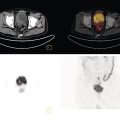
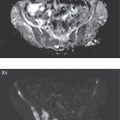
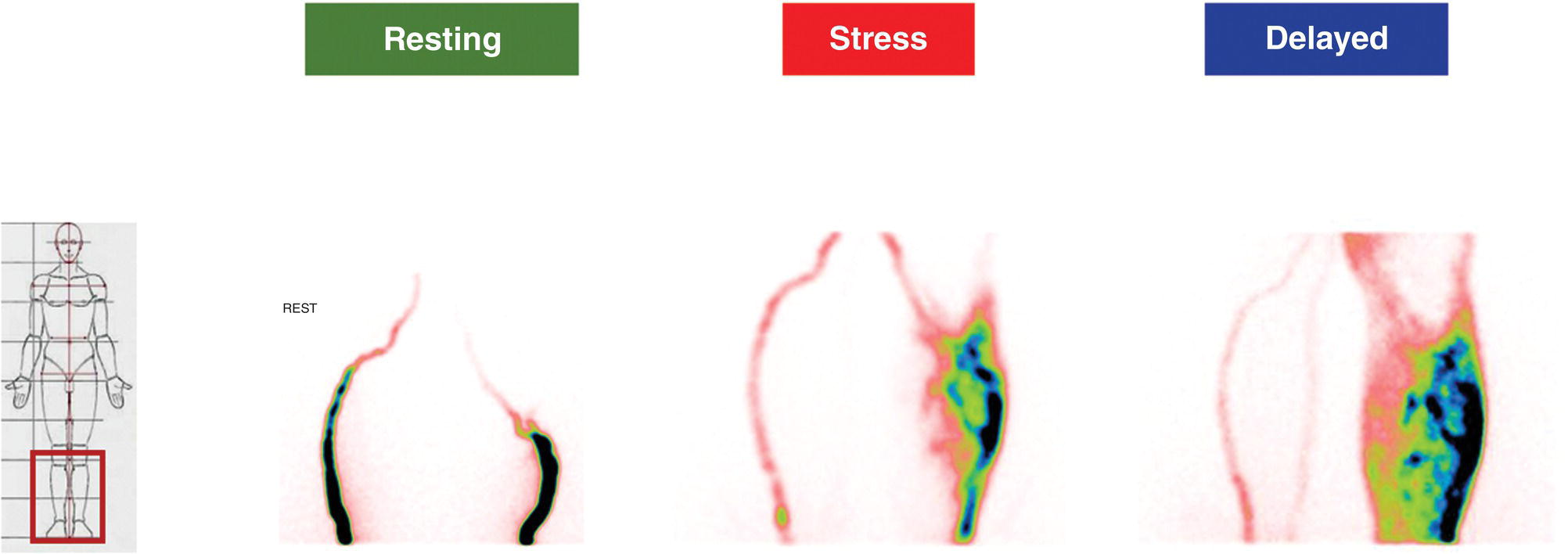
Girolamo Tartaglione1, Marco Pagan1, Francesco Pio Ieria1, Giuseppe Visconti2, and Tommaso Tartaglione3 1 Nuclear Medicine, Cristo Re Hospital, Rome, Italy 2 Plastic Surgery, Lymphedema Center, A. Gemelli Hospital, Sacro Cuore Catholic University, Rome, Italy 3 Radiology, IDI‐ IRCCS, Rome, Italy The lymphatic system is a collection of vessels and lymph nodes (LNs) that is similar yet distinct from the vascular system. It is a complex network composed of lymphatic capillaries, lymphatic vessels, LNs, and other organs (tonsils, adenoids, spleen, thymus, bone marrow) distributed throughout the body. The primary function of the lymphatic system is to maintain fluid balance in the body. It is also an absorptive apparatus of fats and a part of the body’s immune system [1]. Lymph, a clear fluid that derives from plasma filtration across the permeable capillary endothelium, is transported within lymphatic vessels. It contains interstitial fluids, fats, few proteins, unwanted materials (waste and bacteria), and lymphocytes. Lymphatic capillaries are numerous throughout the body, especially in the papillary and reticular dermis of the skin where the superficial and deep plexuses are located [2]. However, lymphatic capillaries are not present in the epidermis. Lymph is transported within lymphatic capillaries at a very low pressure, up to 2 mmHg. The function of lymphatic capillaries is to drain excessive interstitial fluids to lymphatic vessels. Superficial lymphatic vessels are found in subcutaneous tissues, where they run parallel to larger vessels, conducting lymph from the lymphatic capillaries to the large veins of the neck. Lymphatic vessels, in contrast to lymphatic capillaries, have abundant unidirectional valves, as well as thin endothelial walls and a low intraluminal pressure [3]. The lymphatic system lacks a central pump. Lymphatic flow is created by a pressure gradient and aided by unidirectional valves along the lymphatic collectors to prevent backflow. At rest, the peristaltic movement of lymphatic vessels is slow and depends on the intrinsic pumping mechanism of the lymphangion. Lymphatic vessel pressure in a heathy subject is around 25 mmHg in the legs when supine and 30 mmHg when seated []. Lymph drainage is favored by a mechanism such as the surrounding skeletal muscle pump and thoracic pump. With muscular exercise, the pressure within lymphatic vessels increases, allowing lymph to move quickly toward the great veins of the neck. Before returning to the bloodstream, lymph passes through LNs. LNs are little bean‐shaped structures and numbered in the hundreds. They can be found throughout the body, mainly concentrated in the neck, axilla, chest, abdomen, and groin. Lymphatic vessels connect LNs to one another. The lymphatic system plays a key role in the body’s immune system, helping the body to fight infection and disease. The LNs perform a “filtering” action, removing waste, cells, and pathogens from the lymph and releasing lymphocytes, before returning lymph back to the bloodstream. Lymphedema is a condition of swelling caused by lymphatic obstruction, most commonly occurring in the lower limbs. It is produced by a blockage in or damage to the lymphatic system, causing an imbalance between capillary filtration and lymphatic drainage. Moreover, lymphedema negatively affects the quality of life and self‐perception of the patient. The consequence of lymph accumulation in the tissues provokes swelling downstream to the site of obstruction. Edema indicates the presence of excess fluids in the surrounding tissues. In most cases, edema is the collection of extracellular fluids, but in extreme situations of metabolic tissue imbalances, it can involve the intracellular space. Lymphedema is classified into primary and secondary. Primary lymphedema is a hereditary condition caused by poorly developed lymphatic vessels or regional LNs. It is characteristically found in congenital dysplasia, genetic mutations, and hereditary syndromes. Primary lymphedema is relatively uncommon (30 million cases/year worldwide, 1/100 000 people under the age of 20) and can occur at birth, puberty or, more rarely, in adulthood. The specific causes of primary lymphedema include: Secondary lymphedema is far more common than primary lymphedema. In developing countries, filariasis, a parasitic infection of LNs that can limit lymph flow, is the most frequent cause of secondary lymphedema. In the industrialized world, secondary lymphedema is mainly iatrogenic. Secondary lymphedema may result from surgical cancer treatments, radiation, vascular trauma, or tumor cell invasion of LNs. Surgical dissection and radiation of LNs due to cancer are the more common causes of secondary lymphedema. Most frequently, lymphedema begins 12–36 months after a damage to lymphatic vessels. Three‐fourths of patients may develop swelling within 3 years after the injury with a 1% increase risk of lymphedema each year thereafter [5]. Regional LN radiation may increase the risk of lymphedema, particularly radiation of the supraclavicular and posterior axillary LNs. Secondary lymphedema may develop during radiotherapy or a few years after treatment. Tumor metastases may infiltrate LNs, block the lymphatic vessels, and cause lymphedema. Older age, obesity, and rheumatoid or psoriatic arthritis are risk factors that increase the risk of developing lymphedema. Lymphedema is clinically observed as swelling of an arm or leg and, in severe cases, associated with swelling of the fingers and/or foot. Subjective symptoms may include a sense of heaviness or tension, limited movement, discomfort, recurrent infections, and hardening and thickening of the skin. Lymphedema may lead to serious complications. Even the smallest injury of an arm or leg may be an entry point for infection. Possible infections include serious bacterial infections of the skin (cellulitis) and lymph vessel infection (lymphangitis). Lymphangiosarcoma is a rare form of soft tissue cancer that may arise from the most severe cases of lymphedema if left untreated. At clinical examination, lymphedema is typically characterized by a nonpitting edema (negative fovea sign) or swelling of one or more limbs. Physical examination parameters taken include the circumferential measurement of the affected limb compared to the contralateral (if healthy) in proportion to the body weight. The diagnosis of lymphatic insufficiency is confirmed with imaging methods (lymphoscintigraphy). Clinical imaging progressively advanced with the discovery of lymphography in the 1950s [6]. Conventional oil‐contrast lymphography had long been the mainstay for lymphatic imaging. Lymphography is a radiograph of lymphatic channels and LNs, obtained after the injection of a radiopaque contrast agent (ethiodol, the ethyl ester of iodized fatty acids, in 60–90 minutes) in surgically isolated small lymphatic vessels after a previous visualization with subcutaneous injections of patent blue dye (15 minutes earlier). In time, this method was largely abandoned due to complications related to the vital dye and contrast medium: pulmonary embolism, hypersensitivity, intra‐alveolar hemorrhage, and hypothyroidism. In 1953, Sherman et al. first reported that radioactive colloidal gold (198Au), a beta emitter, had a potential for lymphatic system imaging and lymph‐node identification [7]. The interstitial administration of 198Au was followed by a dose of radiation at the site of injection. In time, 198Au was replaced by radiocolloids marked with 99mTc to reduce the dosage of radiation. Lymphoscintigraphy is the gold standard for diagnosis of lymphedema but lacks procedural standardization. Several 99mTc‐based radiopharmaceutical agents have been used for lymphatic imaging worldwide. Many factors may influence the visualization of the lymphatic vessels and the radiotracer uptake by loco‐regional LNs, such as particle size, the amount and volume of injected radiotracer, and particle concentration. The choice of radiopharmaceutical is based on local availability. 99mTc‐antimony trisulfide, containing radioactive particles smaller than 20 nm and an acidic pH, is available in Australia and Canada. 99mTc‐sulfur colloid, with a maximum size of 350–5000 nm and pH of 5.5, is the preferred agent in the United States. After the preparation of the radiopharmaceutical, it should be filtered with a 100–200 nm membrane to select smaller particles. Rhenium sulfide nanocolloid, with a particle size 50–200 nm and pH of 5.5–6, is available in Europe. Because the dermis has abundant neuroreceptors sensitive to pH alterations, the radiopharmaceuticals should be injected subcutaneously to avoid pain at injection site. The radiotracer is drained by lymphatic vessels to LNs, where it is captured by macrophage phagocytosis and/or retained due to particle size. A fraction of the radiopharmaceutical moves on to second‐and third‐echelon nodes downstream. Smaller particles are drained more quickly, whereas larger particles migrate more slowly. For several years, lymphoscintigraphy has been performed using a subcutaneous injection of the radiotracer. It is a minimally invasive method, derived from the radiological experience of lymphography, used to assess lymphatic drainage. It consists of injecting a dose of 99mTc‐labeled colloids subcutaneously in the first interdigital space. Following the subcutaneous injection, the absorption of the radiocolloid is slow and requires prolonged static and total body acquisitions at 2–3 hours or more. In the lower limbs, normal superficial lymphatic drainage occurs via lymphatic vessels running within the superficial venous plexus, draining into the inguinal, iliac, and paraaortic nodes (the axillary, clavicular, and neck LNs in the upper limbs). Traditional lymphoscintigraphy offers a picture of the lymphatic system, showing how it functions by following the tracer as it moves up from the limb toward the central circulation. The tracer may identify the location where lymph is slowed or stopped to determine the extent of obstruction and diffusion of the tracer from the lymphatic vessels. When normal routes are blocked, lymphatic drainage is diverted either into the skin as dermal backflow and/or into the deep subfascial planes, resulting in popliteal (or antecubital) nodal uptake. Lymphoscintigraphy also includes the calculation of several semiquantitative parameters of lymphatic function: where K is lymphatic transport kinetics (0, no delay; 3, low‐grade delay; 5, extreme delay; 9, missing transport), D is distribution pattern (0, normal distribution; 3, partial diffuse; 5, diffuse distribution; 9, transport stop), T is time to appearance of LNs, N is assessment of LNs (0, clearly demonstrated; 3, faint visualization; 5, hardly recognizable; 9, no visualization), and V is assessment of lymphatic vessels (0, clearly demonstrated; 3, faint visualization; 5, hardly recognizable; 9, no visualization). The normal value of TI is <10 (range 0–45). TI combines a visual assessment of five criteria: temporal and spatial distribution of the radionuclide, appearance time of LNs, and graded visualization of LNs and lymphatic vessels. Unfortunately, in severe lymphedema clinical stages, the main limitations of traditional subcutaneous lymphoscintigraphy are the poor anatomical detail of lymphatic collectors and the excessive duration of the examination (up to 6–24 hours). Despite these limitations, traditional lymphoscintigraphy provides functional information that cannot be obtained with other imaging techniques. Lymphatic imaging has greatly improved over the last 20 years as a result of increased interest in lymphatic mapping in cancer treatment. New radiopharmaceuticals with more favorable features such as smaller sized particles, neutral pH, and a better lymph nodal uptake are available to examine the lymphatic system. 99mTc‐human serum albumin‐nanocolloid, the preferred radiopharmaceutical in Europe, has an optimal particle size (4–100 nm, with >95% particles <80 nm) to pass through lymphatic vessels. Nanocolloid is licensed for lymphatic mapping to demonstrate the integrity of the lymphatic system via a subcutaneous injection. It is use is suggested in Sentinel Lymph Node (SLN) imaging as well as off‐license use for intradermal injections (ID). 99mTc‐Tilmanocept, a 7 nm mannosyl diethylene triamine penta‐acetate dextran that targets the CD206 receptor, has been approved by the US Food and Drug Administration (FDA) for lymphatic mapping in 2013. It binds to mannose receptors expressed by reticuloendothelial cells (macrophages and dendritic cells) in LNs. The advantages of this tracer include a rapid clearance from the injection site (when injected intradermally), a higher sentinel node (SN) extraction, and low accumulation in second‐echelon nodes. Thanks to the neutral pH of the injected solution, an intradermal administration does not cause pain at the injection site [8–9]. The lymphatic capillary network of the dermis offers a larger surface area for tracer uptake, allowing for better imaging of lymphatic collectors with a higher target/background ratio in less time. Recently, lymphoscintigraphy for the study of lymphedema has been modified and improved with the use of new radiopharmaceuticals and new methodological approaches that combine the advantages of an ID and muscular exercise. The capturing of images at rest and after exercise (after 2 minutes and 40 minutes) following a single administration of the radiotracer is defined as functional imaging [10, 11]. Table 27.1 Rest/stress intradermal lymphoscintigraphy timeline. After choosing areas of the skin where the dermis is well represented, intradermal administration on the dorsum of the hand or feet should be used to avoid bleeding at injection points. Lymphatic capillaries are abundant in the dermis, where they form superficial and deep plexuses, offering a larger surface for tracer uptake. An ID is associated with a rapid lymphatic transport and a higher target/background contrast. The best results can be obtained by evaluating the effects of muscular exercise. Rest/stress intradermal lymphoscintigraphy evaluates lymphatic flow at rest and after short muscular exercise (weightlifting or stepping) for 2 minutes and prolonged muscular exercise (hand squeezing or walking) for 40 minutes (Table 27.1). Rest/stress intradermal lymphoscintigraphy may improve the examination of patients with severe clinical stages of lymphedema. The method allows for a better visualization of superficial and deep lymphatic vessels and regional LNs and, contemporarily, offers a considerable reduction of examination time (1‐hour intradermal vs. 3–6‐hour subcutaneous). Prior to the examination, the patient should remove tight or elastic clothes, jewelry, and other metallic accessories. In the event of an acute lymphangitis, examination should be postponed until after adequate antibiotic treatment. Two IDs of 99mTc‐HSA‐nanocolloid at the I intermetatarsal space and lateral malleolar area are suggested for the legs and two ID injections at the I and IV intermetacarpal space for the hands. The administered dose is of 50–70 MBq (0.3–0.4 mL) of 99mTc‐HSA‐nanocolloid. For an ID, the needle is angled at 15°, about 1–2 mm below the surface of the skin, with the bevel facing up to form a tiny blister at the injection point. The procedure should be followed by a light massage around the injection site. A series of planar static scans in anterior and posterior views are taken at rest immediately following tracer injection: preset time 5 minutes, matrix 128 × 128, 140 keV ± 10% using a double‐head gamma‐camera equipped with a low‐energy general purpose collimator and parallel holes to increase the sensitivity of the gamma‐camera. In a healthy subject, the rest scan may visualize lymphatic pathways draining the lymph from the hand or foot to regional LNs within a few minutes, whereas in patients with lymphedema, the rest scan may demonstrate a scarce or delayed progression of the radiotracer. In this case, the pressure generated by lymphatic vessel contraction is insufficient to push the lymph forward. A short isotonic muscular exercise may play an important role in squeezing lymphatic vessels and pushing lymph forward. A muscular exercise should be performed for 2 minutes, such as stepping for the lower limbs or weightlifting for the upper limbs. Afterwards, a stress scan is taken of the limbs. In early or intermediate clinical stage lymphedema, a short exercise may display initial drainage of radioactive lymph from the hands or feet. Subsequently, prolonged exercise should be performed for 30–40 minutes, for instance walking for the lower limbs or hand squeezing for the upper limbs. A delayed scan is acquired 1 hour following tracer injection. Late scans may show superficial and deep lymphatic vessels of the limbs as well as inguinal, iliac, and paraaortic (or axillary, clavicular) LNs receiving radioactive lymph. Superficial lymphatic vessels run along large veins, the great and small saphenous veins in lower limbs, and the basilic and cephalic veins in the upper limbs. Usually, superficial lymphatic vessels drain most of the lymph. Deep lymphatic vessels are recognizable by the radioactivity of the popliteal and antecubital LNs in the lower and upper limbs, respectively. In patients with lymphedema, a delayed scan may visualize various patterns (Figures 27.1–27.17). According to the level of severity, several patterns may be observed, such as collateral drainage, deviation, and delayed or decreased lymphatic flow. A crossover drainage by the contralateral limb may occur in secondary lymphedema following unilateral groin dissection, typically performed in melanoma of the lower limb. All previous patterns may be considered minor findings or compensatory mechanisms of lymphedema. On the other hand, the absence or scarce visualization of superficial lymphatic collectors is typical of primary lymphedema. In this case, lymphatic drainage may be partially compensated by deep lymphatic vessels. This may be visualized on delayed scans after prolonged muscular exercise. Major findings of lymphedema are the presence of tracer stagnation areas, dilated lymphatic vessels with a partial extravasation of the tracer, and dermal backflow (distal or proximal). A wide extent of dermal backflow and the absence of draining LN may appear in severe clinical stages of lymphedema. A “no flow” pattern is the absent visualization of superficial and deep lymphatic vessels and LNs at delayed scan. In this case, more superficial and deeper reinjections of microdoses in other sites (ulnar styloid apophysis, subfascial injection, etc.) should be performed to visualize a portion of lymphatic vessels. Table 27.2 Comparison between several methods of lymphatic imaging. CT, computed tomography; ICG, indocyanine green; LN, lymph node; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computerized tomography; SPECT/CT, single‐photon emission computed tomography with integrated computed tomography; SPIO, superparamagnetic iron oxide. Figure 27.1 70‐year‐old man, BMI = 28.9 kg/m2. Lower limbs primary lymphedema. Resting scan shows the lymphatic collector of legs. Stress scan shows a stagnation of tracer in the lymphatic collector of left leg. Rest/stress scans show a progressive dermal backflow of left leg. Functional imaging may play a big role in managing patients at risk of develop secondary lymphedema by evaluating the effects of muscular exercise on lymph flow. Today, patients at risk of developing lymphedema, secondary to surgery or radiation treatments, may perform a personalized exercise program
27
Imaging the Lymphatic System
Lymphatic System
Lymphedema
Lymphography
Subcutaneous Lymphoscintigraphy in Lymphedema
Intradermal Lymphoscintigraphy in Lymphedema
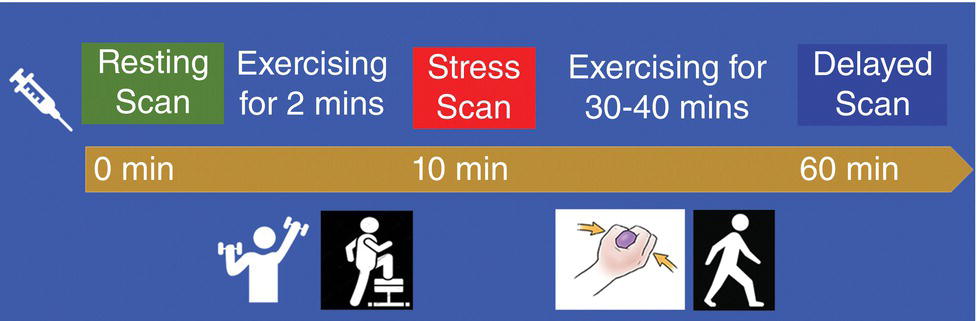
Rest/Stress Intradermal Lymphoscintigraphy in Lymphedema
Techniques
Advantages
Disadvantages
Contrast
Blue dye
Intraoperative visualization of sentinel node
Need to open surgically axilla or groin to visualize lymph‐node
Blue dye
Simple, inexpensive
Not possible distinguish sentinel node from second tier lymph nodes
Allergic reaction
X ray lymphangiography
Higher spatial and depth resolution
Requires surgical intervention to cannulate lymphatic vessel
Ethiodol/lipiodol
Iodine or vital dye allergy
Pulmonary embolism, hypersensitivity, intra‐alveolar hemorrhage, and hypothyroidism
Ionizing radiation
X ray CT
1–3 mm spatial resolution
Cannot visualize normal emphatic vessels
Iopamidal
Visualize LNs and larger lymphatic vessels
Ionizing radiation
Lymphoscintigraphy
Visualize large lymphatic vessel and nodes
Difficult to study area near injection site
99mTc‐radiocolloid/timanocept
Evaluate the effects of exercise on lymph drainage (functional imaging)
Ionizing radiation
Visualize lymph nodes, superficial and deep lymphatic vessels for all their course
Preoperative detection with SPECT/CT of uncommon site of sentinel node
Allow intraoperative radioguided sentinel node biopsy
MRI lymphangiography
1 mm spatial resolution
Difficult to resolve normal lymphatic vessel and node
Gadolinium or iron oxide‐based agents
Imaging lymphatic vessel
Expensive
PET/CT lymphoscintigraphy
May visualize sentinel node in early colon cancer
Preoperative endoscopic injection
89Zr‐Nanocoll
Combined with an intraoperative injection of near‐infrared tracer ICG
ICG fluorescence
Visualize initial lymphatics near the injection site and larger node up to 3 cm depth
Fails to show lymphatic channels located deeper than 1.5 cm below the skin
ICG
Intraoperative visualization of lymphatic vessel and node
Iodine allergy
No radiation
ICG leaking out
May evaluate active lymphatic propulsion
High‐frequency US
Gives high‐resolution imaging at depths up to 3 cm
Depth limited
No contrast
Non‐invasive and non‐radioactive
Real‐time detection of anomalies
SPIO
Easy
Interference of surgical instrumentation with magnetomer
SPIO nanoparticles
No radiation
Intramammary persistence
Cannot be used in patients with pacemakers or metal implants
allergy
Depth limited
CEUS
Real‐time visualization
Operator dependent
Octafluoropropane/sulfurhex afluoride
Cheap
Only for US‐guided biopsy of lymph node
No radiation
3T MR lymphangiography
Images the central lymphatic system
Scarce visualization of peripheral lymphatic system
No contrast
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree