Fig. 1
Relationship between examples of pure malignant germ cell tumors and their secreted marker substances (Data derived from Rice [48] and John Hopkins Pathology, 2001)
Dysgerminoma
Epidemiology
Dysgerminoma is the most common malignant germ cell tumor, accounting for approximately 30–40 % of all malignant neoplasms of germ cell origin [1]; however, it represents only 1–2 % of all ovarian cancers [4, 9]. Seventy-five percent of dysgerminomas occur between the ages of 10 and 30 years and 5 % occur before the age of 10 (years) and rarely after age 50 [1, 9]. After cistoadenoma, it represents the second most frequent tumor diagnosed during pregnancy, accounting for 20–30 % of ovarian neoplasms during gestation [1, 4].
According to a survey conducted in the USA and comprising 1,262 cases of malignant ovarian germ cell tumors registered from 1973 to 2002, the age-adjusted incidence rate of ovarian dysgerminoma was 0.109 per 100.000 women-years [9].
Approximately 5 % of dysgerminomas are diagnosed in phenotypic females with abnormal gonads [1], such as 46XY (bilateral streak gonads), 46X0/46XY (unilateral streak gonad, contralateral testis), and Morris syndrome (46XY, testicular feminization). Therefore, when this tumor is diagnosed in premenarchal patients, the karyotype should be determined.
Approximately 65 % of dysgerminomas are diagnosed at stage I (confined to one or both ovaries). Dysgerminoma is the only germ cell malignancy with a 10–15 % rate of bilaterality, whereas the other germ cell tumors are rarely bilateral [1, 4]. The most common tumor spreading pathway (of the tumor) is through the lymphatic system, particularly to the higher para-aortic nodes, occurring in 25 % of the patients.
Up to 95 % of patients with ovarian dysgerminoma have an elevated serum level of lactic dehydrogenase (LDH) at presentation, with the level varying with the size and stage of the tumor [10, 11]. Determination of this marker may also be useful in detecting tumor recurrence and in monitoring response to therapy. When the neoplasm contains syncytiotrophoblastic giant cells (5 % of cases) [12], it may produce β-human chorionic gonadotropin (βhCG) [3, 9]. Serum levels of CA 125 and placental-like alkaline phosphatase (PLAP) can be elevated in some cases, but CA 125 is an unreliable marker in premenopausal women and PLAP is much more useful as a histochemical marker than as a serum one [3].
Macroscopy
Ovarian dysgerminomas most frequently are solid, well-encapsulated tumors with size ranging from barely visible nodules to masses that virtually fill the entire abdomen and have an average diameter of 15 cm. On cutting surface it appears to consist of different lobules often soft and fleshy, and with a yellow to white to gray to pink appearance. Areas of coagulative necrosis and hemorrhage typically associated with cystic changes may be observed [13, 14].
Microscopy
Histologically identical to seminoma of the testis, ovarian dysgerminomas are composed of a monotonous population of rounded cells resembling primordial germ cells in a predominantly diffuse or insular arrangement. The cells are polygonal, with distinct cell membranes with abundant eosinophilic to clear glycogen-rich cytoplasm. The nuclei are large, central, and rounded, and they contain one or a few prominent nucleoli. Mitoses are often numerous. The nests of tumor cells are separate by fibrous septa frequently infiltrated by T lymphocytes [13]. A scattered multinuclear cells positive for βhCG can be observed, confirming their identity as syncytiotrophoblastic giant cells [12].
Clinical Symptoms
Clinically, ovarian dysgerminomas may be incidentally detected in women with no gynecological symptoms. These tumors grow rapidly; therefore, the most common symptoms are abdominal distension and the presence of a pelvic or abdominal mass felt by the patient herself. Often they can be characterized by the onset of acute pelvic pain caused by the distention of the ovarian capsule, hemorrhage, necrosis, and torsion [15].
When the syncytiotrophoblastic giant cells are present, menstrual and endocrine abnormalities may be observed due to high levels of βhCG resembling pregnancy [12], and extremely rarely precocious puberty can occur due to the presence of yolk sac tumor elements in a mixed tumor with AFP secretion [16].
Prognosis
Clinical characteristics that differentiate dysgerminoma from the other MOGCTs are as follows: (a) it is more likely diagnosed as stage IA, (b) bilateral involvement is more common (10–15 %), (c) retroperitoneal spread is more frequent than intraperitoneal dissemination, and (d) it is radiosensitive [3]. Due to these features, ovarian dysgerminomas have a good prognosis: in patients with initial stage IA disease, unilateral oophorectomy alone results in a 5-year disease-free survival rate greater than 95 % [1].
Lymph node metastasis was reported in the 28 % of dysgerminomas in the Kumar et al. series [17]. Lymph node involvement was an independent predictor of poor survival at multivariate analysis with 95.7 % 5-year survival rate for negative node patients as opposed to 82.8 % for patients with positive nodes [1].
In MITO-9 series, relapse rate was 10.2 and 77 % of these recurrences occurred within 24 months from diagnosis; the 2-year recurrence-free survival was 91.3 %, and the 5-year overall survival (OS) was 97.9 % [18].
Gordon et al. reported a 95 % 5-year overall survival rate in patients with stage IA pure dysgerminoma treated by unilateral adnexectomy alone. Survival in these patients was not affected by tumor size [19].
Historically risk factors associated with a higher recurrence rate are lesion diameter larger than 10–15 cm, age younger than 20 years, and a microscopic pattern picturing numerous mitosis, anaplasia, and medullary pattern [1].
Ninety to 100 % cure rates have been reported in advanced-stage disease with the use of bleomycin–etoposide–cisplatin (BEP) or etoposide–carboplatin (EC) combination regimen therapy [1].
Ultrasound Characteristics
The most common ultrasound features of dysgerminoma are large pure solid adnexal tumor divided into different lobules, with irregular internal echogenicity, smooth lobulated contours, and well-defined borders [4, 9]. Less typical appearance is that of a solid tumor with cystic areas, some of them with irregular internal cystic borders and even papillary projections [9]. In the Guerriero et al. series, half of the patients had free fluid in the pouch of Douglas (common and unspecific ultrasound finding in women of fertile age) and only one presented with ascites [9].
Power Doppler ultrasound images show vascularized tumor with a color scores moderate or abundant. Three-dimensional power Doppler ultrasound volumes revealed densely packed vessels, irregular branching, caliber changes, and tortuosity of tumor vessels in the three dysgerminomas evaluated in Guerriero et al. series [9] (Figs. 2 and 3).
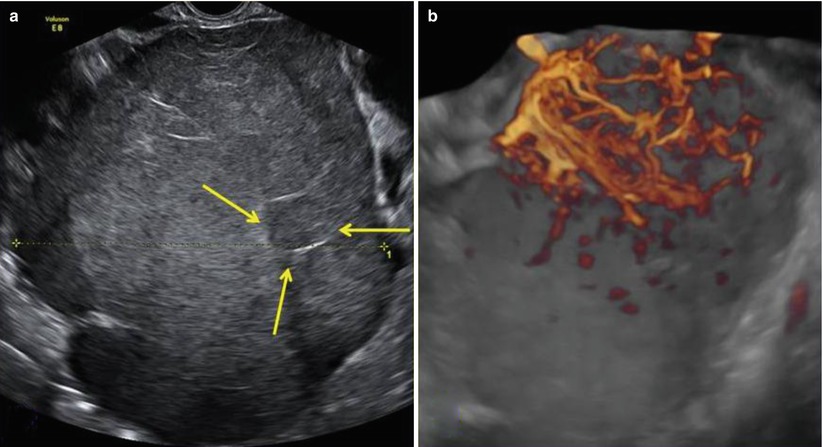
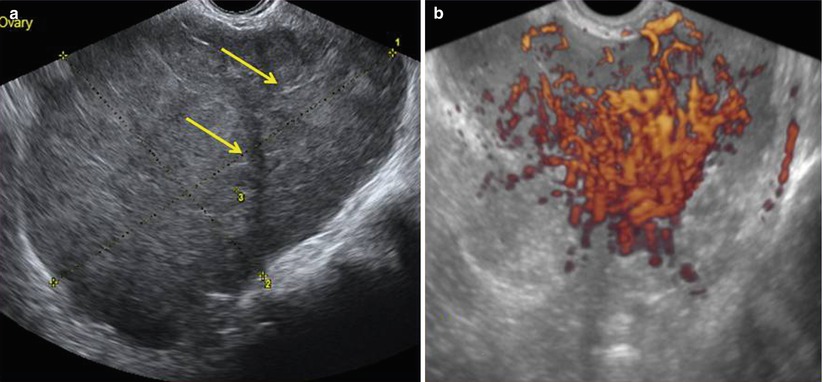

Fig. 2
(a) A large (14 cm) solid ovarian dysgerminoma stage IA with a multilobulated appearance (a lobule is shown between the arrows) and smooth well-defined lobulated contour in a 24-year-old patient. The internal echogenicity is rather irregular. (b) Three-dimensional power Doppler image showing densely packed tortuous vessels with irregular branching and caliber changes in an ovarian dysgerminoma

Fig. 3
(a) Grayscale ultrasound images of an 11 cm ovarian dysgerminoma stage IIIC in a 27-year-old patient with a less-evident multilobulated appearance with well-defined lobulated but smooth contours. Lobules are separated by fine connective tissue (arrows). (b) Three-dimensional power Doppler image showing densely packed tortuous vessels with irregular branching and caliber changes in the same ovarian dysgerminoma
In three reported cases Kim et al. showed prominent arterial flow within the fibrovascular septa of the tumor. The resistive index values ranged between 0.44 and 0.70 (mean 0.59), and the pulsatility index values ranged between 0.60 and 1.32 (mean 0.98) [20].
There are not specific features to distinguish the ultrasound patterns of dysgerminomas from those of other solid malignant ovarian tumors such as solid metastases or lymphoma, but the ultrasound findings of a large, solid, lobulated adnexal mass with irregular internal echogenicity and highly vascularized at color or power Doppler ultrasound in a 20–30-year-old woman should raise the suspicion of ovarian dysgerminoma [9].
Immature Teratomas
Immature teratomas contain tissue elements that resemble those of the embryo. They vary considerably in size and are usually round or oval with mixed content composed of hair and fatty material surrounded by a firm capsule [14]. Immature teratoma elements may occur in combination with other germ cell tumors as mixed germ cell tumors [1].
Epidemiology
Pure immature teratoma accounts for fewer than 1 % of all ovarian malignancies; however, it is the second most common germ cell tumor. In women younger than 20 years, this neoplasm represents 10–20 % of all ovarian malignancies and 30 % of the deaths from ovarian cancer in this age group. Approximately 50 % of pure immature teratomas occur in female patients between the ages of 10 and 20 years; on the contrary, it is extremely rare in postmenopausal women [1, 15].
Survival rate is related to histology grade that depends on the proportion of immature neuroepithelium tissues (in histological sections). Grade 1 has a survival of at least 95 %, whereas grades 2 and 3 appear to have a lower overall survival (approximately 85 %) [1, 15].
Immature ovarian teratomas are associated with gliomatosis peritonei, a favorable prognostic finding if composed of completely mature tissues, with the seemingly unexpected recent discovery, using molecular methods, that these glial “implants” are not tumor derived but represent teratoma-induced metaplasia of submesothelial cells [21].
Immature teratomas can be associated with mature cystic teratomas. Ipsilateral typical mature cystic teratomas are present in 26 % of cases of immature teratoma, whereas 10 % of the patients present with an immature teratoma in the contralateral ovary [22].
Malignant transformation of a mature teratoma is a rare event and the squamous cell carcinoma is the most frequent histological type in this case, but adenocarcinomas, primarily melanomas and carcinoids, may also occur. The risk reported in the literature is between 0.5 and 2 % of teratomas, usually in postmenopausal women [1].
Microscopy
Immature teratoma is composed of immature tissue differentiating toward cartilage, glands, bone, muscle, nerve, and others [14].
In contrast to the other forms of malignant ovarian germ cell tumor, immature ovarian teratoma usually shows relatively minor cytogenetic abnormalities that increase as the grade of the immaturity worsens [21].
The most common monodermal teratoma (composed predominantly or solely of one tissue type) is the struma ovarii, a rare ovarian tumor composed entirely or predominantly of thyroid tissues. It constitutes approximatively 3 % of all ovarian teratomas, 2 % of all germ cell tumors, and 0.5 % of all ovarian tumors. The malignant transformations occur only in about 5 % of all struma ovarii [21, 23]. Histologically it can be described as mature thyroid tissue consisting of colloid-containing follicles of different size lined by a single layer of follicular cells [23].
Macroscopy
Immature teratomas are typically large (14–25 cm) and have a smooth external surface. On section, they are solid or predominantly solid, cystic areas can be present as well and usually filled with serous or mucinous fluid or sometimes with fatty sebaceous material. The solid areas within immature teratomas, which are usually composed predominantly of neural tissue, are typically soft, fleshy, and gray to pink and may be focally hemorrhagic or necrotic. Mature components such as hair, fatty tissue, cartilage, bone, and calcification are usually present [22].
Clinical Symptoms
Immature teratoma is often discovered incidentally during a physical or ultrasound examination of the female pelvis.
Given the large size, patients with immature teratoma can present with the onset of acute pelvic pain due to either rupture or torsion of the ovarian mass. Some patients complain of abdominal distension, pain, urinary or bowel symptoms, and infertility. Rarely patients can experience sexual pseudoprecocity due to the production of steroid hormones [1].
Prognosis
The most important prognostic feature is the grade of the lesions. Patients with stage IA grade 1 tumors have an excellent prognosis, and adjuvant therapy is not required. In case of stage IA grade 2 or 3, adjuvant chemotherapy is commonly recommended. Combination platinum-based chemotherapy is the treatment of choice (BEP regimen) [1, 15].
For patients with all stages, pure immature teratomas’ 5-year survival rate is 70–80 %, and it is 90–95 % for patients with surgical stage I lesions. The presence of residual disease at the end of primary surgery has been reported to significantly lower 5-year survival rate from 94 to 50 % [1].
Ultrasound Characteristics
The ultrasound appearance of immature teratoma is nonspecific, and it is very difficult to differentiate them from the benign counterparts.
Three ultrasound patterns most commonly occur in mature teratoma. The most common setting is a cystic lesion with a densely echogenic tubercle (Rokitansky nodule) projecting into the cyst lumen; the second one is a diffusely or partially echogenic mass with the echogenic area usually demonstrating sound attenuation owing to sebaceous material and hair within the cyst cavity; and the third one consists of multiple thin, echogenic bands due to the presence of hair in the cyst cavity [22].
There are different echogenicities depending on the characteristics of the material filling the dermoid cyst: pure sebum may be hypoechoic or anechoic; fluid–fluid levels result from sebum floating above aqueous fluid, which appears more echogenic than the sebum layer; dermoid plug is echogenic, with shadowing due to adipose tissue or calcifications within the plug or to hair arising from it. Diffuse echogenicity in these tumors is caused by hair mixed with the cyst fluid [22].
The immature teratomas typically are large masses with heterogeneous, partially solid lesions. Scattered calcifications are usually presents. Ultrasonographically, these features can appear as heterogeneous internal signal intensity with punctate high signal intensity or echogenic mass with sound attenuation or heterogeneous mass containing echogenic reflectors representing hair [22] (Fig. 4).
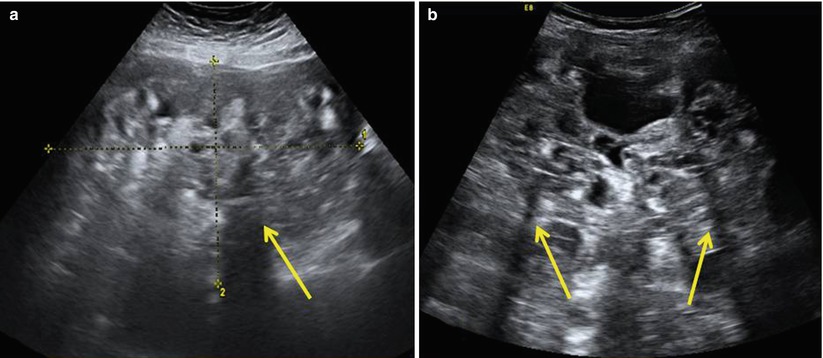

Fig. 4
Large prominent solid pure ovarian immature teratomas in a 50-year-old patient with a 24 cm mass (a) and in a 12-year-old girl with a 28 cm mass (b) with a heterogeneous appearance and cystic elements. The internal echogenicity is rather irregular; echogenic sebaceous material and scattered calcifications determining cone shadows (arrows) are present; contours are undefined
Endodermal Sinus Tumor
It is also called yolk sac carcinoma since it derives from a multipotential embryonal carcinoma by selection and dedifferentiation toward primitive yolk sacs structures; it is the third most frequent malignant germ cell tumor of the ovary [1, 14]. It usually occurs in the second decade of life with a median age of 18 years. Approximately one-third of the patients are premenarchal at the time of initial presentation [1, 24].
Endodermal sinus tumor often is characterized by extremely rapid growth and extensive intra-abdominal spreading sometimes characterized by poor prognosis [24]. The tumor appears to involve a single ovary, but it grows rapidly and aggressively [14]. Abdominal or pelvic pain is the most frequent presenting symptoms (75 % of cases), whereas an asymptomatic pelvic mass is documented only in 10 % of patients [1].
The tumor is frequently encapsulated, rounded, oval, or globular. On section, they are solid and frequently present with cystic areas [4]. Extensive areas of hemorrhage and necrosis are common. A honeycomb appearance due to many small cysts may indicate the presence of a polyvesicular-vitelline component. The histological feature is a glomerulus-like structure composed of a central blood vessel surrounded by germ cells within a space similarly lined by germ cells (Schiller–Duval body). Similar structures are observed in the yolk sac of the rat placenta, but in the tumor, loose meshwork of communicating spaces lined by primitive tumor cells with clear or vacuolated cytoplasm is observed. Conspicuous intracellular and extracellular hyaline droplets are present in all neoplasms [14, 24]. The tumor is rich in AFP and α1 antitrypsin. The serum levels of these markers, particularly AFP, are useful in the diagnosis and monitoring of a patient’s response to treatment. It may rarely produce detectable serum levels of α1 antitrypsin [1].
The standard treatment of this tumor consists of surgical exploration, unilateral salpingo-oophorectomy, resection of any gross metastases if feasible, followed by adjuvant cisplatin-based combination chemotherapy, such as BEP, that is considered the standard of care. Most patients have early stage disease: 71 % stage I, 6 % stage II, and 23 % stage III. Nowadays, cure rate approaches 100 % for early stage disease and at least 75 % for more advanced stages [1, 15].
Ultrasonographically, endodermal sinus tumors range from entirely solid to predominantly cystic patterns, but heterogeneous appearance consisting of a mixture of solid and cyst features has been reported. The cystic component might be due to cystic degeneration or necrosis [24].
It has been reported that this tumor shows an intense vascularization with vessels with low blood flow resistance detected at the color Doppler flow velocity measurement [24] (Fig. 5).
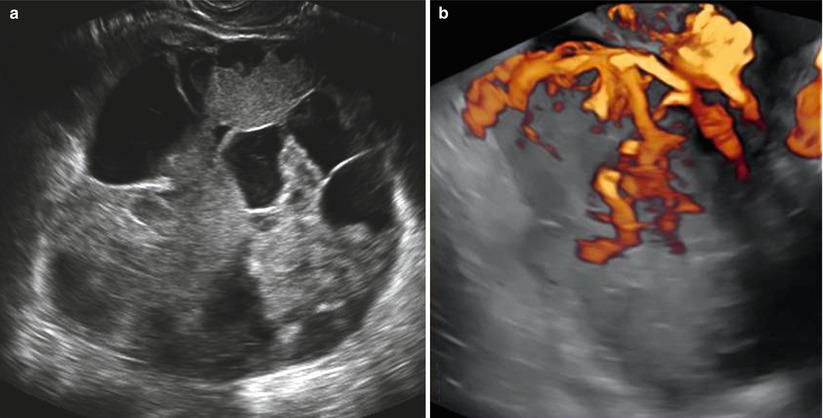

Fig. 5
(a) Grayscale ultrasound images of a large (19 cm) stage IIB endodermal sinus tumor in a 19-year-old patient with a preeminent solid round-shaped mass with cystic areas representing necrosis. (b) Three-dimensional power Doppler image showing intense vascularization, tortuous vessels with irregular branching, and caliber changes in the endodermal sinus tumor
Rare Germ Cell Tumors of the Ovary
Embryonal Carcinoma
Embryonal carcinoma of the ovary is an extremely rare tumor characterized by large primitive cells resembling those of the embryonic germ disk and growing in solid, papillary, and glandular patterns [1]. It is rarely diagnosed as a pure form, and most commonly it occurs as a component of a germ cell tumor [25]. Patients are very young, ranging in age from 4 to 28 years with a mean age at diagnosis of 14 years [15]. Patients may present with precocious pseudopuberty or with abnormal uterine bleeding since embryonal cell carcinoma may produce estrogen [25]. Moreover, these lesions frequently secrete AFP and hCG that are useful markers to follow the response to subsequent therapies [26].
The most common symptoms described in the literature are abdominal or pelvic mass, abdominal pain, fever, and marked weight loss. Signs of abnormal hormonal production can occur such as precocious puberty manifested by bilateral breast development, vaginal bleeding, and growth of hair on the vulva in prepubertal girls and amenorrhea, irregular vaginal bleeding, infertility, and mild hirsutism in postmenarchal woman [27].
The primary lesions tend to be large, and approximately two-thirds of them are confined to one ovary at the time of presentation [1]. In Kurman et al. series, the greatest tumor diameter ranged from 10 to 25 cm, the median being 17 cm [27]. At surgery spread to the peritoneum is observed in 40 % of the cases, sometimes accompanied by parenchymal involvement of pelvic or intra-abdominal viscera [25].
Macroscopically the tumors had smooth, glistening external surfaces with a yellow to gray tissue on transection, solid for the most part, but cystic spaces containing mucoid material are frequently observed as well [27].
Microscopically, the embryonal carcinomas present solid proliferation of cells and isolated clusters of syncytiotrophoblast. The cells of this tumor, like those of dysgerminoma, tend to form sheets but are easily distinguished from dysgerminoma by their larger size, more hyperchromatic nuclei, and tendency to form a glandular pattern. The stroma of embryonal carcinoma is either loose with foci of hemorrhage and necrosis or more collagenous. The overall microscopic appearance of embryonal carcinoma suggests a uniphasic overgrowth by cytotrophoblast with scattered, isolated syncytiotrophoblast cells [27].
Women with embryonal carcinomas and other germ cell tumors should be usually treated with conservative surgery followed by BEP regimen chemotherapy. While there are some data to suggest that the presence of embryonal carcinoma in mixed tumors is associated with a higher relapse rate following conservative treatment than that of other germ cell tumors, those neoplasms appear to respond to chemotherapy as their counterparts in males and the other ovarian germ cell tumors do [25]. Surveillance for recurrence should be intensive in the first 2 years, with a more relaxed schedule thereafter. Ongoing surveillance for second malignancies and chemotherapy-related toxicity should be included in the follow-up of these patients [25].
Choriocarcinoma of the Ovary
Non-gestational choriocarcinoma of the ovary can be pure or mixed with other germ cell tumors. Pure type is less frequent than mixed type and the diagnosis of non-gestational choriocarcinoma of the ovary is very difficult in the reproductive period [28]. A primary germ cell origin may be difficult to prove purely on a histological basis except in those cases that develop in premenarchal or virginal patients [29]. The majority of patients with this cancer are younger than 20 years [1]. The distinction between gestational versus non-gestational choriocarcinoma is important because a non-gestational choriocarcinoma carries a worse prognosis than the gestational type [19, 30]. Another poor prognostic factor is the presence of metastases to other organs (parenchyma) at the time of initial diagnosis [1].
Similarly to other ovarian neoplasms, the presenting symptoms of non-gestational choriocarcinoma are nonspecific [19] such as abdominal pain, irregular bleeding, precocious puberty, and positive pregnancy test without pregnancy because of the markedly elevated tumor-associated HCG levels [19]. The presence of HCG can be useful in monitoring the patient’s response to treatment [1].
On gross examination, the pure choriocarcinoma is typically solid, hemorrhagic, and friable.
Histologically, non-gestational pure choriocarcinoma of the ovary presents the same appearance as the gestational choriocarcinoma metastatic to the ovaries [28].
There are only a few limited reports on the use of chemotherapy for these non-gestational choriocarcinomas, but complete responses have been reported to the MAC regimen (methotrexate, actinomycin D, and cyclophosphamide) used in the same manner as described for gestational trophoblastic disease [28]. These tumors are so rare that definitive data are not available, but other chemotherapy regimens such as BEP or cisplatin, vincristine, methotrexate, bleomycin, actinomycin D, cyclophosphamide, and etoposide (POMB-ACE) have shown a positive impact on survival (Fig. 6).
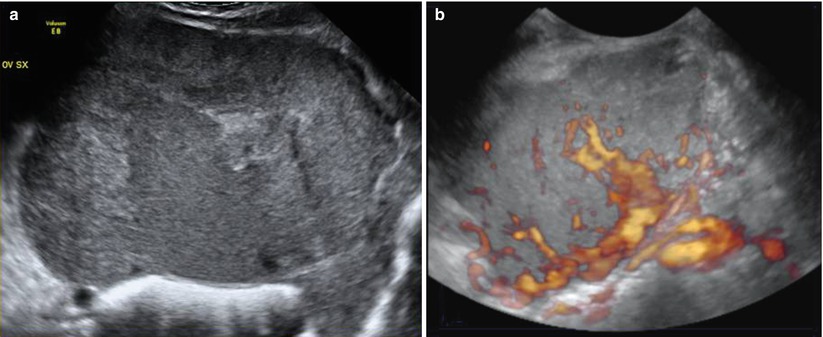

Fig. 6




(a) Grayscale ultrasound images of a large (14 cm) stage IA mixed germ cell tumor consisting of choriocarcinoma, embryonal carcinoma, and a majority amount of clear cell carcinoma with a solid appearance and inhomogeneous echotexture due to hemorrhage and necrosis. Margins are rather regular and well defined. (b) Three-dimensional power Doppler image showing intense vascularization, tortuous vessels with irregular branching
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree