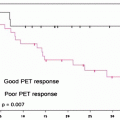
Fig. 10.1
(a) Five-year observed survival by combined AJCC stage squamous cell carcinoma of the lip, 1998–1999. (b) Five-year observed survival by “combined” AJCC stage squamous cell carcinoma of the oral cavity, 1998–1999. (From Edge S, et al. editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010, with permission of Springer Science + Business Media)
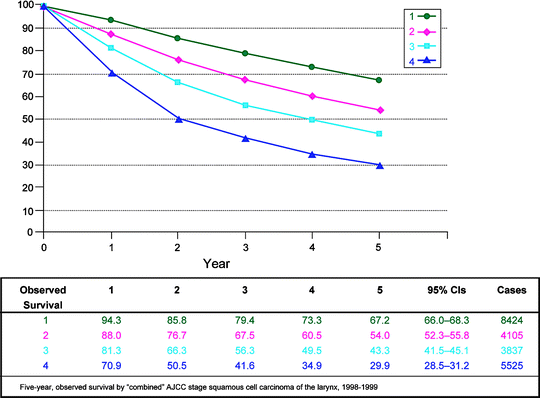
Fig. 10.2
Five-year observed survival by combined AJCC stage squamous cell carcinoma of the larynx, 1998–1999. (From Edge S, et al. editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010, with permission of Springer Science + Business Media)
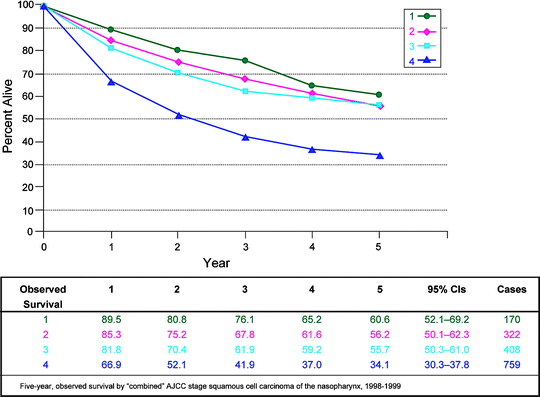
Fig. 10.3
Five-year observed survival by combined AJCC stage squamous cell carcinoma of the nasopharynx, 1998–1999. (From Edge S, et al. editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010, with permission of Springer Science + Business Media)
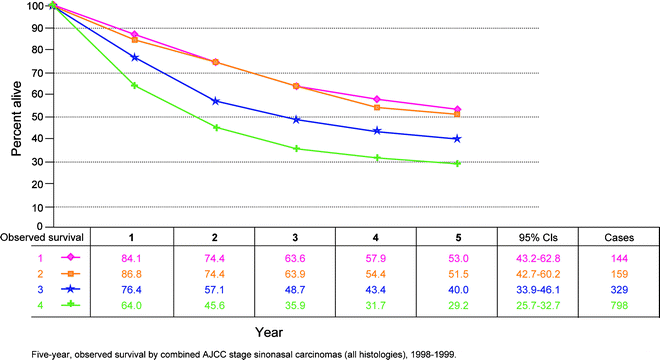
Fig. 10.4
Five-year observed survival by combined AJCC stage sinonasal carcinomas (all histologies), 1998–1999. (From Edge S, et al. editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010, with permission of Springer Science + Business Media)
Traditionally, the most important risk factors for HNSCC were tobacco use and alcohol consumption, which seem to have a synergistic effect. In recent years, a new disease entity has become more common: HNSCC of the oropharynx secondary to infection with human papillomavirus (HPV) [2]. HPV is a known cause of cervical cancer in females. Transmission to the oral cavity occurs through oral sex. In the USA, approximately 60% of oropharynx cancers are positive for HPV-16. The overall incidence of HNSCC is still rising in the industrialized world, because the substantial increase in HPV-positive cases (in particular males with carcinoma of the palatine tonsil and base of tongue) exceeds the decline in HPV-negative cases. Most HPV-positive cases express the viral oncoproteins E6 and E7. E6 oncogene expression induces degradation of p53 and E7 inactivation of the retinoblastoma protein (RB). Under normal conditions, p53 induces cell cycle arrest in the G2 phase to allow for repair of damaged DNA. It also is a master regulator for apoptosis [3]. RB normally binds to, and inactivates, the E2F transcription factors, which control the transition from the G1 to the S phase of the cell cycle. Only under the influence of mitogen signals, this “brake” of RB on E2F is released, thus permitting cell cycle progression. Binding of the viral oncoprotein E7 to RB therefore causes disinhibition and uncontrolled cell cycle progression [3, 4]. Table 10.1 summarizes epidemiologic and biologic features of HPV-positive and HPV-negative HNSCC [3, 5].
Table 10.1
Epidemiology of HNSCC circa 2011
HPV-positive HNSCC | HPV-negative HNSCC | |
|---|---|---|
Incidence | Increasing | Decreasing |
Anatomical site | Oropharynx (tonsil, tongue) | All sites |
Histology | Nonkeratinized | Keratinized |
Age | Younger; <60 years | Older; >60 years |
Sex ratio | 3:1 Men | 3:1 Men |
T stage | Tx, T1, T2 | Any |
N stage | Advanced, often cystic–necrotic, multilevel | Any |
Risk factors | Sexual behavior | Alcohol, tobacco |
Etiology | HPV virus | |
Prognosis | Favorable | Unfavorable |
New approaches | De-escalation of standard therapy? vaccination? oral Pap smear? | Early identification of nonresponders to standard therapy → clinical trials |
Patients with HPV-positive HNSCC have a better prognosis than patients with HPV-negative disease [6, 7]. This was shown in a phase 2 trial, which employed induction chemotherapy followed by concurrent chemoradiotherapy in patients with stage III or IV HNSCC of the oropharynx or larynx. Response rates to induction chemotherapy were 82% versus 55%, 2-year overall survival was 95% versus 62%, and the 2-year progression-free survival was 86% versus 53% [7]. Similarly, in a large retrospective analysis [6] the 3-year overall survival was 82% in the 206 patients with HPV-positive tumors as compared to 57% in the 117 patients with HPV-negative tumors. Corresponding rates for progression-free survival were 74 and 38%. Even after adjusting for age, race, T and N stage, tobacco exposure, and treatment modality (standard fractionation vs. accelerated fractionation radiotherapy), patients with HPV-positive tumors still showed a 58% reduction in the risk of cancer death. Using a risk classification that considered HPV status, T and N stage, and smoking status, patients could be characterized as low, intermediate, or high risk. Whereas the 3-year survival was 93% in the low-risk group (HPV + tumor and <10 pack years smoking history), it was only 46% in the high-risk group (HPV-negative, ≤10 pack years and T4, or >10 pack years and any TN). Collectively these data, and those forthcoming from other groups, suggest increased sensitivity to chemo- and radiotherapy in HPV-positive tumors. Accordingly, there is now great interest in developing new treatment paradigms in HNSCC that may decrease the amount and duration of standard chemoradiotherapy in low-risk patients (to maintain high cure rates but reduce potential treatment associated long-term side effects, such as dysphagia, xerostomia, feeding tube dependency, or chronic aspiration), and to find more successful therapies for high-risk patients, including early participation in clinical trials [8, 9]. Patients with persistent HPV expression after completion of therapy may require closer surveillance [10]. The potential role of an HPV vaccine for men and the role of an “oral Pap smear” for early detection of oropharynx cancer are under study.
An important consideration in head and neck cancer is the concept of field cancerization. The occurrence of multiple synchronous or metachronous primary tumors in the upper aerodigestive tract was first described by Theodor Billroth more than 100 years ago. Over the past decades knowledge has expanded, and a recent study showed that at least 35% of cases of oral and oropharynx cancers demonstrate genetic abnormalities in their surrounding (histopathologically normal) mucosa [11]. This is often addressed by the clinical term “biologically positive margins,” suggesting that local recurrence is likely even though standard histopathology shows no cancer cells at or near the surgical margins of the tumor specimen [12].
As for many other malignancies, genetic alterations and activation or inhibition of cellular signaling pathways are important for the development and progression of many HNSCC. Some signaling factors are of particular interest because they are potential targets for drug therapy, including the epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and molecules involved in regulating the PI3K–PTEN–AKT pathway as well as Ras–MAPK pathway.
Treatment Strategies
The treatment approach varies with disease stage and site(s) of disease in the head and neck [13]. Approximately 1/3 of patients with HNSCC present with early stage disease. Depending on tumor location and institutional preferences, they are treated surgically or with radiotherapy, and approximately 80% are cured. Patients with locoregional advanced disease, which is surgically unresectable, and patients in whom definitive treatment is administered with an attempt at organ preservation (e.g., oropharyngeal and laryngeal carcinomas) undergo treatment with concurrent chemoradiotherapy [14, 15]. Cisplatinum is the drug with the most randomized clinical trial data to support its use as a drug enhancing the effects of radiotherapy in this setting. The larynx-preservation paradigm is supported by the results of a study that randomized 547 patients with stage III–IV supraglottic and glottic larynx cancer into three treatment arms: concurrent chemoradiotherapy, induction chemotherapy consisting of cisplatinum and 5-fluorouracil (5-FU) followed by radiotherapy, or radiotherapy alone. After a median follow-up of 3.5 years, the rates of locoregional control were 78, 61, and 56% for the three treatment arms, respectively. The fraction of patients who maintained an intact larynx at 2 years (and thus the ability to speak and swallow after the end of therapy) was also better with the concurrent regimen (88, 75, and 70% for the three treatment arms, respectively) [15]. Overall survival rates were similar in all three groups. The utility of concurrent high-dose cisplatinum for other subsites was established in a randomized study of 295 patients with unresectable head and neck cancer [16]. Concurrent chemoradiotherapy is therefore now widely applied as the definitive treatment of choice for locoregional advanced HNSCC. If residual disease is detected after the end of therapy or during follow-up, salvage surgery (e.g., laryngectomy) may be offered.
The management of the neck when using an organ-preservation approach has remained somewhat controversial. Complete response rates in irradiated cervical lymph nodes vary between 59 and 83% and to some degree are related to nodal size, dose of radiotherapy, as well as the time point when response is determined: complete response rates are almost 100% in N1 disease, higher in N2 than in N3 disease, and better when the largest metastatic node is <3 cm [17]. In N2–N3 disease, residual cancer in neck nodes has been reported in 16–39% of patients achieving a clinical complete response (no overt residual neck mass) [17–19]. Over the past decade, locoregional control rates with concurrent chemoradiotherapy have improved. It is now widely accepted that routine neck dissection is not appropriate in this setting. Instead, [18F]FDG PET/CT has emerged as a gatekeeper for the management of the neck in this setting [13] (see following discussion).
Newer biologic therapies in HNSCC include angiogenesis inhibitors and drugs targeting the EGFR. EGFR is overexpressed and/or activated in the majority of HNSCC relative to normal tissue, and high expression is associated with poor disease control [20, 21]. While these data suggest HNSCC as an ideal malignancy for treatment with EGFR inhibitors, the selection of appropriate patients remains challenging. In studies of anti-EGFR agents in patients with advanced HNSCC, the objective response rates have been approximately 10%, depending on the specific agent [22]. Cetuximab, a chimeric IgG1 antibody against the extracellular domain of EGFR, is the most widely studied agent and has been tested in a number of clinical trials in patients with advanced disease. Cetuximab also enhances the efficacy of radiotherapy. In a randomized study of 420 patients [23], the addition of cetuximab to radiotherapy improved locoregional tumor control and overall survival without increasing mucositis and dysphagia when compared to radiotherapy alone. The corresponding median progression-free survival was 24 months versus 15 months, and the median overall survival was 49 months versus 29 months. This study showed that concurrent radiotherapy plus cetuximab is a viable treatment option in locoregional advanced HNSCC. Whether this alternate regimen improves outcome vis-à-vis the standard approach with cisplatinum based chemotherapy and concurrent radiotherapy is under investigation. Other ongoing trials are investigating the role of adjuvant cetuximab in patients with recurrent or metastatic HNSCC undergoing platinum-based chemotherapy. Preliminary data show a survival benefit over chemotherapy alone (reviewed in [24]). Antiangiogenic therapy has mainly been studied using Bevacizumab, a recombinant humanized, monoclonal IgG antibody against VEGF-A, usually in combination with chemotherapy or in combination with cetuximab. Preliminary data suggest that partial responses or disease stabilization can be achieved in >75% of patients [25]. Other drugs under study include various tyrosine kinase inhibitors. Recent improved understanding of relevant signaling pathways may open up new avenues for targeted drug therapies in HNSCC. For instance, downregulation of the transforming growth factor-β (TFG-β) receptor is frequently found, and the PI3K/PTEN/Akt/m-TOR pathway (important for increased proliferation, evasion of apoptosis, and increased survival) is frequently upregulated. A number of drugs interfering with these tumor pathways are under study [24].
Imaging of Head and Neck Cancer
Standard imaging studies for the staging and follow-up of head and neck cancer include contrast-enhanced CT, MRI, and 18F-FDG PET/CT. CT or MRI is essential for characterizing the primary tumor and in defining its extent. Bone involvement is better detected by CT and early bone marrow disease better with MRI. MRI can better define the interface between normal muscle and tumor. It is essential in staging nasopharyngeal carcinoma (NPC) and also has particular advantages for detecting intracranial tumor spread, perineural spread, and for differentiating entrapped secretions in paranasal sinuses from adjacent tumor.
Lymph node staging is done equally well with either CT or MRI. Traditionally, both imaging tests have relied on the assessment of lymph node size in order to identify metastatic disease. In general, lymph nodes ≥1.5 cm in levels I and highest level II, as well as lymph nodes ≥1.0 cm in all other locations, are considered abnormal. This approach is suboptimal because metastasis can be present in normal size lymph nodes and enlarged nodes may not contain tumor. Depending on the chosen cutoff for lymph node size, one can achieve high sensitivity at the expense of specificity or vice versa [26]. Secondary criteria are presence of grouped lymph nodes (usually >3 nodes in one location), heterogeneous nodal enhancement, stranding of surrounding fat indicating extracapsular spread, and demonstration of central necrosis (the most reliable criterion for nodal metastasis). Special MRI sequences, such as diffusion-weighted imaging (DWI), or newer MR contrast agents (including ultrasmall superparamagnetic iron oxide particles or more recently various nanoparticles) may improve the characterization of nodes. However, they are not yet routinely employed, and all available data are preliminary.
The vast majority of head and neck cancers show intense [18F]FDG uptake; however, uptake in salivary gland tumors may be variable (high [18F]FDG uptake in some benign tumors, low uptake in some malignant tumors). Combined PET/CT is a more accurate than PET alone and has beneficial effects on patient management [27, 28]. We perform [18F]FDG PET/CT imaging from the skull base to the floor of the pelvis for appropriate N and M staging in patients with head and neck cancer. Both distant metastases and potential synchronous primaries in the aerodigestive tract are hypermetabolic and therefore easily detectable. In order to save cost and potentially radiation dose, some investigators have proposed a limited field of view scan (e.g., ending at the level of the diaphragm) for patients with head and neck cancer. We, however, believe that a different approach should be taken: rather than using [18F]FDG PET indiscriminately in all patients, the test should only be used in subsets of patients where imaging findings are most likely to alter management, and in these cases it remains important to image the entire torso. For instance, it is essential in surgical patients with locally advanced disease. In addition, we use [18F]FDG PET/CT in all patients who are candidates for definitive chemoradiotherapy for the purpose of staging and radiotherapy planning; these scans are performed in the radiotherapy position and mask. PET/CT is also used for response assessment in patients with advanced disease undergoing chemotherapy or enrolled on experimental protocols.
Interpretation of head and neck PET/CT requires special expertise in head and neck anatomy and an understanding of physiologic [18F]FDG uptake in normal tissues and awareness of the many potential normal variants. Scan interpretation is primarily based on visual assessment, although SUV numbers may be helpful in estimating biologic aggressiveness and for response assessment. SUV is related to lesion size and may be underestimated in small primary tumors. Physiologic [18F]FDG uptake is nearly always noted in lymphoid tissues (tonsils) composing “Waldeyer’s ring,” including the pharyngeal tonsil (upper nasopharynx), symmetrical uptake in the palatine tonsils (unless the patients is status posttonsillectomy or suffers from unilateral tonsillitis or tonsillar carcinoma), and the lingual tonsil at the base of tongue. Variable uptake is noted in the tongue and floor of mouth muscles and is usually more intense if the patient was moving the tongue or swallowing during the uptake phase. Asymmetrical uptake in normal tongue can be noted after hemiglossectomy or even resection of smaller unilateral tongue lesions, leading to increased muscle activity and [18F]FDG uptake in the contralateral tongue. This is also noted after unilateral denervation, e.g., after hemimandibulectomy or due to hypoglossal nerve paralysis, where fatty atrophy and lack of [18F]FDG uptake in the denervated hemitongue is associated with relatively intense uptake in the contralateral normal tongue. Asymmetrical uptake, due to spasm, can sometimes be observed in pterygoid or temporalis muscles. Uptake along scalene muscles is frequently seen, either symmetrical or asymmetrical, and is easily characterized as normal variant on fusion images. Retained mucous in paranasal sinuses shows no [18F]FDG uptake unless there is severe inflammation, infection, or tumor. Similar to MRI (but not CT) [18F]FDG PET/CT differentiates well between tumor and entrapped secretions due to sinus outflow obstruction. While mucous retention or small polyps are fairly common in the paranasal sinuses, fluid retention in mastoid air cells should prompt careful assessment of the oropharynx to identify possible reasons for obstruction of the Eustachian tube.
Normally, symmetrical [18F]FDG uptake is seen in vocal cords and other laryngeal muscles (mild uptake is usually noted even if the patient does not vocalize during the uptake phase). Asymmetrical vocal cord uptake suggests vocal cord paralysis, usually due to damage of the ipsilateral recurrent laryngeal nerve. Secondary CT signs are sometimes helpful in confirming suspected vocal cord paralysis, including a paramedian position of the paralyzed vocal cord, tilting of the thyroid cartilage, displacement of the ipsilateral arytenoid cartilage, dilatation of the ipsilateral pyriform sinus, and prominent laryngeal ventricle. Long-standing vocal cord paralysis may lead to atrophy of the posterior cricoarytenoid and thyroarytenoid muscles [29, 30]. In searching for possible reasons for vocal cord paralysis, attention should focus on the upper mediastinum as well as lower neck to identify possible tumors or metastasis. Other reasons for vocal cord paralysis include nerve damage due to fibrosis (e.g., after radiotherapy) and chemotherapy (vinca alkaloids can cause transient vocal cord paralysis [31]). Asymmetrical or unusual focal [18F]FDG uptake can be observed in patients who received instillation of foreign material (e.g., Radiesse) for attempted medialization of a paralyzed cord. In this case the [18F]FDG uptake occurs on the treated site, likely due to foreign body reaction and inflammation. The instilled dense material is easily identified on the CT images. Large salivary glands usually show symmetrical [18F]FDG uptake (submandibular gland > parotid gland). A common cause for unilateral focal [18F]FDG uptake in parotid glands are pleomorphic adenomas (the most common benign tumor in the parotid gland) or “Warthin’s tumors” (which are entrapped normal lymphoid tissue). The submandibular gland is usually removed as part of neck dissections. Asymmetrical uptake in the contralateral remaining normal gland should not be confused with lymph node metastases. Postsurgical muscle flaps can show (normal) very intense [18F]FDG uptake, in particular at the flap pedicle, presumably related to hyperemia. This should not be confused with tumor recurrence. Some of the aforementioned normal findings and normal variations are described in detail in several review articles [32, 33].
Neck Metastases from an Unknown Primary Tumor
This condition, also known as carcinoma of unknown primary, accounts for 3–15% of all cancer diagnoses and for approximately 1–2% of head and neck cancers. For practical purposes this entity should be defined as the combination of no history of previous malignancy, no clinical or laboratory evidence for primary neoplasm, and a neck mass that is histologically or cytologically proven to be carcinoma. Occurrence of nodal metastases in neck levels I–III increases the likelihood for a primary HNSCC. However, in 5–40% of cases a primary malignancy is not identified during diagnostic evaluation and long-term follow-up. Provided a primary tumor cannot be identified, in many institutions these patients are treated with extensive radiotherapy fields that cover the entire pharyngeal mucosa, the larynx, and bilateral necks. This is obviously associated with considerable morbidity. Identification of a primary tumor will direct rational therapy to a single mucosal site and the ipsilateral neck (unless the primary tumor is in, or close to, the midline). Detection of the primary tumor, in general, also allows for less extensive treatment (e.g., smaller radiation fields) and may potentially improve locoregional control. Therefore, these patients undergo extensive work-up. In some cases, the “unknown primary” is already identified in a thorough clinical exam by an experienced head and neck surgeon or oncologist; all other patients should undergo panendoscopy of upper aerodigestive tract and [18F]FDG PET/CT.
The literature is replete with studies in patients with unknown primary. Unfortunately, most study populations are very heterogeneous, and the definition of unknown primary and methods of verification vary considerably. For this chapter, I have only considered studies in which patients had at least undergone clinical exam, CT or MRI, and office endoscopy prior to PET (Table 10.2). The percentage of primary tumors detected is quoted as 14–37% [34–45]. When reviewing the literature, it should be noted that the sensitivity of PET is oftentimes stated incorrectly: since all neck metastases must have an underlying primary tumor, the denominator for calculating sensitivity should be the total number of patients studied (not the much smaller number of patients in whom the primary is eventually detected by imaging and panendoscopy). Therefore, it appears overall less controversial and more meaningful to simply state the rate of primary tumors that were only detected by PET (Table 10.2). Depending on the interpretation criteria, some studies report a high number of false-positive findings. It is therefore necessary to strike a reasonable balance between the desire to identify the location of the unknown primary on PET and the danger of calling every little variant suspicious. Potential reasons for a false-negative PET studies include a small primary tumor (although lesions as small as 4 mm can be detected as long as there is intense [18F]FDG uptake); low metabolic activity (cystic/necrotic tumor or lymph nodes) or the primary tumor was accidentally removed at time of neck dissection.
Table 10.2
[18F]FDG PET and PET/CT for the detection of unknown primary tumors in patients with neck lymph node metastases
Author | N | Prior panendoscopy | PET or PET/CT | Total detection rate with all tests | Total PET uptake | PET detection rate for primary | PET false-positive rate % | Distant metastases with PET | Comments |
|---|---|---|---|---|---|---|---|---|---|
Kole [40] | 14 | None (was done after PET) | PET | 7/14 (50%) | 4/14 | 4/14 (25%) | N/R | N/R | Management change in 21% of patients |
Jungehülsing [39] | 27 | All | PET | 7/27 (26%) (5 in HN) | N/R | 7/27 (26%) | N/R | N/R | Management change in 29% of patients |
Johansen [38] | 42 | All | PET | 10/42 (24%) (7 in HN) | 20/42 | 10/42 (24%) | 10/42 (24%) | N/R | |
Fogarty [35] | 21 | N/R | PET | 3/21 (14%) (One path confirmed, two probable) | 8/21 | 3/21 (14%) | 5/8 (62%) | 3/21 (14%) | PET detected more regional neck dissection in 2/21 |
Wong [44] | 17 | All | PET | N/R | 8/17 | 5/29 (17%) | 3/8 (37%) | Treatment plan changed in 53% of patients | |
Bohuslavizki [34] | 44 | All | PET | 22/42 (53%) | 22/44 (13 in HN) | 15/44 (34%) (8 HN = 18%) | 5/13 (38%) | N/R | Mixed patient population |
Miller [41] | 31 | None (CT/MR, office endoscopy; panendoscopy after PET) | PET | 14/31 (45%) | 10/31 | 9/31 (29%) | 1 (3%) | N/R | Four additional found by panendoscopy, size 0.8–5 mm |
Gutzeit (data shown for patients with neck metastases only) [36] | 18 | N/R | PET/CT | 6/18 (33%) | 7/18 | 3/18 (33%) | 3/16 (19%) | N/R | PET/CT slightly better than CT or PET alone |
Wartski [43] | 38 | All | PET/CT | 14/38 (38%) | 26/38 | 13/38 (34%) | 4/17 Patients w/further w/u (23%); additional nine patients had no further w/u | N/R | Management change in 60% of patients |
Johansen [37] | 60 | 41 | PET or PET/CT | 21/60 (35%) | 30/60 (22 in HN) | 18/60 (30%) Total; (missed 3 HN primaries later found by panendoscopy) | 12/30 (40%) Total; 10/22 (45%) in HN; 1/8 pre-endoscopy; 11/22 postendoscopy | 12/22 (54%) with HN primary | Management change in 25% of patients |
Roh 2009 [42] | 44 | None (was done after PET) | PET/CT | 16/44 (36%) | 19/44 | 14/44 (32%) | 5/14 (35%) | 6/44 (14%) | Subtracting tumors seen on contrast CT, PET detected 7/37 primaries (18%) |
Yabuki [45] | 24 | All | PET | 9/24 (37%) | 12/24 | 9/24 (37%) | 3/12 (25%) | N/R |
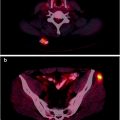
In our institution, patients with unknown primary, negative clinical exam and office endoscopy first undergo [18F]FDG PET/CT, before panendoscopy; panendoscopy is defined as nasopharyngoscopy, direct laryngoscopy, and upper esophagoscopy (in the absence of specific symptoms or imaging findings, a bronchoscopy is no longer done routinely because the yield is exceedingly low). This approach may reduce the number of necessary panendoscopies and avoid false-positive [18F]FDG uptake after manipulation during endoscopy and biopsy (Fig. 10.5). Of note, other imaging studies are usually not needed, because it is extremely unlikely that they may identify a primary tumor that cannot be detected by PET. Statistically, most primary tumors originate in the ipsilateral palatine tonsil, base of tongue, pyriform sinus, nasopharynx (more commonly in East Asian populations), and larynx. Particular attention should therefore be focused during PET/CT interpretation to these sites. If the PET study is positive, biopsies should be obtained from the suggested location. If the PET is negative, panendoscopy with biopsy sampling from suspected locations (based on the location of the lymph node and statistical considerations) should be performed. Depending on the results of these biopsies, the patient may proceed to surgery or radiation therapy.
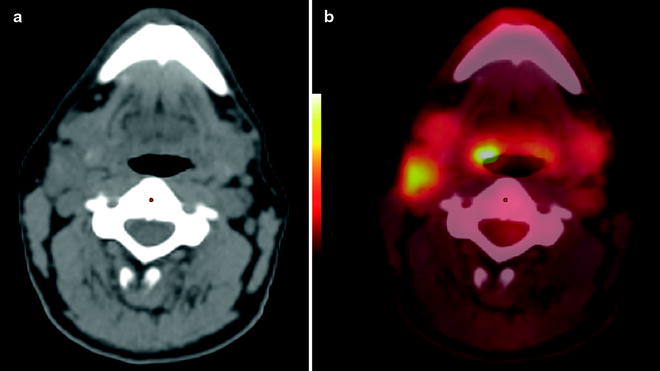

Fig. 10.5
Carcinoma of unknown primary in a 46-year-old man who presented with a right neck mass from an unknown primary squamous cell carcinoma. Clinical exam and office endoscopy were negative. [18F]FDG PET was performed for localization of primary tumor. Non-contrast CT shows enlarged right neck node (a); PET/CT fusion image shows abnormal [18F]FDG uptake in right level two node and at the right base of tongue primary (b)
Primary Tumor Staging
Most HNSCC are diagnosed clinically (Tables 10.3, 10.4, 10.5, and 10.6). The primary goal of imaging studies is therefore not tumor detection, but rather to define the extent of the primary tumor, in particular with regard to structures whose involvement may alter the surgical approach (e.g., depth of soft tissue infiltration, bone invasion, orbital invasion, skull base invasion, tumor “tracking” along nerves and blood vessels).
Table 10.3
AJCC anatomic stage and prognostic groups
Group | T category | N category | M category |
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage III | T3 | N0 | M0 |
T1 | N1 | M0 | |
T2 | N1 | M0 | |
T3 | N1 | M0 | |
Stage IVA | T4a | N0 | M0 |
T4a | N1 | M0 | |
T1 | N2 | M0 | |
T2 | N2 | M0 | |
T3 | N2 | M0 | |
T4a | N2 | M0 | |
Stage IVB | Any T | N3 | M0 |
T4b | Any N | M0 | |
Stage IVC | Any T | Any N | M1 |
Table 10.4
AJCC anatomic stage and prognostic groups for pharynx
Group | T category | N category | M category |
|---|---|---|---|
Nasopharynx | |||
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T1 | N1 | M0 |
T2 | N0 | M0 | |
T2 | N1 | M0 | |
Stage III | T1 | N2 | M0 |
T2 | N2 | M0 | |
T3 | N0 | M0 | |
T3 | N1 | M0 | |
T3 | N2 | M0 | |
Stage IVA | T4 | N0 | M0 |
T4 | N1 | M0 | |
T4 | N2 | M0 | |
Stage IVB | Any T | N3 | M0 |
Stage IVC | Any T | Any N | M1 |
Oropharynx, hypopharynx | |||
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage III | T3 | N0 | M0 |
T1 | N1 | M0 | |
T2 | N1 | M0 | |
T3 | N1 | M0 | |
Stage IVA | T4a | N0 | M0 |
T4a | N1 | M0 | |
T1 | N2 | M0 | |
T2 | N2 | M0 | |
T3 | N2 | M0 | |
T4a | N2 | M0 | |
Stage IVB | T4b | Any N | M0 |
Any T | N3 | M0 | |
Stage IVC | Any T | Any N | M1 |
Table 10.5
AJCC anatomic stage and prognostic groups for larynx
Group | T category | N category | M category |
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage III | T3 | N0 | M0 |
T1 | N1 | M0 | |
T2 | N1 | M0 | |
T3 | N1 | M0 | |
Stage IVA | T4a | N0 | M0 |
T4a | N1 | M0 | |
T1 | N2 | M0 | |
T2 | N2 | M0 | |
T3 | N2 | M0 | |
T4a | N2 | M0 | |
Stage IVB | T4b | Any N | M0 |
Any T | N3 | M0 | |
Stage IVC | Any T | Any N | M1 |
Table 10.6
AJCC anatomic stage and prognostic groups for nasal cavity and paranasal sinuses
Group | T category | N category | M category |
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage III | T3 | N0 | M0 |
T1 | N1 | M0 | |
T2 | N1 | M0 | |
T3 | N1 | M0 | |
Stage IVA | T4a | N0 | M0 |
T4a | N1 | M0 | |
T1 | N2 | M0 | |
T2 | N2 | M0 | |
T3 | N2 | M0 | |
T4a | N2 | M0 | |
Stage IVB | T4b | Any N | M0 |
Any T | N3 | M0 | |
Stage IVC | Any T | Any N | M1 |
Head and neck cancers tend to spread locally with invasion of adjacent structures. For instance, many cancers of the oral tongue tend to extend into the floor of the mouth. Local staging of HNSCC is routinely done by either CT or MRI, not PET. However, occasionally [18F]FDG PET may visualize small submucosal primary tumors that are difficult to distinguish from adjacent normal tissues with anatomic imaging studies. In a study of 48 patients with HNSCC (mostly tumors of the oral cavity), [18F]FDG PET detected 35/40 primaries known or still present at that time (88%), whereas contrast-enhanced CT of the neck identified a primary tumor only in 18 of the 35 patients (51%) with known or present primary tumors. Of the 17 primaries not detected by CT, 11 were clearly visualized by [18F]FDG PET [46]. PET/CT readers should pay attention to secondary tumor signs. For instance, cancers at the anterior floor of the mouth can cause obstruction of Wharton’s duct, through which the submandibular gland empties into the oral cavity. Ductal dilatation and enlargement of the gland, sometimes associated with increased [18F]FDG uptake, may be noted. Perineural spread can occur in various locations in the head and neck but is particularly common with adenoid cystic carcinoma (submucosal cancers that are common in the hard palate). Therefore, PET/CT readers should also be familiar with the normal branching patterns of cranial nerves relevant for innervation of the head and neck, in particular the facial nerve and trigeminal branches V/2 and V/3.
Lymph Node Staging
The risk of lymph node metastases is related to the anatomic location of the primary tumor. Tumors originating in sites that are rich in lymphatics, such as the tongue and floor of mouth, metastasize earlier and more often than those in other locations, including the hard palate or upper gum. The presence of nodal metastases is an independent prognostic factor for survival in patients with head and neck cancer; it decreases the overall survival by approximately one-half [47]. These historical data were corroborated by a recent retrospective study of 3,800 patients, in which the presence of neck node metastases lowered the 5-year disease-specific survival rate by approximately 30% [48]. The prognosis worsens additionally with the number of lymph nodes involved [49], with extracapsular spread of nodal disease [50], and with metastases located in the lower neck [51, 52]. The presence and extent of nodal metastases may affect the surgical or radiotherapy approach. Early and complete removal of neck node metastases is a prerequisite for cure. Accurate nodal staging of the neck is therefore particularly important.
The clinical neck exam and anatomic imaging studies suffer from a lack of sensitivity and specificity in assessing the extent of nodal disease [26, 53]. Numerous studies have compared the accuracy of anatomic imaging modalities and [18F]FDG PET for the detection of nodal metastases in the neck [46, 54–59] (Fig. 10.6). In most studies PET had a higher sensitivity and/or specificity than CT, MRI, or ultrasound. In 124 patients with oral cavity cancer, the authors compared [18F]FDG PET and neck CT or MRI with surgical histopathology of each dissected neck level; metastatic nodes were found in 95 of 493 dissected neck levels. Overall, and for each neck level, [18F]FDG PET showed a higher accuracy than CT or MRI. The overall sensitivity, specificity, and accuracy for PET versus CT/MR were 75% versus 53%, 93% versus 94%, and 89% versus 86% [59]. However, these numbers may be somewhat optimistic, because the same authors later published data in 471 patients with oral cavity cancer and found a suboptimal sensitivity and specificity of 73 and 58% on a per-patient basis [60]. In a meta-analysis of 35 studies comparing the diagnostic performance of [18F]FDG PET or PET/CT with that of neck CT, MRI, or ultrasound-guided fine needle aspiration (USFNA), [18F]FDG PET had a combined sensitivity and specificity in the range of 73–82% and 86–89%, as compared to 74-76% for CT and 78-80% for MRI. For unclear reasons, four studies comparing PET and USFNA showed a surprisingly low sensitivity of ∼45% for both modalities (probably due to methodological issues or selection bias); as expected, USFNA had a near perfect 96% specificity because it is based on cytology of aspirates [61].
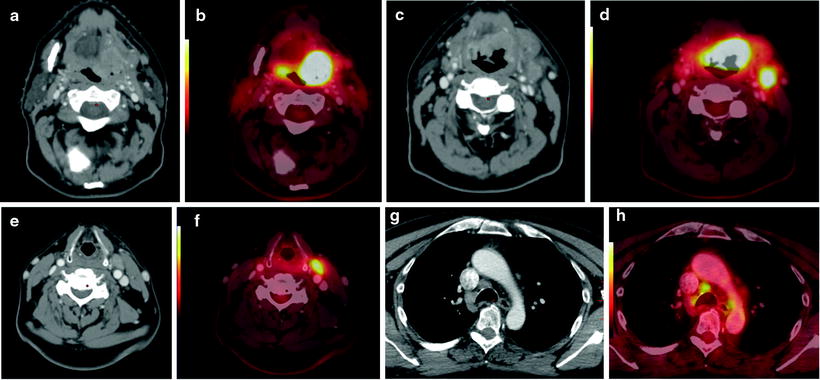

Fig. 10.6
Staging studies in a 57-year-old man with base of tongue cancer. Contrast-enhanced CT of the neck shows a large enhancing mass at the left base of tongue (a), which is intensely hypermetabolic on PET/CT fusion image (b). Intense [18F]FDG uptake in lymph node metastases in the left neck level I (c, d) and level III (e, f). In addition, the staging scan showed enlarged hypermetabolic nodes in the mediastinum (g, h). Because of a potential change in management, these mediastinal nodes were biopsied: histopathology showed granulomatous disease (i.e., false-positive [18F]FDG uptake)
It is important to understand that sensitivity and specificity of [18F]FDG PET were probably overestimated in earlier studies, when the technique had just been introduced. This is common phenomenon applicable to any new imaging technique. In addition, CT and MRI techniques have vastly improved over the past 10 years. Readers are encouraged to read the methods section of published studies carefully; the most rigorous assessment would require analysis for each neck level (rather than analysis per patient or per neck), comparison with surgical histopathology of neck dissection, and at least 6–12 months of follow-up for nondissected neck sites. Nevertheless, PET probably provides some (difficult to quantify) added value in the initial nodal staging of HNSCC.
False-positive [18F]FDG uptake can occur in inflamed, reactive lymph nodes, a common phenomenon in the “classical” (HPV-negative) patient with HNSCC, secondary to poor oral and dental hygiene. Other reasons for false-positive [18F]FDG uptake include granulomatous disease (Fig. 10.6) and uptake in the inflamed wall of branchial cleft cysts. The latter may be difficult to distinguish from cystic/necrotic lymph nodes, in particular since these nodal metastases often originate from small, indolent, and clinically occult primary tumors in the oropharynx [62]. Although various SUV cutoff have been proposed, in clinical reality no single SUV number can reliably distinguish between metastatic and benign lymph nodes. Reasons for false-negative [18F]FDG PET studies may include a small tumor burden in metastatic nodes, cystic degeneration of metastatic nodes only surrounded by a small rim of viable tumor tissue, low tracer uptake in the metastatic node, and imaging artifacts. Nodal metastases in close proximity to the primary tumor may not be detectable as separate hypermetabolic focus when the primary show very intense tracer uptake.
Detection of Distant Metastases and Synchronous Second Primary Malignancies
Distant metastases are rare in patients with head and neck cancers, but the frequency increases with higher T stage and size and number of tumor-involved lymph nodes and with jugular vein invasion. The risk for distant disease also increases with nodal metastases in the lower neck or supraclavicular region, in primary tumors in the hypo- and oropharynx, and in the setting of locoregional recurrence. The most common location is the lung, followed by bone and liver (with an estimated frequency of 66, 20, and 10%; [63]). In contrast to most squamous cell cancers, adenoid cystic and neuroendocrine carcinomas tend to metastasize early and widely, even in the absence of advanced local disease. Although the lung is the most common site of distant disease, a chest CT is insufficient for comprehensive staging if the probability for distant disease is reasonably high, because potential metastases at other sites, including bone and liver, remain undetected [64]. [18F]FDG PET/CT clearly improves the detection rates.
Patients with HNSCC have a higher propensity for synchronous (within 6 months of primary diagnosis) or metachronous (>6 month after primary diagnosis) second primaries as compared to many other malignant tumors. This is likely related to synergistic effects from exposure to carcinogens in tobacco and alcohol. In a large retrospective study of 1,100 patients, synchronous second primaries were noted in 7% of patients and metachronous second primaries in another 9% of patients (at a median of 3.5 years after detection of the first head and neck cancer) [65]. However, in other analyses the reported rates for second primary malignancies in patients with HNSCC vary widely, from 7 to 30%, probably related to selection bias [66–70]. Rather than recording the rate of second primaries, it may be more informative to record a standardized incidence ratio (the ratio between the number of observed to expected primary cancers in patients with prior HNSCC, using a cohort of similar age, sex, and ethnicity as standard of reference) [71]. In a recent analysis of the SEER database [71], the highest standardized incidence ratio was found for primary HNSCC in the hypopharynx and the lowest for primaries in the larynx. Patients with oral cavity or oropharynx index cancer most commonly develop a second primary at other subsites of the head and neck. Interestingly, the rate of second primaries in patients with index tumor in the oropharynx has declined over the past decade, presumably related to the increasing number of HPV-positive tumors (which carry a better prognosis in general and apparently also lower risk for a second primary). Indeed, in a study of 746 patients with oropharynx primary [6], the rate of second primaries at 3 years was 8.9% in total, but only 5.9% in patients with HPV-positive tumors as compared to 14.6% in patients with HPV-negative tumors. Likewise, the 3 year rate of distant metastasis was 10% overall, and was 8.7% in HPV-positive as compared to 14.6% in HPV-negative tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree