultrasound in the Diagnosis and Management of GTN
Gestational trophoblastic neoplasia (GTN) includes invasive mole, choriocarcinoma, PSTT, and ETT. Choriocarcinoma arises from both cyto- and syncytio-trophoblast and produces high levels of hCG. Choriocarcinoma is associated with early metastatic spread but is generally highly sensitive to chemotherapy. Unlike choriocarcinoma, PSTT and ETT arise from extravillous intermediate trophoblasts and produce low levels of hCG. Unfortunately and importantly, both PSTT and ETT are relatively resistant to chemotherapy unlike the other types of GTN. Ultrasound is the imaging modality of choice for the initial evaluation of the uterus and adnexa when a patient has been diagnosed with GTN. Ultrasound is not able to differentiate between types of GTN. Therefore, correlation with clinical history and hCG is critical. Betel et al. compared 17 cases of GTD to 14 cases of retained products of conception sonographically and found that GTD cases were more likely associated with a larger mass (>3.45 cm), thin endometrium (<12 mm), myometrial based mass and vascular lakes [44]. Non-gestational conditions that have been described to mimic GTD sonographically include uterine leiomyomas and an adenomyomatous polyp [26, 45]. In addition to the increased vascularity, Doppler can also illustrate focal areas of increased flow within the myometrium in cases of invasive molar pregnancy and choriocarcinoma [46]. Measurement of uterine artery Doppler indices including the resistive index (RI) and the pulsatility index (PI) has been studied in GTN. RI and PI tend to be very low in GTN and changes within these indices correlate with response to treatment and resolution of GTN over the course of follow-up with the indices increasing as the hCG levels decline [47, 48]. Nonetheless, some sonographic findings may be clues to the diagnosis.
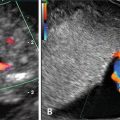
Invasive molar pregnancy may appear as intrauterine mass(es) with anechoic areas and also often demonstrates focal areas of increased echogenicity within the myometrium or can appear as heterogeneous lesions containing fluid-filled cavities representing invasion (Fig. 18.6).
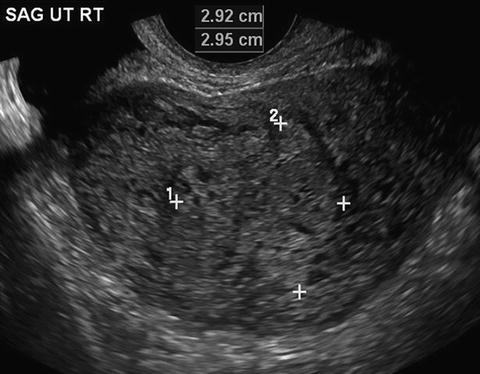
Fig. 18.6
Invasive complete molar pregnancy with intrauterine mass containing diffuse vesicular changes invading into the myometrium. Figure provided by Dr. Carol B. Benson, Director of Ultrasound, Department of Radiology, Brigham and Women’s Hospital and Professor of Radiology, Harvard Medical School, Boston, MA
Choriocarcinoma may appear as an enlarged uterus containing a semisolid heterogeneous echogenic mass with areas of necrosis and hemorrhage. Choriocarcinoma nodules are hypervascular showing increased vascularity on color Doppler. Choriocarcinoma can also be seen invading the myometrium or even out to the parametria.
Placental site trophoblastic tumor may also appear as small heterogeneous echogenic areas with fluid filled cysts, but generally has less necrosis than choriocarcinoma. PSTT may also appear as a solid tumor with or without cystic spaces in the uterus or invading the myometrium. These masses can demonstrate a wide range of vascularity from a non-vascularized mass to a high degree of vascularity [48, 49].
Epithelioid trophoblastic tumor appear early in the course of disease with irregular echolucent lacunae within the myometrium on transvaginal ultrasound, but later in the disease with a well-circumscribed solitary echogenic lesion in the fundal myometrium without blood flow [50].
Ultrasound to Assess Presence and Volume of Intrauterine Disease
Transvaginal ultrasound (TVUS) is the preferred technique to detect the presence of invasive GTN [29, 51, 52]. TVUS findings of GTN are described as hypoechoic areas in the endometrium and intramyometrial nodules [53], clusters of high amplitude echoes within the myometrium representing invasive tumor with echo-free areas representing hemorrhage [54], and multiple “serpinginous anechoic channels” throughout the central part of the uterus [51].
Ultrasound Evaluation for Postmolar GTN
Following diagnosis and evacuation of a CHM or PHM, serial hCG levels are used to determine the development of postmolar GTN. The diagnosis of postmolar GTN is made when hCG levels plateau for more than three consecutive weeks, or rise for more than two consecutive weeks [1]. Once the diagnosis of GTN is made, ultrasound is one of the imaging tests used for identifying the location and extent of disease. In one study of 33 patients with GTN, ultrasound findings were reviewed and in 17/33 (51.5 %) patients the ultrasound demonstrated uterine disease that correlated 100 % of the time with pathology from an endometrial curettage or hysterectomy. Of the 16/33 patients who did not have evidence of intrauterine disease on ultrasound, the endometrial curettings were positive for fragments of trophoblastic tumor in only 6/16 (37.5 %) [55].
Color Doppler in Diagnosis of GTN
The vascular nature of GTN makes the use of color Doppler in conjunction with TVUS ideally suited for evaluating the presence and extent of intramyometrial disease. The use of Doppler can aid in the identification of myometrial invasion sonographically, a feature of GTN that was previously often only made histologically after hysterectomy. Doppler color flow mapping is seen as abnormal flow through the myometrium in cases of myometrial invasion [20, 28, 51, 56]. TVUS with Doppler can identify small foci of resistant intrauterine disease [29] and can be used to evaluate depth of myometrial invasion which may also be prognostic for resistant disease [34]. Doppler can also be utilized to monitor and measure uterine artery blood flow and a RI and PI can be calculated. In pregnancy the resistance to blood flowing into the uterus drops dramatically with the development of uteroplacental blood vessels and with the hypervascularity of GTD the resistance is even less. In fact, resistive indices progressively decrease as one goes from a nonpregnant uterus, to a normal pregnancy to molar pregnancy to invasive molar pregnancy and choriocarcinoma [27, 57–59]. Thus, in cases where the diagnosis is not clear use of the color Doppler may demonstrate invasion into the myometrium and measurement of uterine artery pulsations may be low suggestive of a diagnosis of GTN.
Ultrasound in the Follow-Up of Molar Pregnancy and GTN
Use in Determining Need for Surgical Intervention
Although the mainstay of treatment for GTN is chemotherapy, in select circumstances surgical intervention is indicated [60]. Ultrasound has proven to be very useful in identifying patients who may benefit from surgery. First, patients presenting with bleeding from persistent intrauterine disease may benefit from a repeat D&C, local resection, or hysterectomy. Second, patients undergoing chemotherapy whose hCG levels indicate chemoresistance may benefit from local resection or hysterectomy, particularly in those women where future fertility is no longer desired [61, 62]. Transvaginal ultrasound with color Doppler should be the first line imaging modality to assess for intrauterine disease when there is concern for heavy vaginal bleeding or drug-resistant uterine tumor.
Uterine Artery Pulsatile Index as a Predictor of Chemotherapy Resistance in GTN
The association of low uterine artery Doppler pulsatile indices and need for chemotherapy has been well established as described above. Thus, several investigators have queried whether the use of these resistive indices might be helpful in identifying patients at risk for chemotherapy resistance. Approximately, 30 % of patients who are considered to have low-risk disease (FIGO risk scores <7) will become resistant to first line single agent chemotherapy and require subsequent alternative chemotherapy [63]. Several studies have demonstrated Doppler indices can be predictive of chemotherapy resistance with patients requiring multi-agent chemotherapy as opposed to single agent chemotherapy having lower resistive indices [34, 64]. Agarwal et al. investigated whether the uterine artery pulsatility index (UAPI) was a predictor of chemotherapy resistance. Initially, they found in 164 patients that those with a UAPI < 1 had 2.68 greater odds of developing methotrexate resistance as opposed to those with inital UAPI > 1 [65]. A subsequent study by the same investigators showed a UAPI < 1 as compared to >1 was predictive of chemotherapy resistance (64.6 % vs. 35.4 %) in multivariate analyses. Patients with a FIGO score of 6 and UAPI ≤ 1 had a 100 % rate of single agent methotrexate resistance [66]. Thus, there may be a role for measurement of the UAPI in addition to calculation of the FIGO risk score in patients requiring chemotherapy for GTN.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree