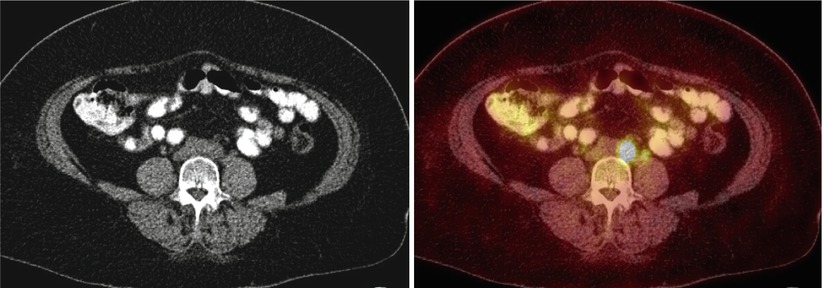
Fig. 1
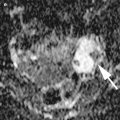
A 83-year-old woman with newly diagnosed left ovarian cancer (SUV max 8.7)
Technique for PET/CT
Proper patient preparation and scanning protocol are needed for optimal diagnostic accuracy. Considerations that are unique to FDG-PET/CT systems include bowel preparation techniques, reduction of FDG activity in the urinary bladder, and reconstruction techniques to minimize metallic artifacts.
Bowel definition can be enhanced by using low-density barium for oral contrast material at CT, which does not cause significant artifacts at FDG-PET [11]. Oral hydration is recommended as long as the fluids do not contain glucose.
It is also important to reduce FDG accumulation within the urinary bladder. The patient is asked to void completely immediately prior to the start of scanning and by imaging the pelvis early in the study.
Metallic prostheses can cause foci of apparent increased FDG uptake due to attenuation correction [12]. This artifact can be reduced by minimizing patient motion. When CT is used for attenuation correction, an attenuation-weighted iterative reconstruction technique can also be used to minimize this artifact, although more studies are needed to optimize reconstruction factors such as segmentation, number of iterative steps, and preprocessing of attenuation date [12]. At our institution, an ordered subset expectation maximum iterative reconstruction algorithm with three iterations and 21 subsets is used. Examination of non-attenuation-corrected images is also very helpful in avoiding this pitfall.
Imaging technique at our institution for FDG-PET/CT is described in the following paragraphs and summarized in Table 1.
Table 1
Technique used at the author’s institution for FDG-PET/CT
Patient preparation |
Make sure the patient has ingested no food or drink for at least 4 h prior to scanning |
Check blood glucose level prior to radiotracer injection |
One hour prior to scanning, inject a weight-based dose of FDG (0.22 mCi/kg [8.1 MBq/kg]) and administer 450 mL of low-density barium orally for the patient to drink as a glucose-free solution |
Place the patient in a quiet, dimly lit room with instructions not to talk or move |
Immediately prior to scanning, have the patient void completely |
Place the patient head first into the gantry with both arms above the head |
CT scanning |
Scanner: 64-section multidetectora |
Scan coverage: skull base to midthigh |
Detector row configuration: 2 × 2 mm |
kVp = 120 |
Weight-adjusted milliampere-second mean = 200 mAs |
Gantry rotation speed: 0.5 s per revolution |
Pitch: 1.75 |
Image reconstruction: 512 × 512 matrix, 70-cm field of view (FOV) |
PET scanning |
Scanner: 64-section multidetectora |
Scan coverage: skull base to midthigh in a caudal-to-cranial direction |
Coverage per table position: 17.0 cm |
Number of table positions: 5–7 |
Acquisition time: 3 min per table position |
Section thickness: 2 mm |
Image reconstruction: 168 × 168 matrix, ordered subset expectation maximum iterative reconstruction algorithm (three iterations, 21 subsets), 5-mm Gaussian filter, 70-cm FOV |
Patient Preparation
The patient is asked to take nothing by mouth for at least 4 h prior to examination. Upon the patient’s arrival at the radiology department, his or her weight and height are obtained and a weight-based (0.22 mCi/kg [8.1 MBq/kg]) dose of FDG is prepared. A 22- or 24-gauge intravenous line is placed, usually in the upper extremity contralateral to any prior surgery (e.g., lymph node resection) to ensure proper distribution of radiotracer. The patient’s blood glucose is determined to ensure that he or she is not hyperglycemic. At our institution, a serum glucose level of 200 mg/dL is used as a maximum cutoff point. One hour prior to imaging, the FDG dose is injected and 450 mL of low-density barium (Read-Cat 2.1 % weight/volume barium sulfate suspension; E-Z EM, Lake Success, NY) is administered orally for the patient to drink as a glucose-free solution. The patient is then placed in a quiet, dimly lit room for the remainder of the uptake phase. To minimize skeletal muscle uptake during this period, the patient is instructed not to talk and to keep the arms at the sides and the legs uncrossed. Just prior to the start of scanning, the patient is asked to void completely to minimize bladder activity.
Scanning Protocol
For scanning, the patient is positioned supine and head first on a PET/CT system with a single gantry and table. The arms are raised above the head (if the patient can tolerate this position), and he or she is allowed to breathe quietly. CT is performed first. At our institution, a 64-section multidetector Siemens Biography PET/CT system is used with the following parameters: detector row configuration of 2 × 2 mm, 120 kVp, weight-adjusted milliampere-second mean 200 mAs, gantry rotation speed of 0.5 s per revolution, and pitch of 1.75. A tomogram is used to adjust the FOV so that it extends from the skull base to the midthigh. The images are reconstructed on a 512 × 512 matrix with a 70-cm FOV.
After CT is completed, the table is moved into the PET scanner. The images are acquired in a caudal-to-cranial direction from the midthigh to the skull base to minimize bladder filling. Three-minute emission acquisitions per FOV are obtained in three-dimensional mode. Transverse PET scans are obtained per FOV, with a section thickness of 2 mm. Five to seven table positions are usually needed, with each position allowing coverage of 17.0 cm. The PET scans are reconstructed on 168 × 168 matrix, with an ordered subset expectation maximum iterative reconstruction algorithm (three iterations, 21 subsets), a 5-mm Gaussian filter, and a 70-cm FOV.
The CT transmission map is used for attenuation correction. Attenuation correction accounts for differences in activity due to location within the body (i.e., photons from deep sites are attenuated to a greater degree than photons from superficial sites). Attenuation-corrected and uncorrected PET scans, along with the CT and PET/CT scans, are reviewed on a workstation.
Normal FDG Activity
FDG, an analog of glucose, is distributed via the bloodstream after being glycolytically active tissues. Imaging is typically started at least 60 min after injection to allow sufficient blood pool clearance, thereby improving the target-to-background ratio. Normal physiologic uptake is seen in the brain and myocardium and, to a lesser extent, in the liver, spleen, bone marrow, gastrointestinal tract, testes, and skeletal muscles. Myocardial uptake is variable in fasting patients but often intense in nonfasting individuals. Skeletal muscle uptake is dependent on recent use of the muscle group. Activity within the blood pool, particularly in the mediastinum, can also be seen [13]. Other less frequent sites of uptake include the endometrium, breast, major and minor salivary glands, and brown fat in the supraclavicular and paraspinal regions [13–16]. Because FDG is excreted by the kidneys, intense activity is normally seen in the renal collecting system, ureters, and bladder [13] (Fig. 2).
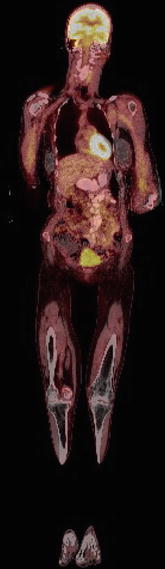

Fig. 2
Normal FDG physiologic activity
Increased FDG uptake can also be seen in many benign processes. Increased uptake has been reported in healing fractures, granulomatous diseases, inflammatory/infectious processes, and foci of wound healing and repair such as ostomy sites [13] (Figs. 3, 4, and 5).
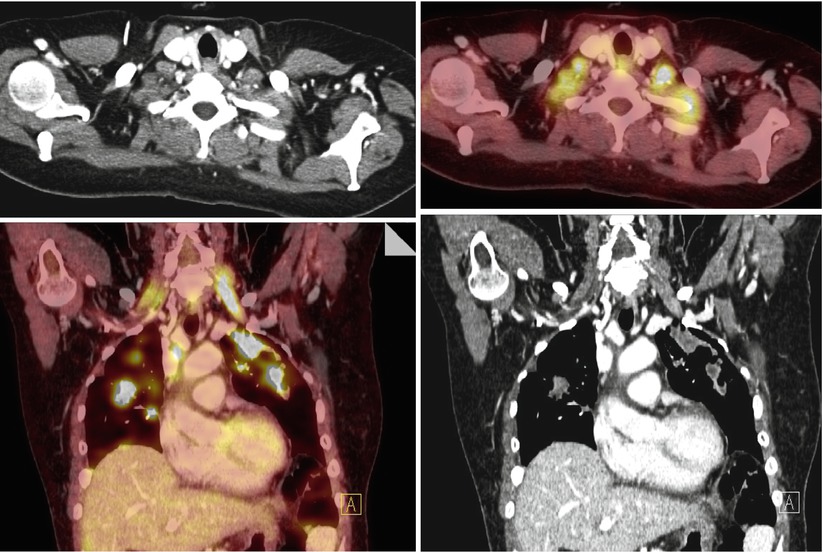
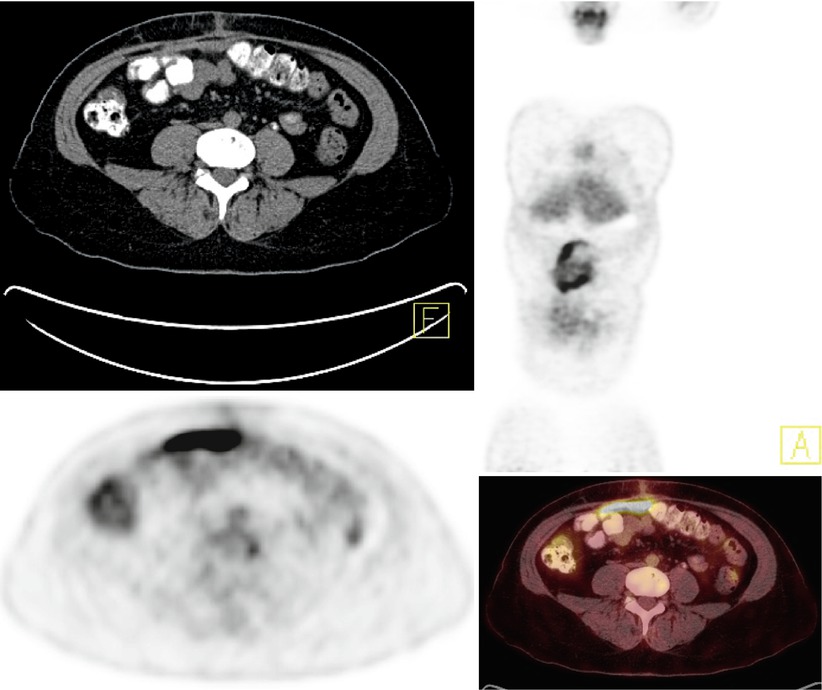
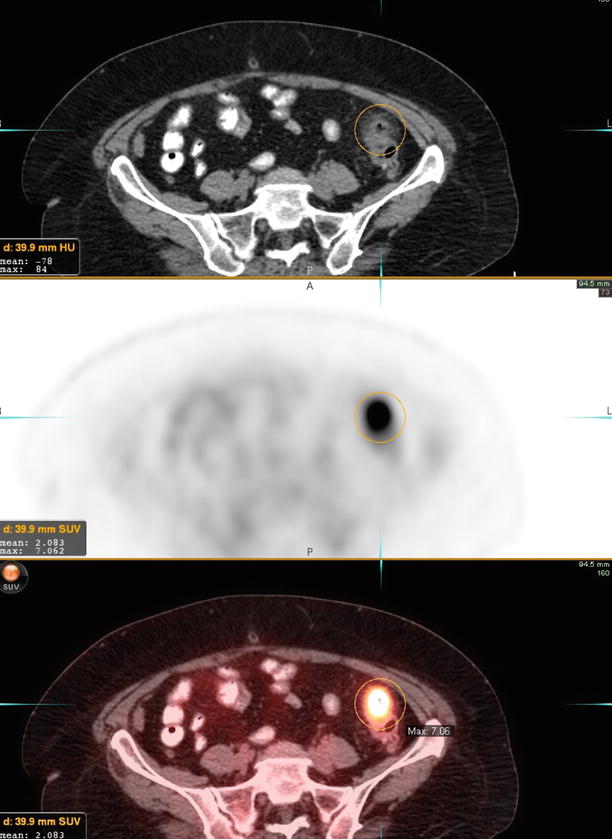

Fig. 3
A 47-year-old woman with history of ovarian cancer. Restaging PET/CT shows extensive hypermetabolic pulmonary metastases. Diffuse physiologic muscular activity is also noted in bilateral scalene muscles

Fig. 4
A 54-year-old woman with history of ovarian cancer. Restaging PET/CT shows new moderately intense activity in the anterior abdomen (SUV max up to 5.7), corresponding to new ventral hernia mesh-related inflammation. This finding could mimic peritoneal metastasis

Fig. 5
A 58-year-old woman with history of ovarian cancer. Restaging PET/CT images demonstrate intensely active wall thickening of the distal descending colon (SUV max 7.1) with perifat stranding, consistent with diverticulitis
Imaging Features
Physiologic Versus Pathologic Uptake
Ovarian cancer generally appears as a focal FDG-positive area but many other benign lesions or physiologic conditions may have a similar appearance: benign cystadenomas, teratomas, schwannomas, endometriosis, inflammatory processes, normal ovaries in menstruating women (follicular cysts and corpus luteal cysts between days 10 and 25 of the menstrual cycle), as well as normal physiologic activity in bowel, bladder diverticula, pelvic kidney, and focal retained activity in ureters [17]. In this setting, the anatomic correlation provided by the CT images help establish the correct diagnosis.
In premenopausal women, the amount of FDG uptake in the ovaries varies with the phase of menstrual cycle, with physiologic uptake particularly present in the early luteal phase (Fig. 6 – corpus luteal cyst). This can be avoided by performing the scan just subsequent to menstruation. Moreover, the typical appearance of physiologic uptake in the spherical or discoid with smooth margins and is located above the urinary balder or around the uterus, which can be used to distinguish it from pathologic changes. Characteristic CT appearance of normal benign ovarian tissue include the observance of a small rim enhancing cyst, the absence of any lymph nodes, and concordance with the appropriate stage of menstrual cycle are also helpful in recognizing the benign nature of the uptake [18]. In postmenopausal ovaries, however, any ovarian uptake is pathologic, whether benign or malignant (Figs. 7 and 8).
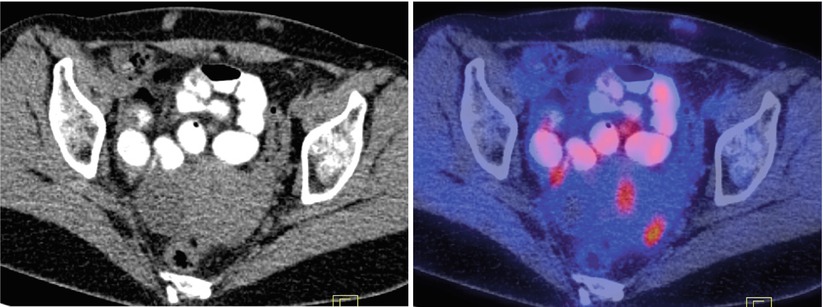
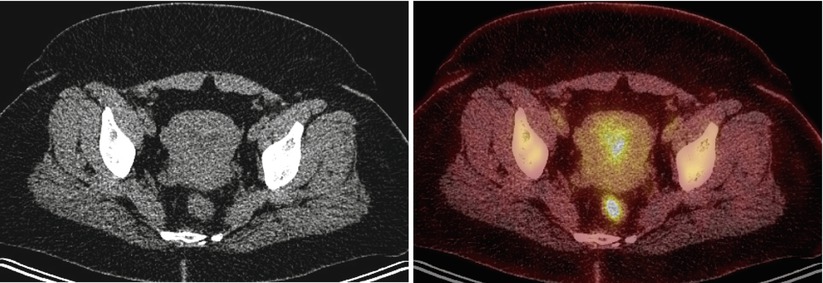
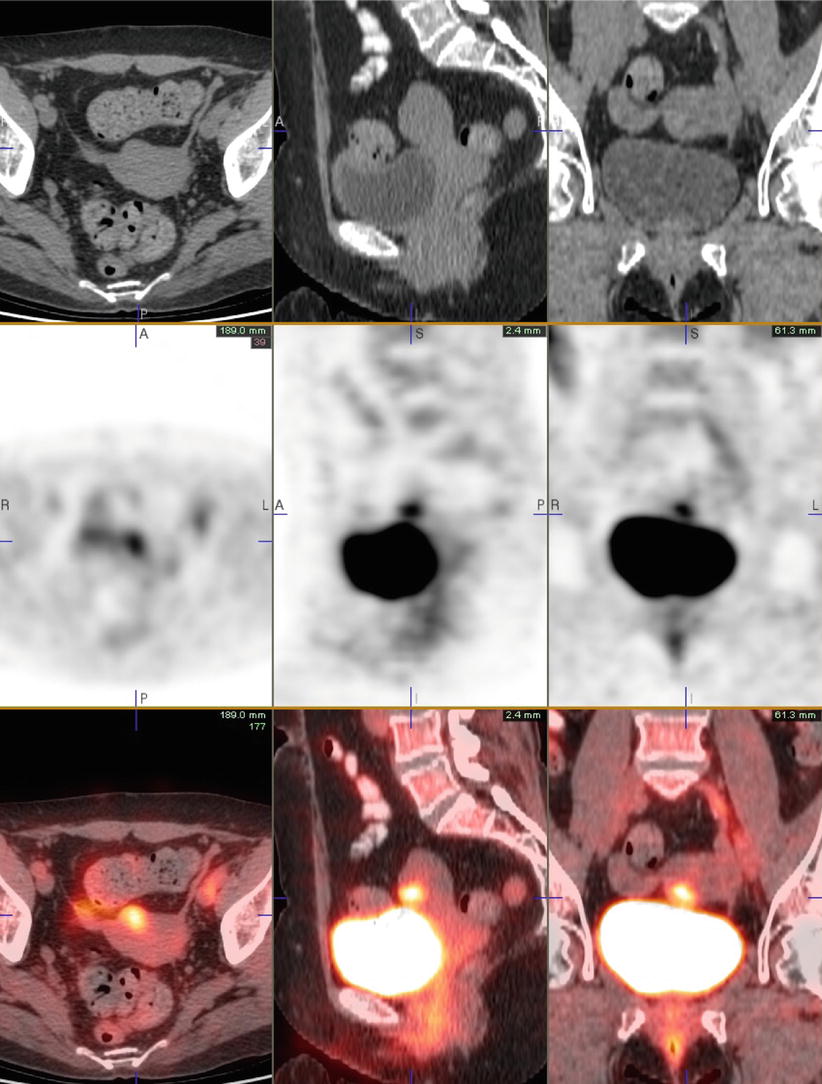

Fig. 6
A 42-year-old premenopausal woman with breast cancer. Axial images show presence of physiologic PET activity in the left ovary, corresponding to corpus luteal cyst on concurrent CT. A few small, mildly active subcutaneous densities in the abdominal wall represent sites of Lovenox injection

Fig. 7
A 37-year-old premenopausal woman with history of lymphoma. Endometrial activity consistent with menstrual-cycle-related changes

Fig. 8
A 41-year-old woman with history of lymphoma, currently in remission. Restaging PET/CT shows moderately active exophytic fibroid in the right anterior aspect of the uterus which appears less intense on the subsequent follow-up study. No evidence of malignancy is present
Benign Versus Malignant Lesions
Studies evaluating the role of PEt alone for early diagnosis of ovarian cancer have reported a low sensitivity (58 %) and specificity (76 %) [19]. The advent of combined structural and metabolic data acquired contiguously with PET/CT helps to precisely localize suspicious areas of increased FDG uptake and assess incidental ovarian findings. Ovarian lesions will show variable increase in FDG uptake on PET/CT. Castellucci et al. demonstrated a sensitivity of 87 % and specificity of 100 % for differentiating benign from malignant ovarian cancer [20]. These results were achieved in part by characterizing a focal increased standardized uptake value (SUV) of 3 or higher in the ovary as positive for ovarian malignancy, whereas an SUV of 2.7 or lower was considered benign. Benign and malignant ovarian FDG uptake on PET were also differentiated using concurrently acquired CT, the pattern of FDG uptake and absence of lymphadenopathy in patients with benign lesions.
In another study, PET/CT was found to have a sensitivity of 80 % and specificity of 97 % for diagnosis of synchronous malignancy in the contralateral ovary in patients with primary ovarian cancer [21]. Although PET/CT has a high specificity for lesions greater than 5 mm in size, its sensitivity is much lower than that of sonography for the assessment of small lesions (<5 mm). Therefore, PET/CT has not replaced sonography for the screening of ovarian cancer. However, given its high specificity, FDG-PET/CT is used to assess for occult metastases before the patients undergo surgical treatment.
A study performed by Karantanis et al. showed that FDG-PET/CT is unable to distinguish tumor grade and histologic type among epithelial ovarian carcinoma. There is no statistically significant relationship between SUVmax value and tumor grading or between SUVmax value and histologic type (particularly for serous endometrioid subtypes). However, there is a significant correlation between FDG uptake, SUV mean, tumor grade, clinical stage, cell proliferation index, and GLUT-1 expression in epithelial ovarian cancer [22].
Some pathologic conditions may be missed due to the lack of FDG uptake as in borderline ovarian tumors and mucinous adenocarcinoma [23]. A definite diagnosis of a pelvic mass requires a surgical specimen. FDG-PET/CT is a useful noninvasive tool to characterize adnexal lesions in case of inconclusive transvaginal ultrasound in the presurgical phase [20, 23, 24]. In addition, FDG-PET/CT also has a great value in staging.
FDG-PET for Primary Diagnosis of Ovarian Cancer
Currently, the initial investigations of ovarian masses include physical examination, biochemical analysis of tumor markers such as CA-125, and transvaginal ultrasound (TVUS). TVUS is the preferred imaging modality for initial evaluation because of its availability, high resolution, and lack of ionizing radiation. Using ultrasound, a wide range of sensitivities (87–100 %) and specificities (57–90 %) have been reported for detection of ovarian malignancies [19, 20, 25–27]. A systemic review revealed a sensitivity of 87 % and a specificity of 90 % for ultrasound combined with color Doppler in the diagnosis of ovarian cancer [28]. Magnetic resonance imaging (MRI) is often used for further characterization of indeterminate adnexal masses, with a sensitivity and specificity of 100 and 94 %, respectively [29]. Although MRI can be helpful in cancer detection, the major contribution of MRI in adnexal mass evaluation is its ability to provide a confident diagnosis in many common benign adnexal abnormalities [30].
The role of FDG-PET in the detection of primary ovarian cancer has been evaluated for almost two decades. Ovarian cancer is typically characterized by increased glucose metabolism and therefore demonstrates increased FDG uptake, whereas benign tumors are usually negative on PET imaging. Early studies were predominantly undertaken with 1st-generation whole-body PET scanners, which had a lower sensitivity and spatial resolution compared to the latest PET/CT technology. Nevertheless, results are still comparable to more recent reports using PET/CT. In 2000 and 2002, two studies assessing women with asymptomatic adnexal masses found FDG-PET to have a sensitivity of 58 % and specificity of 76–80 % [19, 25]. Similarly, two further studies evaluated FDG-PET for characterization of a suspicious pelvic mass and found sensitivities and specificities to range from 58 to 78 % and 78 to 87 %, respectively [26, 31]. Despite the relatively low sensitivity of FDG-PET in the detection of primary ovarian cancer, all of the false negative results were either small invasive stage I tumors or lesions of low malignant potential.
Data with fusion of anatomic and functional imaging using PET/CT resulted in more precise localization of suspicious areas of increased FDG uptake and enabled a better assessment of incidental ovarian findings. In 97 patients, FDG-PET/CT demonstrated areas of abnormally increased metabolic activity in 60 patients, whereas lesions were considered benign in 37 patients [32]. The sensitivity and specificity for FDG-PET/CT were 100 % (57/57) and 92.5 % (37/40), respectively. This was higher than all other imaging modalities including US, CT, MRI, and FDG-PEt alone. The group from Bologna found in 50 consecutive patients a sensitivity, specificity, and accuracy of 87, 100, and 92 %, respectively, compared with 90, 61, and 89 %, respectively, for TVUS. The authors concluded that FDG-PET/CT provides additional information to TVUS for differentiating benign from malignant pelvic lesions as well as to CT for the staging of ovarian cancer patients. These findings were further supported by a prospective study of 133 women with suspected ovarian cancer [24]. Histopathology showed benign tumors in 25 patients, borderline tumors in 13 patients, and malignant tumors in 95 patients. The accuracy of FDG-PET/CT (0.92) was higher compared to pelvic ultrasound (0.83) and contrast-enhanced CT or pelvic MRI (0.75; p = 0.013).
FDG-PET and FDG-PET/CT, however, do have certain limitations, which also need to be considered. FDG-PET is often not able to detect small tumor deposits (less than 5 mm in size) [20]. Ovarian cancer shows variable levels of increased FDG uptake depending on the cellular composition. Abdominal or pelvic masses containing large cystic components as well as mucinous tumors will often not be metabolically active. False negative results are also seen in borderline ovarian tumor and cystadenocarcinoma of the ovary [19, 31, 33]. In particular, differentiating benign from borderline ovarian tumors is often difficult on FDG-PET. Even though borderline ovarian tumors demonstrate low-grade malignancy, they can be associated with aggressive biologic behavior in the form of peritoneal deposits and lymphadenopathy. However, borderline ovarian tumors are often non-FDG avid or demonstrate low-grade FDG uptake due to their mucinous and serous cellular composition [32].
Although generally false-positive findings in the abdomen and pelvis are rare, early studies showed that in patients with pelvic masses a number of benign conditions can cause increased FDG uptake. These include benign cystadenomas, dermoid tumors, teratomas, schwannomas, endometriosis, and inflammatory process [19, 31, 33] (Fig. 9 – bilateral salpingitis). Increased FDG uptake in normal ovaries is found in premenopausal women; this is related to the time point of ovulation. Two studies reported increased FDG uptake in the ovary and uterine endometrium in the late follicular and early luteal phases of the menstrual cycle [34, 35].
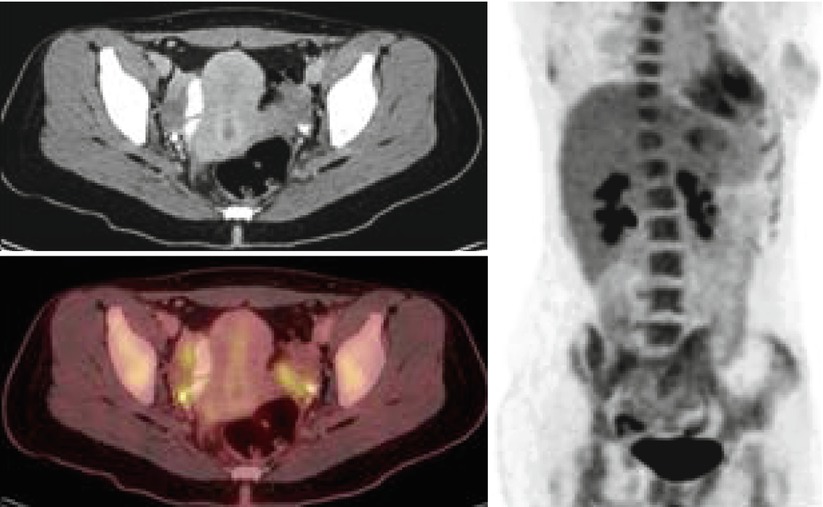

Fig. 9
Bilateral tubo-ovarian activity in a patient with history of breast cancer and chronic salpingitis
In order to reduce errors in interpretation, specific emphasis needs to be directed towards a good technique when undertaking pelvic PET/CT imaging. Ideally, the bladder should be empty to avoid artifacts on PET images from a high concentration of radioactivity in the bladder. It is suggested that the patient is scanned in a caudal-to-cranial direction, thus imaging the pelvis at the beginning of the study. The CT portion of PET/CT is often helpful to identify bladder diverticula and focal retained activity in ureters. Pelvic imaging can be improved by intravenous injection of furosemide (20–40 mg) to reduce tracer retention in the urinary system. The CT portion of PET/CT is also helpful in identifying normal physiologic activity in bowel, endometrium, and blood vessels. Bowel preparation can be performed with oral hydration as well and some groups recommend the use of oral contrast [36]. The administration of butylscopolamine (20–40 mg) at the time FDG injection has been reported to reduce FDG uptake in the bowel [37].
FDG-PET for Initial Staging of Ovarian Cancer
The most important prognostic factor for ovarian cancer patients is the tumor stage: the 5-year survival rate is 93, 70, 37, and 25 % for stage I, stage II, stage III, and stage IV, respectively [38]. Ovarian cancer is typically staged by exploratory laparotomy at the time of primary debulking in accordance with the recommendations of the International Federation of Gynecology and Obstetrics (FIGO) [39]. Debulking surgery followed by adjuvant chemotherapy is considered the standard approach in newly diagnosed ovarian cancer. Studies have shown that when using platinum-based chemotherapy in patients with advanced ovarian disease, maximal cytoreduction, correlated to the minimal residual tumor mass after surgery, is one of the most powerful determinants of survival [40].
For preoperative staging of ovarian cancer, CT and MRI have been accepted as being the most useful imaging techniques. Studies evaluating performance of CT in preoperative staging of ovarian cancer have found sensitivities and specificities ranging from 72 to 82 % and 53 to 81 %, respectively [41, 42]. Recent studies have shown that, when used in conjunction with CT, FDG-PET is beneficial in evaluating distant metastases and equivocal lesions [20, 21, 42].
An initial study of 15 patients found that the addition of FDG-PET to CT improved the accuracy in preoperative staging of ovarian cancer from 53 to 87 % [42]. In a later study, 40 ovarian cancer patients underwent FDG-PET/CT with intravenous contrast-enhanced CT for staging prior to primary debulking surgery [21]. An increase in sensitivity, specificity, and accuracy was found for combined FDG-PET/CT compared to CT alone from 37.6 to 69.4 %, 97.1 to 97.5 %, and 89.7 to 94.0 %, respectively. FDG-PET/CT findings and the final pathological staging were in concordance in 75 % of cases.
More recent data has shown that using FDG-PET/CT can result in a stage migration in a significant number of patients. Out of 66 ovarian cancer patients, 64 were initially stage III and 2 were stage IV [43]. However, after PET/CT, 51 % (39/66) were restaged as stage III and 41 % (27/66) as stage IV (Fig. 10).


Fig. 10
A 85-year-old woman with newly diagnosed ovarian serous carcinoma. The right adnexal cystic lesion measures approximately 8.2 cm AP × 7.9 cm transverse × 7.8 cm craniocaudal, with intensely active soft tissue component in the superoposterior wall (maximum SUV of 5.9), consistent with primary tumor
PET/CT Findings of Ovarian Cancer Metastases
Lymph Node Metastases
The presence of metastatic lymph nodes is another important prognostic factor for patients with ovarian cancer. Recognizing the precise location of metastatic nodes is therefore crucial for selecting appropriate treatment and predicting the possibility of optimal resection. A recent meta-analysis compared the diagnostic performances of CT, MRI, and PET or PET/CT for detection of metastatic lymph nodes in patients with ovarian cancer [44]. Eighteen eligible studies were included, with a total of 882 patients. FDG-PET or PET/CT was more accurate with a sensitivity of 73.2 % and a specificity of 96.7 % than CT (sensitivity, 42.6 %; specificity, 95.0 %) or MRI (sensitivity, 54.7 %; specificity, 88.3 %). When interpreting results from imaging studies the reference standard used is important. Generally, FDG-PET or PET/CT excels compared to other imaging modalities; however, when findings are compared to histopathology, the sensitivity tends to be lower. This is often related to the inability of FDG-PET to detect small and microscopic tumor deposits, which applies to all noninvasive imaging techniques.
Lymphatic dissemination to the pelvic and para-aortic lymph nodes is common, particularly in patients with advanced disease (Fig. 11 – retroperitoneal and mesenteric nodal metastases). Spread of disease through the lymphatic channels of the retroperitoneal lymph nodes and diaphragm may lead to dissemination into the supraclavicular or superior mediastinal lymph nodes and pleural space, or rarely, the internal mammary lymph nodes [45]. Furthermore, involvement of the rectosigmoid colon by ovarian carcinoma is associated with a high incidence of mesenteric nodal metastasis [46]. Lymph node metastasis is present in 10–20 % of patients with presumed early-stage ovarian cancer, and it is present in 40–70 % of patients with advanced-stage disease. Because PET/CT has a low negative predictive value in the pelvis and skip metastases may occur in as many as 60 % of patients, the lack of hypermetabolism in pelvic nodes does not preclude the presence of pathologic para-aortic nodes [47, 48].
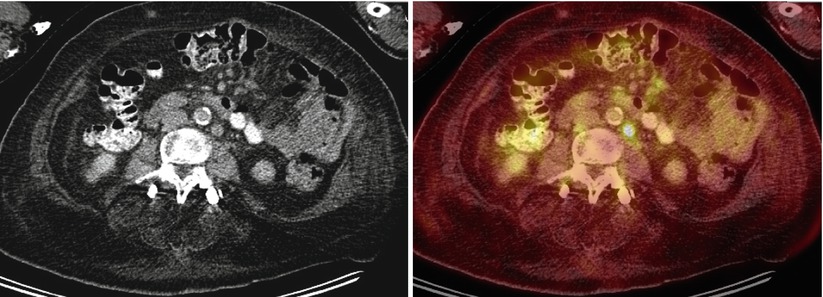

Fig. 11
A 75-year-old woman with history of ovarian cancer. Multiple normal-sized, hypermetabolic retroperitoneal and mesenteric lymph nodes are seen (SUVmax 6.8 in the left para-aortic region), consistent with metastatic disease
FDG-PET/CT accurately depicts metastatic disease on the basis of significantly increased metabolic activity, even in normal-sized nodes (Fig. 11 – active normal-sized nodes). However, the evaluation for detection of small or necrotic lymph nodes or early nodal involvement with PET/CT is limited, with high false-negative rates [49].
In a study of 30 women with advanced-stage (IIC–IV) epithelial ovarian cancer, pretreatment FDG-PET/CT was performed and detected supradiaphragmatic lymph node metastases (LNM) in one or more locations in 67 % (20/30) of patients, whereas conventional CT found LNM only in 33 % (10/30) of patients. Fourteen patients had parasternal, 14 cardiophrenic, 8 other mediastinal, 6 axillary, and 1 subclavian LNM. The patients with supradiaphragmatic LNM had significantly more ascites (p < 0.01), higher CA-125 levels, and more frequent peritoneal carcinomatosis (p < 0.03) compared to patients without supradiaphragmatic LNM in preoperative FDG-PET/CT. These findings suggest that the route of epithelial ovarian cancer cells from the peritoneal cavity to the lymphatic system permeates the diaphragm mainly to the cardiophrenic and continues to the parasternal lymph nodes [50] (Fig. 12).