VDO should be thought of as a continuum of disease, with both entities occurring at different times in pathogenesis of infection based on the age of the patient. In children younger than 4 years old, primary hematogenous disc infection happens first, followed by bone infection (osteomyelitis) (3), whereas in adults, osteomyelitis of the end plates occurs first, followed by discitis (4). Differences in age-related vascularity of the intervertebral disc can explain the pattern of microbial spread. In infants and young children, numerous anastomoses exist between arteries in the vertebral bone marrow and the periosteal arteries in the peripheral third of the disc. These interosseous arteries start regressing after age 7, are nearly gone by age 15, and completely involute by adulthood (5).
Infection can be hematogenous or nonhematogenous. Arterial hematogenous spread is most common, with pathogens deposited in the end arterioles of the end plates in adults (6) and in the disc itself in children less than 4 years old (3). The presence of microbes in the end plate alone does not cause osteomyelitis, but rather is associated with osseous infarction. Venous hematogenous spread is less common and occurs via retrograde transmission of microbes through valveless veins in the vertebral bodies. These veins also anastomose with the paravertebral venous plexus of Batson (7). Pelvic and urinary infections are highly associated with this route of spread.
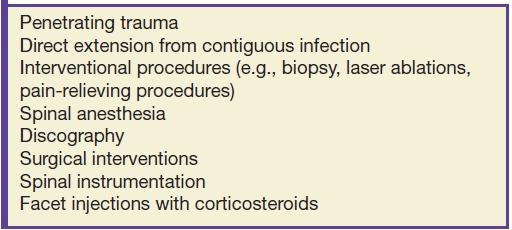
Nonhematogenous routes of spread can be via direct inoculation, such as from penetrating trauma. Table 14.2 lists multiple ways by which direct spread of infection occurs (7). Direct inoculation accounts for 20% to 30% of VDO (8), so a careful search for clinical or imaging evidence of prior spinal interventions is important.
Table 14.2 Nonhematogenous Routes of Infection Propagation
Pyogenic vertebral discitis–osteomyelitis
Staphylococcus aureus (S. aureus) is the most common cause of pyogenic VDO and occurs both by a hematogenous route (up to 84% of cases of nontuberculous VDO) (6) and via direct inoculations (up to 33%) (9). Hematogenous spread of Enterobacter species along with other less common species including Haemophilus influenza, Staphylococcus epidermidis, and Streptococcus subtypes accounts for the remainder (6). Sickle cell disease patients may be infected with Salmonella. The most common gram-negative bacterium is Escherichia coli (E. coli), although diabetic patients tend to get anaerobic infections (10). Patients with VDO usually present with back pain and muscle spasm 90% of the time, with leukocytosis and fever in 40% to 50% of cases (6). Both erythrocyte sedimentation rate (ESR) (11) and C-reactive protein (CRP) levels are increased, with the latter normalizing precipitously with effective therapy (12).
Spine radiography is a poor imaging modality for VDO because it shows signs of demineralization, disc space narrowing, and endplate erosion only after 2 to 8 weeks following the onset of infection and symptoms (6). CT has limited utility in acute VDO, showing endplate erosion, paraspinal soft tissue enlargement, and vertebral body demineralization earlier than radiographs (6). Indium 111 scintigraphy (13) shows greater specificity than the highly sensitive, but nonspecific combination of technetium 99m and gallium 67 scintigraphy (14); however, this study can take hours to days to complete, limiting its utility in the emergent setting.
MRI is the imaging study of choice to detect VDO and has been shown to be more sensitive and specific compared to nuclear scintigraphy (15). Acute pyogenic VDO shows T1 hypointense signal of the end plates, adjacent vertebral bodies, and the infected disc, with corresponding irregular T2 hyperintense signal. The end plates are irregular and ill defined. Following contrast administration, there is enhancement of the disc and affected end plates (6) (Fig. 14.1). The abovementioned findings are however seen together only 50% of the time because the T2 marrow signal may not be present if there is significant VB sclerosis (affects the trabeculae). If enough sclerosis is present, only profound T2 hypointense signal will be present due to lack of any remaining marrow (16). In any event, STIR imaging, which nulls out the signal from marrow fat, shows T2 changes to best advantage. Extraosseous spread can extend into the paraspinal tissues, the epidural space (i.e., spinal epidural abscess [SEA]), and the psoas musculature, with abscess formation (Fig. 14.2).
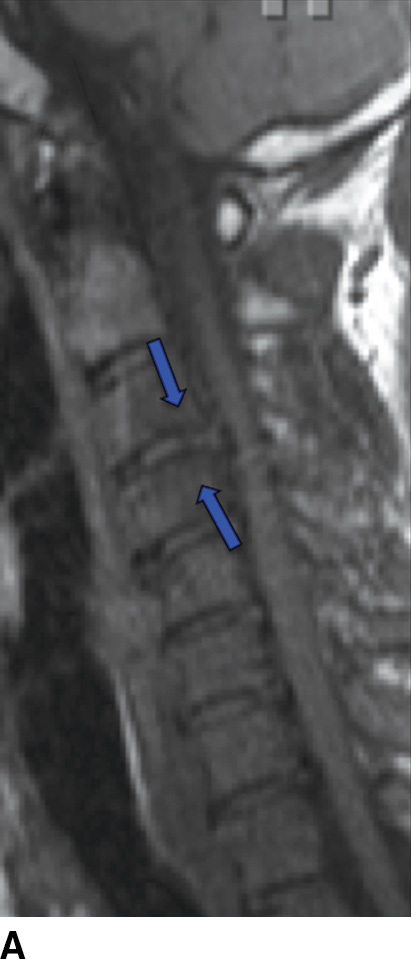
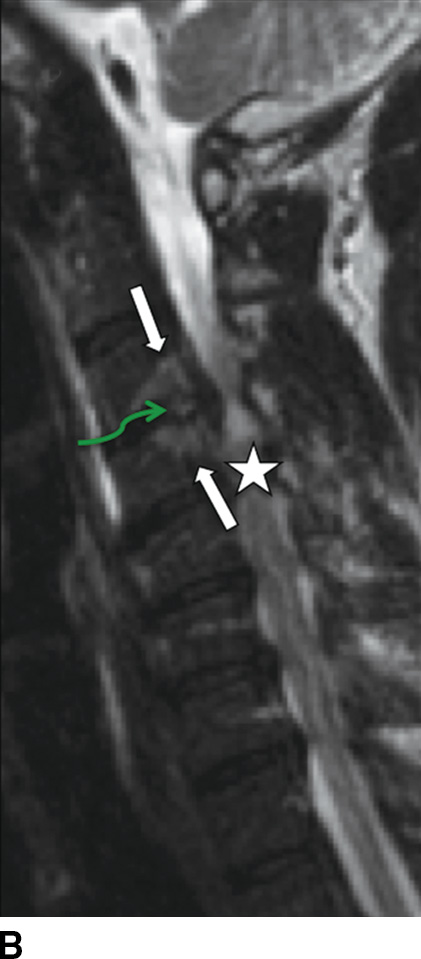
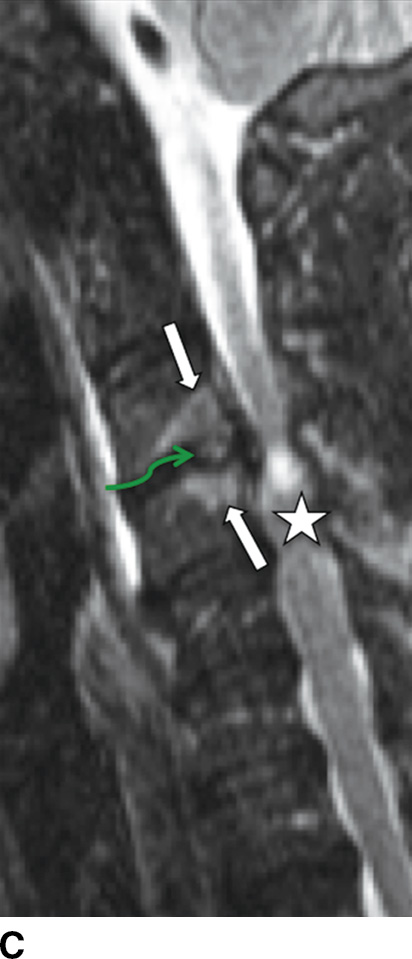
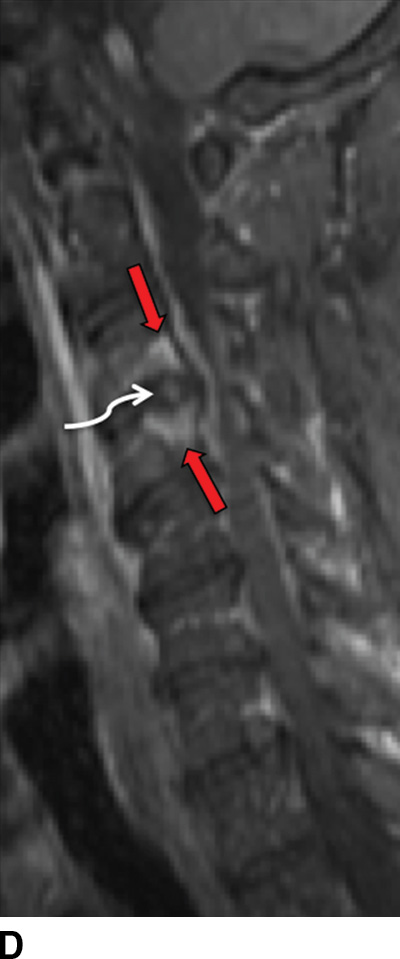
FIG. 14.1 Acute vertebral discitis–osteomyelitis (VDO). Sagittal T1-weighted (A), T2-weighted (B), T2-weighted FS (C), and postcontrast T1-weighted FS (D) MR images show endplate irregularity and adjacent T1 hypointense (blue arrows) and T2 hyperintense (white arrows) marrow signal abnormality and abnormal enhancement (red arrows), with prevertebral edema. T2-weighted signal hyperintensity of the intervening disc (green arrows in B and C) shows abnormal enhancement (white arrow in D). There is resultant compression of the cord; incidental disc herniation and T2-weighted cord hyperintensity, which could be myelomalacia or cord edema (just superior to the stars in B and C). The ventral epidural enhancement is engorgement of the venous plexus and not epidural phlegmon. T1 weighted, T1 weighted; T2 weighted, T2 weighted; FS, fat saturated. (Courtesy of Nafi Aygun, MD, The Johns Hopkins Hospital, Baltimore, MD.)
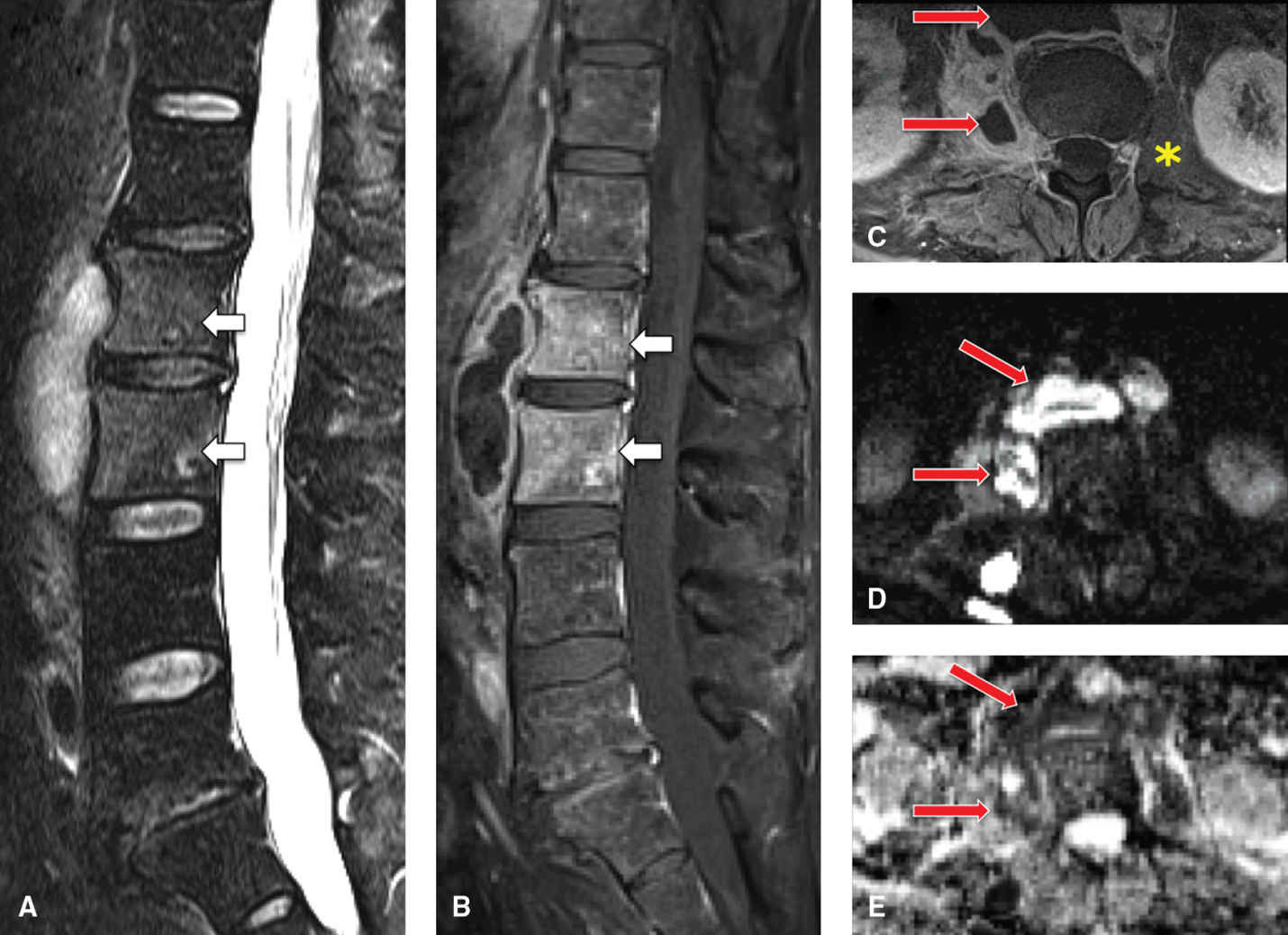
FIG. 14.2 Psoas and retroperitoneal abscess in HIV-positive patient. Sagittal T2-weighted (A) and postcontrast T1-weighted FS (B) images show a rim-enhancing, T2-weighted hyperintense collection anterior to the L1 and L2 VB, which occurred first and spread to the adjacent spine. These VBs are also infected (osteomyelitis) and show abnormal enhancement and T2 hyperintense signal (arrows). C: Axial postcontrast T1-weighted FS image shows thick-rimmed peripherally enhancing collections in the retroperitoneal/prevertebral space, extending into the right psoas muscle (arrows). Note the normal left psoas muscle (asterisk) for comparison. Axial DWI (D) and ADC (E) images show DWI hyperintensity and ADC hypointensity (arrows), indicating restriction of water diffusion and corresponding to the areas of collection that do not enhance. This signifies highly viscous pus in the abscesses. Lack of intervertebral disc involvement is due to early osteomyelitis due to direct extension of extraosseous retroperitoneal infection.
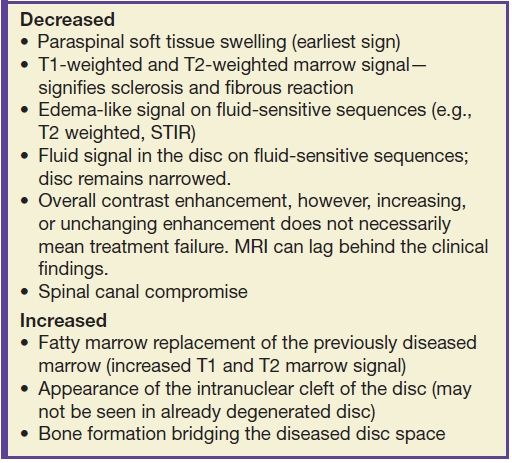
MR imaging findings often lag behind both early clinical signs of VDO and after the healing phase has commenced. Signs of healing on MRI have been variable with conflicting information in the literature (17). Table 14.3 lists signs of healing that can be seen. In fact, MR imaging during the healing phase can appear to show increased involvement due to ongoing bone necrosis when the patient may actually be improving clinically. CT can show endplate sclerotic changes and irregularity.
Table 14.3 Signs of VDO “Healing” on MRI
Postoperative VDO is usually due to direct inoculation and can be difficult to diagnose. MRI findings lag behind clinical symptoms by 3 to 4 weeks. Patients present with muscle spasm, back pain, and positive leg raise test. Intervertebral disc and endplate postcontrast enhancement and signal changes can be seen in postoperative asymptomatic patients and infected patients (18). The lack of these two findings and paraspinal soft tissue swelling makes VDO unlikely. CT or fluoroscopic-guided needle biopsy may on occasion be needed for confirmation (6).
Patients with SEA associated with VDO present with back pain (71%), fever (66%), progressive myelopathy or radiculopathy, and/or tenderness (2). In subacute or chronic cases, fever may be absent (19); however, other laboratory markers for infections such as sedimentation rates will be positive. The vast majority of cases are due to S. aureus (18), of which 15% are methicillin resistant (i.e., MRSA) (20). MRI is 91% to 100% sensitive for SEA (2) and often shows a soft tissue mass with tapering edges in the spinal epidural space with mass effect on the thecal sac and spinal cord. SEA appears as isointense to cord on T1 and hyperintense to cord on T2 sequences (21). Postcontrast enhancement can be (a) thin, peripheral, (b) heterogeneous, or (c) diffuse and smooth (21). With an ever-increasing prevalence in both the HIV and non-HIV population (6), recognition of SEA is critical to combating the 18% to 30% mortality (22) associated with this surgical lesion. An important distinction to be made on imaging is between a phlegmon versus an SEA, since the latter may require surgical drainage (Fig. 14.3).
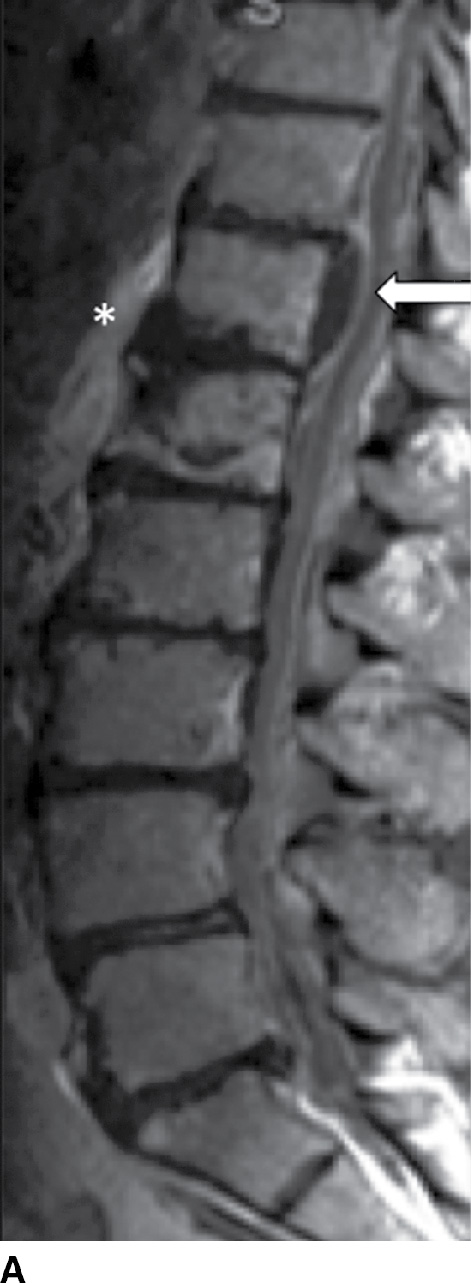
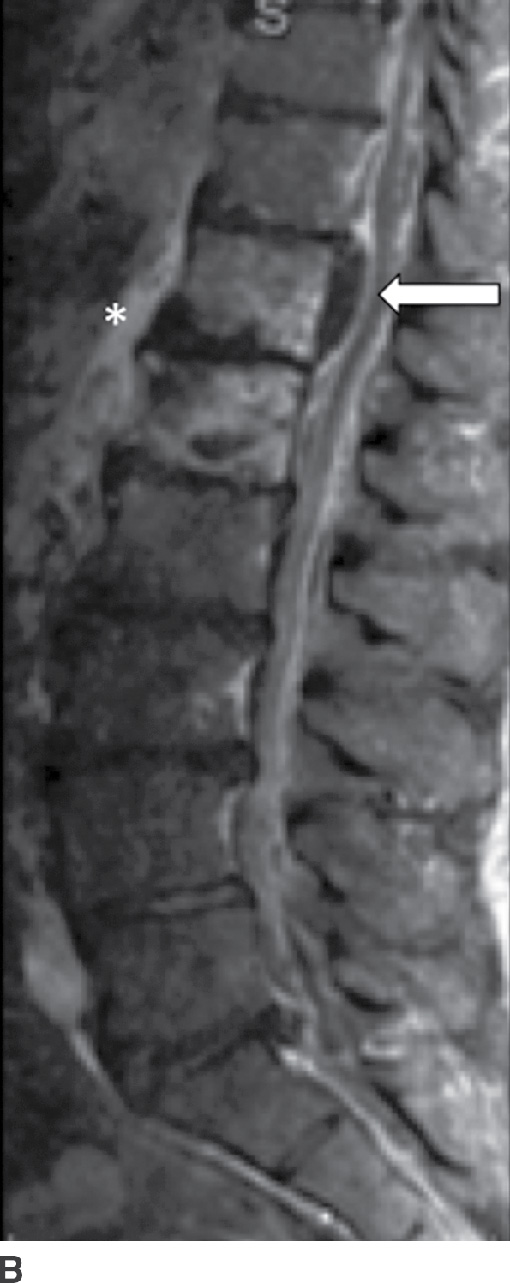
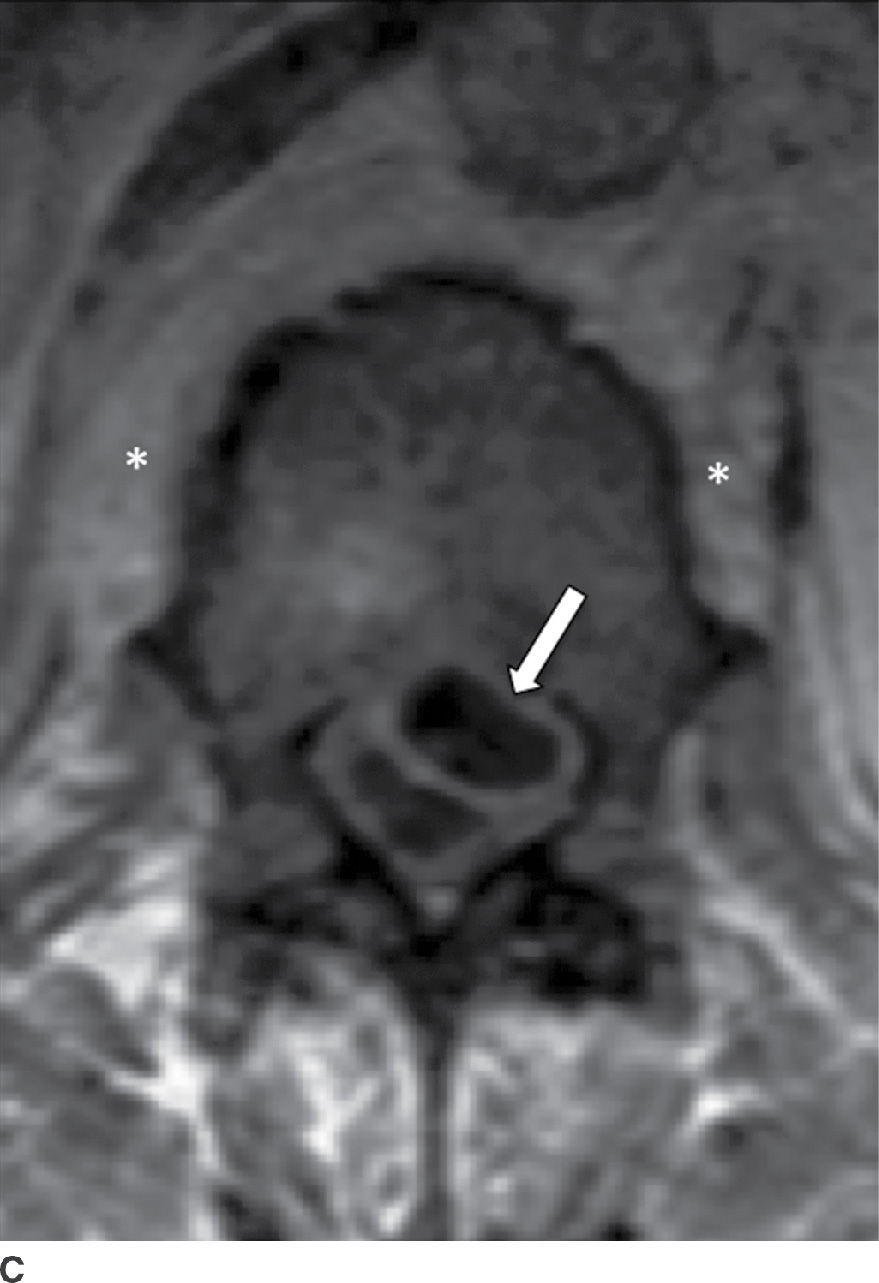
FIG. 14.3 Spinal epidural abscess (SEA). Slight parasagittal postcontrast T1-weighted without (A) and with (B) FS and axial postcontrast T1-weighted (C) MR images show a ventral epidural rim-enhancing collection, compatible with SEA (arrows). Resultant compression of the thecal sac is present, in addition to VB marrow enhancement (osteomyelitis) and paraspinal phlegmon (asterisk). VB, vertebral body.
Mycobacterial and Brucella spondylitis
Mycobacterial spondylitis is predominantly due to Mycobacterium tuberculosis, also known as Pott disease, and has shown increased international prevalence. The bacteria spread hematogenously and the clinical course can be indolent, with symptoms initially lasting for 2 to 3 months to many years (23). Usually starting in the anteroinferior portion of the VB, TB spondylitis spreads under the anterior longitudinal ligament (ALL) and involves contiguous VBs (24). Due to a lack of proteolytic enzymes, the intervertebral disc is usually spared until later in the disease (25).
MRI is the study of choice for TB spondylitis. T1 hypointense and T2 hyperintense signal is present in the VB, with a paucity of hyperintense signal in the intervertebral disc and variable loss of VB cortical distinction compared to pyogenic VDO. The posterior elements are involved more often with TB spondylitis than with pyogenic VDO (26). These findings can make differentiation of TB spondylitis from tumor difficult, especially when there is preservation of the intervertebral disc space. Anterolateral extraosseous spread of infection occurs, with rim-enhancing intraosseous and paraspinal collections more commonly with TB spondylitis than other forms of spondylitis (6); however, by itself, this is not a discriminator (Fig. 14.4). The thoracolumbar junction is the most common segment affected, with partial destruction of VB often causing focal kyphotic deformity (i.e., Gibbus deformity) (27).
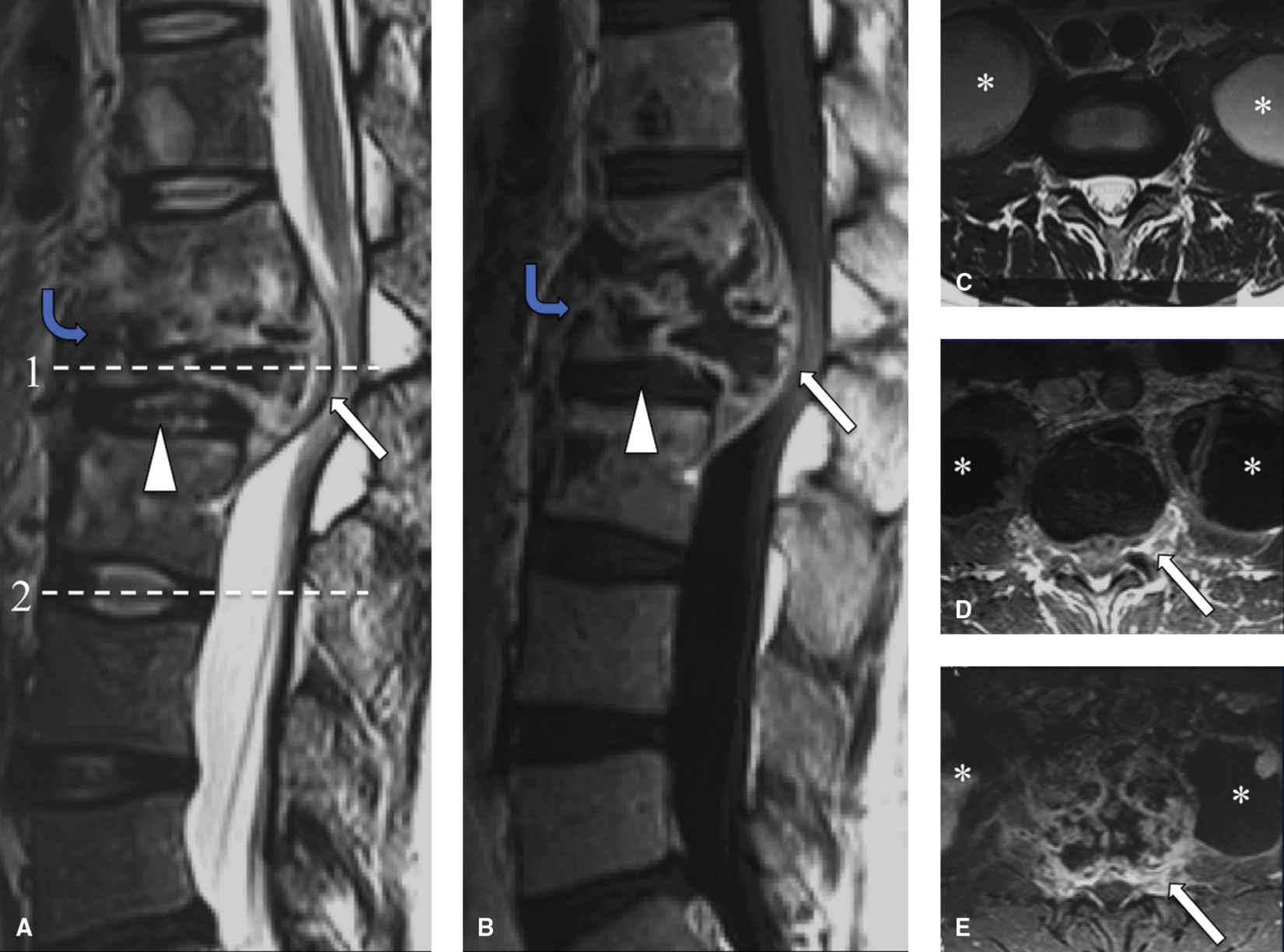
FIG. 14.4 Tuberculous (TB) spondylitis. Sagittal T2-weighted (A) and postcontrast T1-weighted images (B) show destruction of the T12 VB (with involvement of T11 and L1 VB), with a complex epidural abscess (white arrows) compressing the cauda equina. Note subligamentous extension of infection (blue arrows) and sparing of the disc space (arrowhead). Axial T2-weighted image at level 2 (C) shows large bilateral psoas collections (asterisk), with irregular, thick rim enhancement (white arrow ) on postcontrast T1-weighted images (D, E), compatible with huge psoas abscesses (level 1) become infected. Late in the disease, the intervertebral disc can become infected.
Gram-negative bacteria of the Brucella species cause brucella spondylitis, or brucellosis. It is endemic in developing countries and seen in dairy workers, farmers, slaughterhouse workers, and veterinarians. The lumbar spine is most commonly involved (28). The focal form of spinal disease can be confined to the anterior VB, whereas the diffuse form can spread through the entire VB, disc, paraspinal tissues, and into the epidural space. Although the imaging appearance can be similar to TB spondylitis, VB collapse is rare (29). Brucella can cause total ankylosis of the involved VBs, in effect stabilizing the spine. Formation of an anterior osteophyte can be seen, mimicking a “parrot’s beak” (28). Features of arachnoiditis or radiculitis may also be present.
Fungal spondylitis
Fungal infection of the spine can present as discitis, meningitis, or osteomyelitis. Aspergillus and Candida species are most common, causing abscess and granuloma formation (4). Incidence of fungal spondylitis is increasing, predominantly in the immunocompromised population (30). Inoculation can be direct, hematogenous, or through local soft tissue invasion, with a particular predilection for vascular invasion that can lead to cord infarction (30).
Imaging findings can differ compared to pyogenic VDO, especially in immunocompromised individuals. T2 signal changes are less pronounced, with almost normal appearance of the disc intranuclear cleft (31), although disc destruction can occur (Fig. 14.5). Posterior element involvement is present, as in TB spondylitis. Imaging findings different from pyogenic VDO are attributed to lack of appropriate host response to the organism. Biopsy is often needed for definitive diagnosis (6).
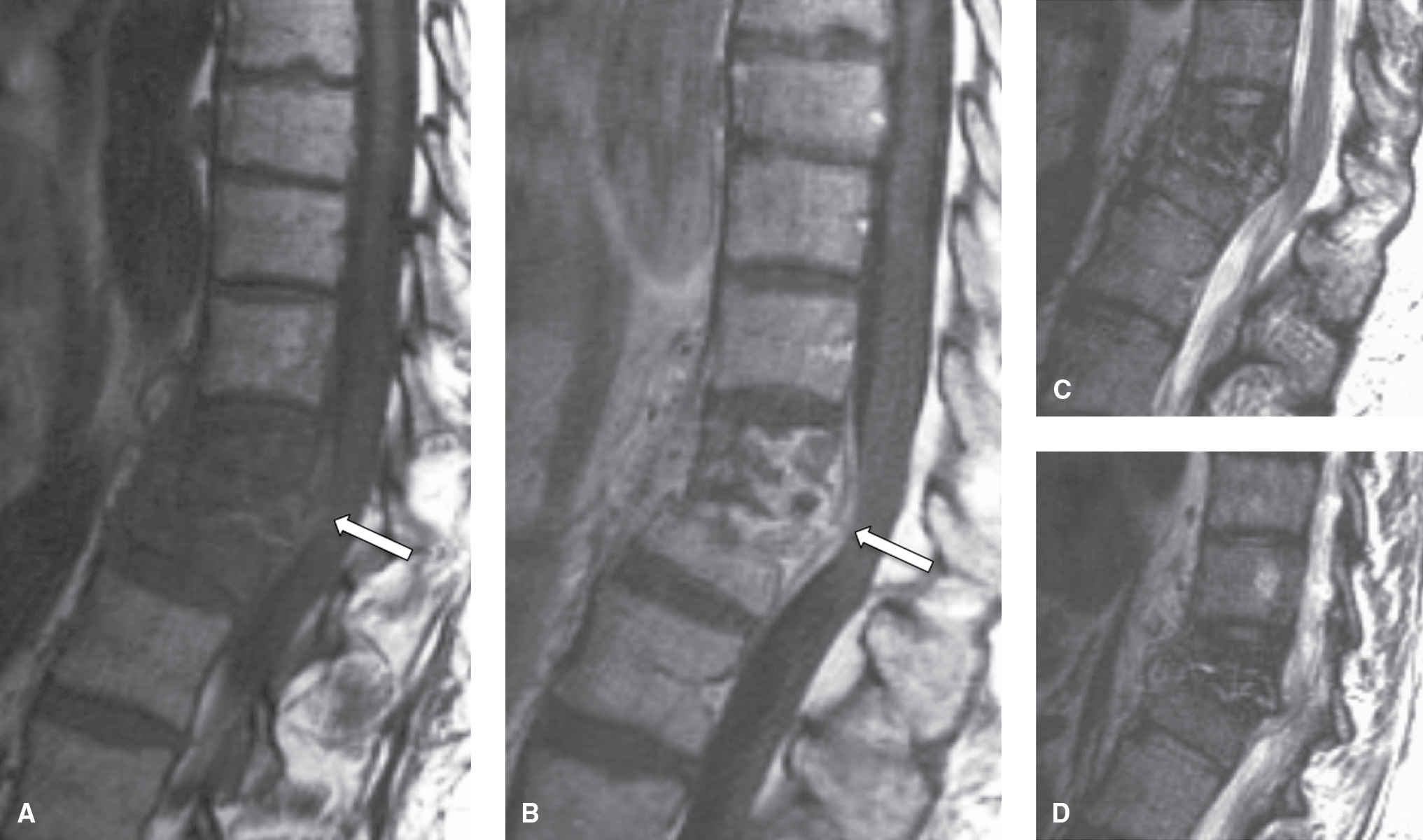
FIG. 14.5 Fungal spondylitis (Aspergillus species). Sagittal T1-weighted (A), T1-weighted postcontrast (B), T2-weighted (C), and parasagittal T2-weighted (D) images show destruction of the T11-T12 disc space, with early kyphosis at this level. Note that the T2 hyperintense signal is less pronounced than seen in pyogenic VDO. Epidural extension causes compression of the lower thoracic spinal cord (arrows). In this immunocompromised patient, biopsy was performed and revealed aspergillosis. (Courtesy of Nafi Aygun, MD, The Johns Hopkins Hospital, Baltimore, MD.)
Hydatid disease
During its cystic stage, the parasitic tapeworm Echinococcus causes hydatid disease. The cause for human infection is ingestion of food contaminated by canine feces. The disease is endemic in Central Europe, Latin America, Australia/New Zealand, and the Mediterranean Basin, predominantly affecting prepubescent and adolescent males (32). Spinal involvement is rare; however, when present, it usually affects the thoracic spine (50%), followed by the lumbar, sacral, and cervical segments of the spine (33).
CT and MRI show intraosseous cystic lesions, sometimes in a “bunched grape” appearance, without associated enhancement. Degenerating cysts can show heterogeneous signal on T1 and T2 sequences. MRI is preferred for evaluation of any associated mass effect on the cord and exiting nerves (34). Due to its expansive and destructive appearance, spinal hydatid disease can resemble primary bony malignancy (e.g., osteosarcoma, chondrosarcoma, or aneurysmal bone cyst), metastatic disease (e.g., renal cell or anaplastic thyroid carcinoma), or spondylitis (pyogenic or TB) (32).
Inflammation
Ankylosing spondylitis
AS is the prototypical HLA B-27 positive, seronegative (rheumatoid factor negative) spondyloarthropathy (35). There is a 0.1% to 0.2 % prevalence, with males affected more than females. A bimodal distribution exists, with a primary peak at 26 years and a second resurgence after 39 years of age (36). Onset of AS is insidious, lasting at least 3 to 4 months, with morning stiffness that improves with exercise (37). Sacroiliac joint involvement occurs early in the disease, generally followed by an ascending pattern of involvement from the lumbar to cervical spine and a pattern that is more common in men.
Radiography can show erosions of the end plates with sclerotic margins at the corners of the vertebral bodies, called the “shiny corner sign” or “Romanus lesion” (38) and a squaring of the vertebral bodies. Although radiography can show ankylosing of the SI joints and syndesmophyte formation of the spine, CT is better for anatomic evaluation, especially for spinal canal stenosis, the formation of pseudoarthrosis, facet joint degeneration, and fractures (38). The rigidity of the fused spine makes it particularly susceptible to fractures (39), even in the instance of relatively mild trauma.
AS-related sacroiliitis showing abnormal synovial enhancement on MR along with early cartilage damage, bone erosion, and subchondral bone destruction (40). T2 hyperintense marrow signal can indicate edema or revascularized marrow stroma. In patients with long-standing disease, there is varying degrees of bony fusion, spinal canal stenosis, and possible spinal cord compression. “Romanus lesion(s)” can be present (Fig. 14.6). The intervertebral disc may be involved with irregularity of the end plates, mimicking VDO. Unlike VDO, however, AS does not present with an extraosseous component.
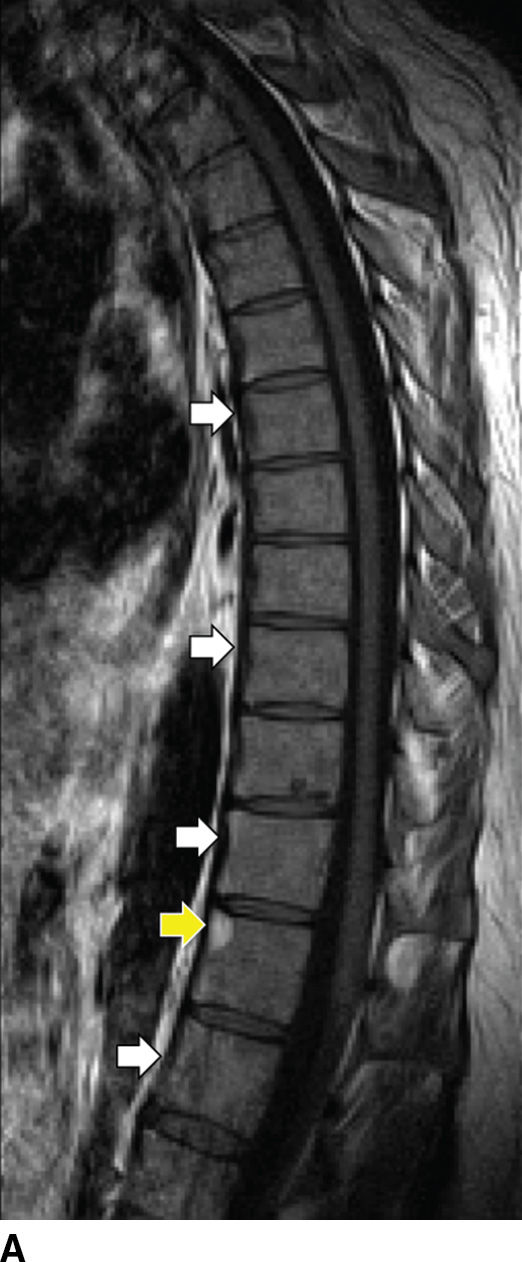
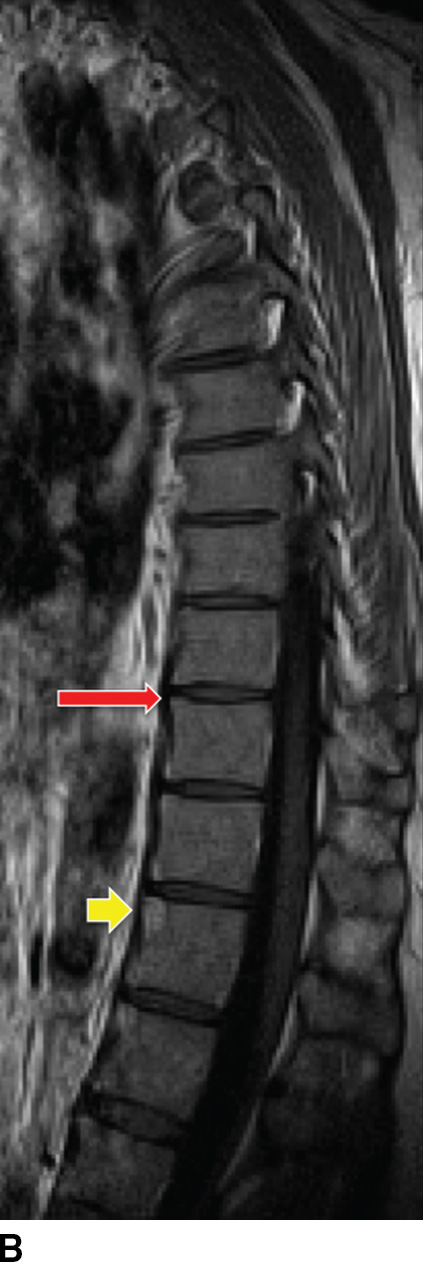
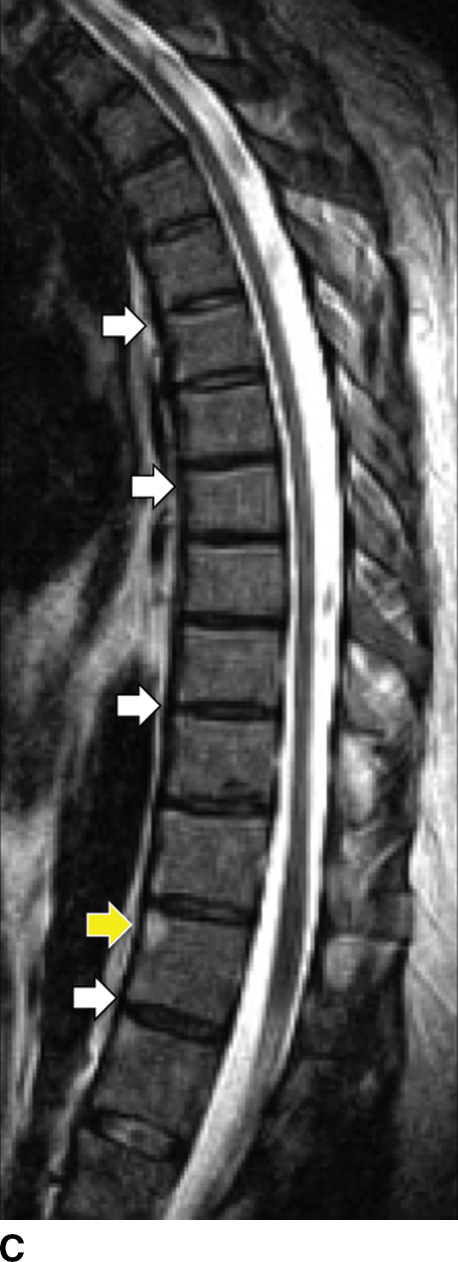
FIG. 14.6 Ankylosing spondylitis. Sagittal T1-weighted (A), parasagittal T1-weighted (B), and sagittal T2-weighted (C) MR images show multilevel syndesmophytes fusing the anterior longitudinal ligament (ALL) (white arrows). Red arrow in B shows a paramedian syndesmophyte bridging the disc space. A T1/T2 hyperintense lesion of the superoanterior portion of the T11 VB represents a chronic Romanus lesion (yellow arrows). (Courtesy of Mahmud Mossa-Basha, MD, University of Washington, Seattle, WA.)
Psoriatic arthritis
Psoriatic arthritis (PA) is usually a manifestation of psoriasis, with an incidence up to 1% in that population. Typically involving only a few joints, 20% of these patients progress to polyarticular involvement (41). On MRI, PA appears similar to RA (see below), with synovial inflammation extending beyond the joint capsule and involvement of the paraspinal soft tissues and ligamentous structures. Ill-defined areas of bony destruction are seen on STIR or fat saturation T2-weighted images with enhancement on postgadolinium studies. Radiographically and by CT, asymmetric spondylitis and arthritis of the spine are noted, contrary to the symmetric, smooth involvement of AS (41) (Fig. 14.7).
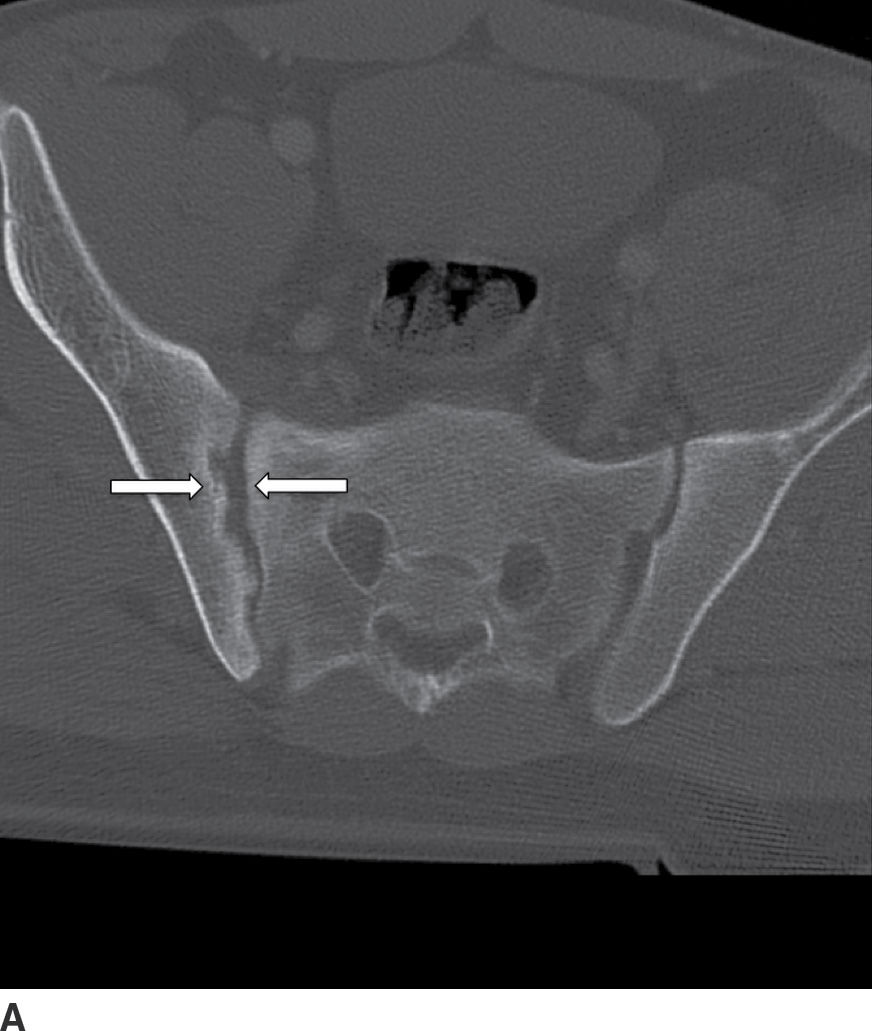
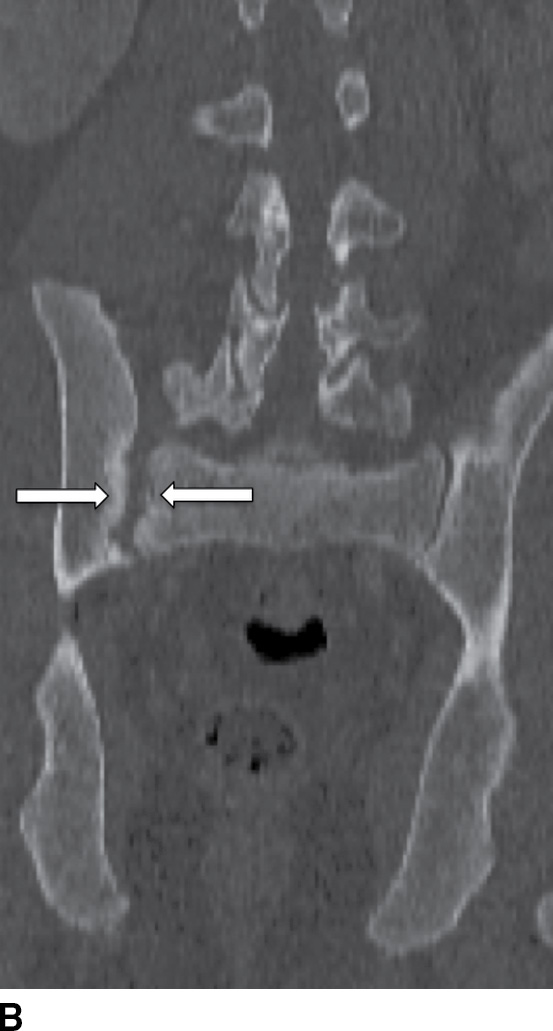
FIG. 14.7 Psoriatic sacroiliitis. Axial (A) and coronal (B) CT images of the pelvis show unilateral erosive changes of the right sacroiliac joint (arrows), in this patient with psoriasis. (Courtesy of Nafi Aygun, MD, The Johns Hopkins Hospital, Baltimore, MD.)
Reactive arthritis
Reactive arthritis (Reiter syndrome, associated with HLA-B27) has a prevalence of 3.5 per 100,000 individuals and occurs predominantly in white males. Clinical presentation is variable, ranging from constitutional symptoms of fever and malaise to urethritis in 1% to 3% affected patients (42). MRI shows no specific pattern of abnormality; however, radiography and CT can show paraspinal calcification (comma shaped), distinct from syndesmophytes, with associated unilateral or bilateral sacroiliitis (43).
Rheumatoid arthritis
RA is a multisystem disease with a prevalence of 1% worldwide and affects women three times as often as men (43). RA predominantly affects the cervical spine, with rare involvement of the remainder of the spine. The pathophysiology includes an aggressive synovitis that causes pannus formation, which may extend into the adjacent soft tissues and ligaments, resulting in laxity and rupture (Fig. 14.8). Osteoporosis is present, as are bony erosions and fractures, with associated compression of the vertebral arteries, spinal cord, and spinal canal (44). Cervical instability results from laxity and rupture of the transverse ligament, which holds the odontoid process against the anterior arch of C1. Atlantoaxial subluxation can occur due to involvement of the atlantoaxial, atlanto-occipital, and atlanto-odontoid joints (45). Persistent synovitis is a characteristic feature of RA.
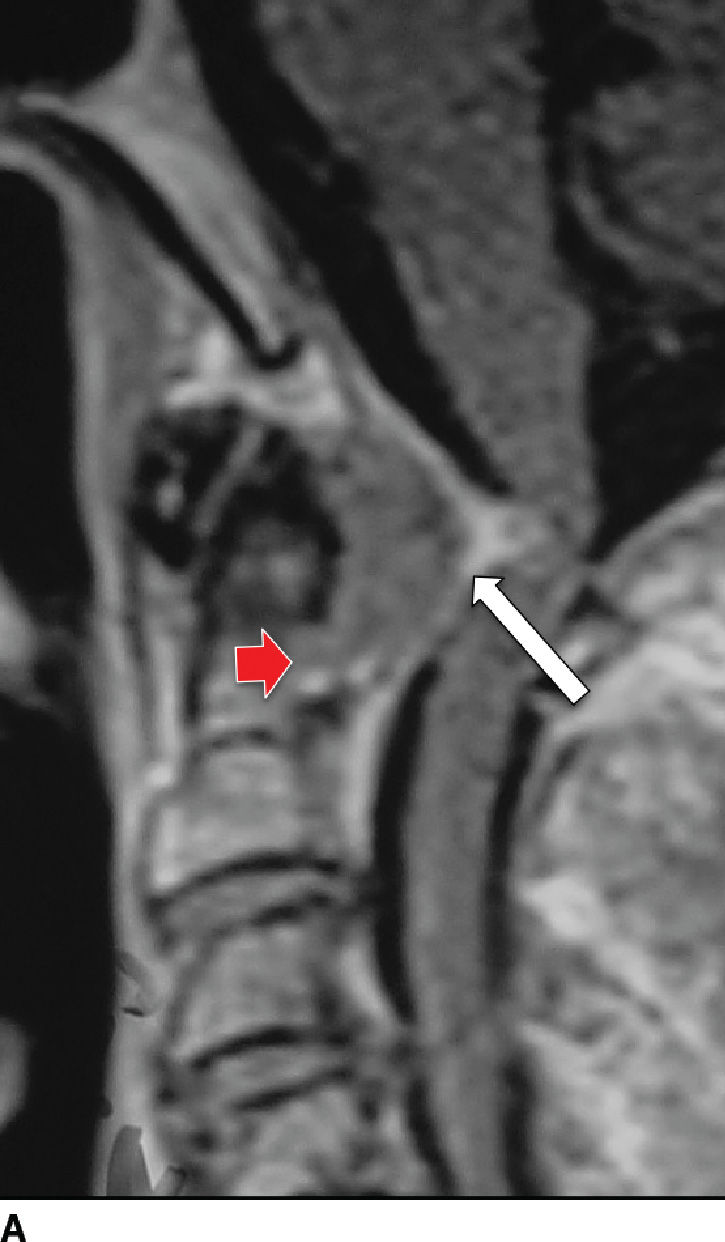
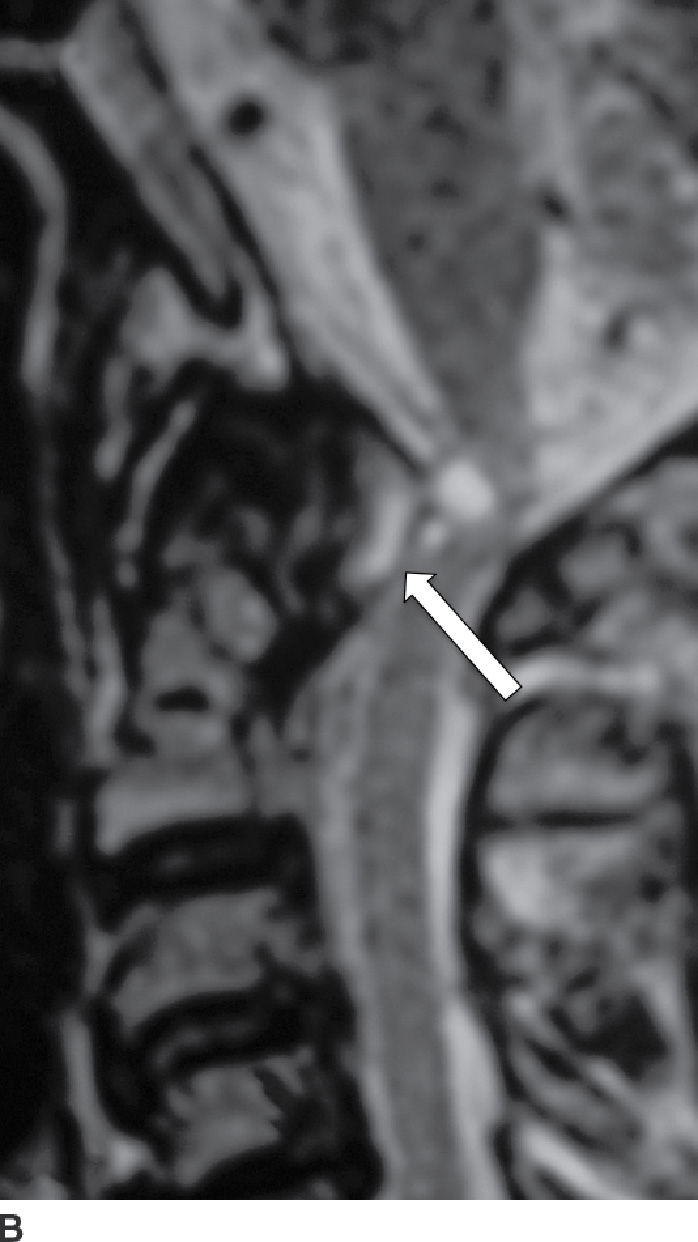
FIG. 14.8 Rheumatoid arthritis. Sagittal postcontrast T1-weighted (A) and T2-weighted (B) MR images show a large enhancing soft tissue mass (pannus) eroding the dens (red arrow) and compressing the thecal sac, with mass effect on the cervical spinal cord (white arrows). (Courtesy of Nafi Aygun, MD, The Johns Hopkins Hospital, Baltimore, MD.)
Radiography shows destructive changes of the cervical spine, especially the atlantoaxial joints, and instability as demonstrated on flexion–extension views (46). CT helps identify the extent of destruction and best visualizes the atlantodental space, which normally measures less than 2.5 mm in adults. MRI is ideal for evaluation of cord, nerve, and vascular compression, unrelenting suboccipital pain, and progressive subluxations on radiography or CT. In addition to destructive changes of the uncovertebral joints and facet joints, atlantoaxial joint destruction, cord compression, and cord edema can be evaluated; the latter sign portends a poor prognosis (44).
Sarcoidosis
Sarcoidosis is one of the great mimickers of other diseases such as TB and lymphoma, with the spinal column infrequently involved (47). Multiple lytic lesions with sclerotic margins are seen on CT, and these lesions show enhancement on MRI (Fig. 14.9). Relative sparing of the discs is present, despite multiple-level vertebral body involvement (48). Biopsy is usually needed for definitive diagnosis.
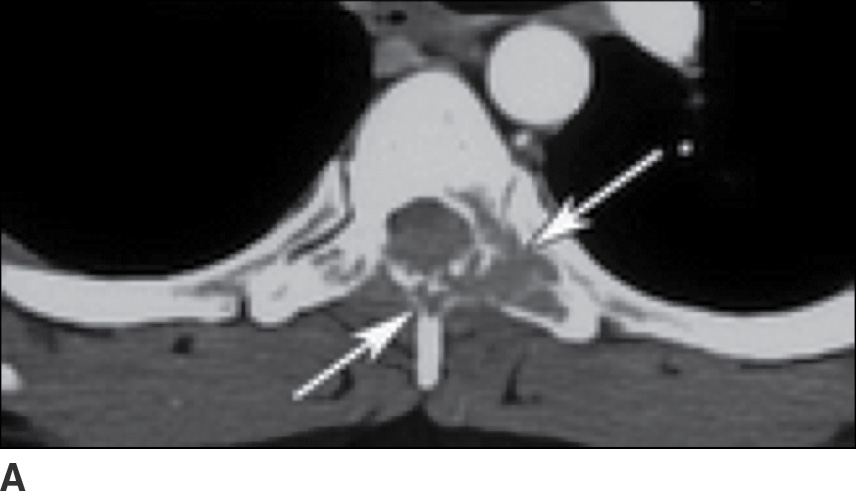

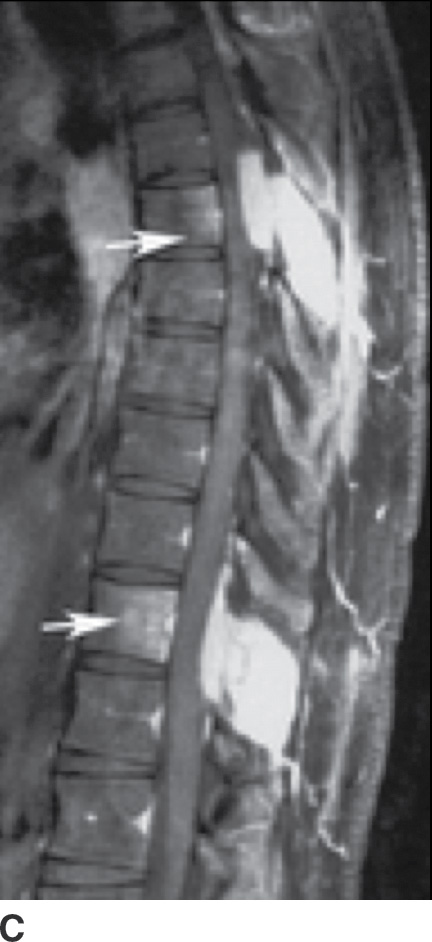
FIG. 14.9 Sarcoidosis. A: Axial CT image of the upper thoracic spine shows destructive changes of the posterior elements of this VB (arrows). B: Sagittal T1-weighted MR image shows multiple enhancing lesions in the spinous processes (arrows), with extraosseous extension into the dorsal epidural space (arrowheads). C: Postcontrast T1-weighted FS also shows mild enhancement of VBs (arrows), not seen on the precontrast image (B), indicative of early VB involvement. (Courtesy of Nafi Aygun, MD, The Johns Hopkins Hospital, Baltimore, MD.)
Dialysis-Related Amyloidosis
A complication of long-term hemodialysis, dialysis-related amyloidosis (DRA) (amyloid spondyloarthropathy) is the result of amyloid deposition by circulating β2-microglobulin, with associated macrophage reaction. Deposition occurs in the intervertebral disc, the apophyseal joints, and the ligaments (49). The appearance is that of an aggressive spondyloarthropathy (50). The cervical spine is usually involved (Fig. 14.10); however, cauda equina compression can sometimes occur from lumbar spine involvement (50).
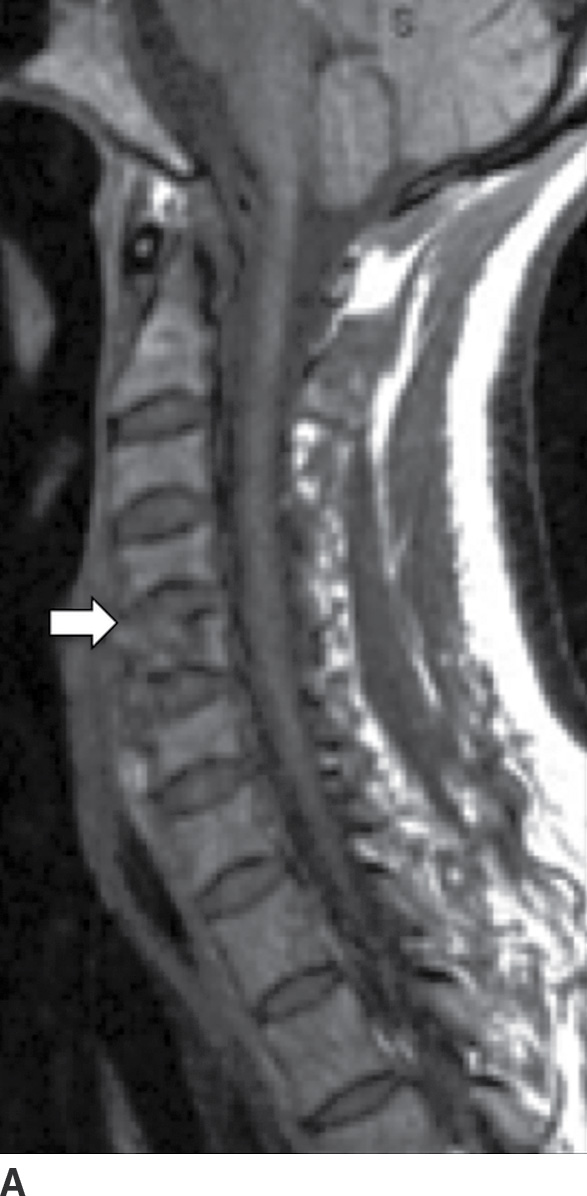
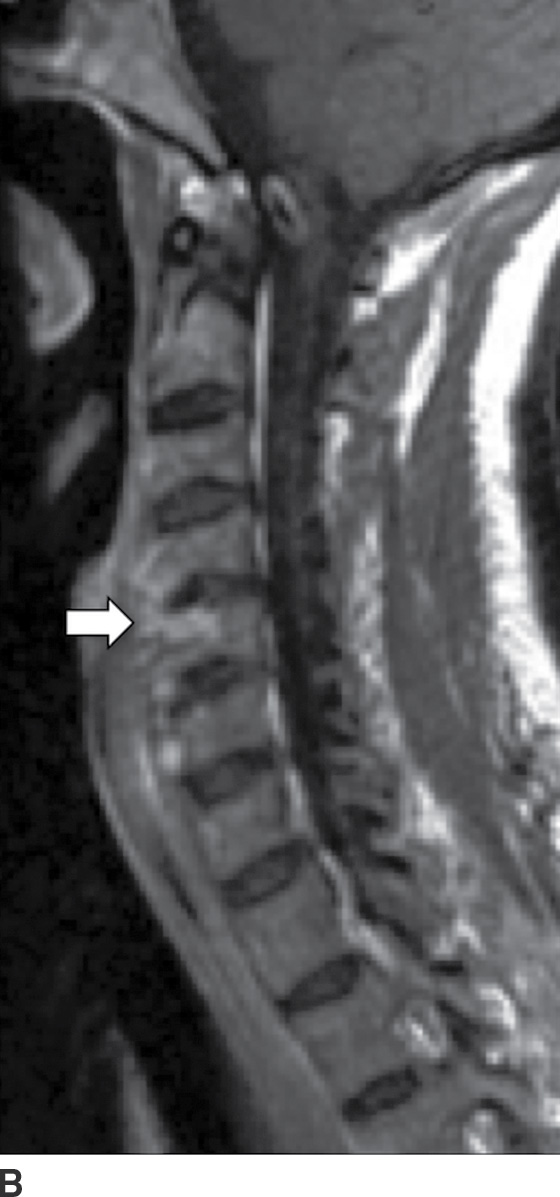
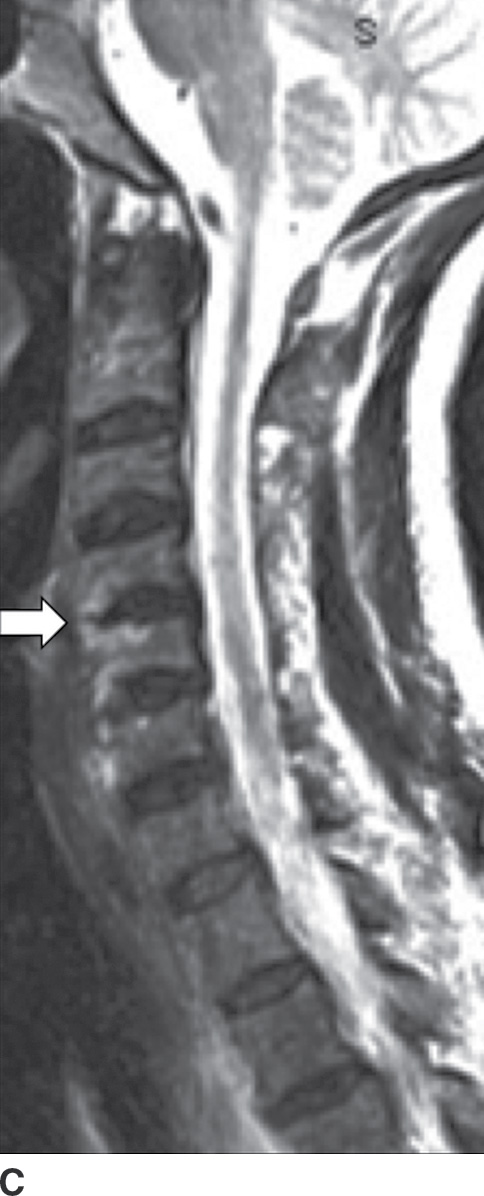
FIG. 14.10 Dialysis-related amyloidosis (DRA). Long-term hemodialysis patient with end-stage renal disease, presenting with vague, nontraumatic neck pain. Sagittal T1 (A), postcontrast T1-weighted (B), and T2-weighted (C) MR images of the cervical spine show abnormal contour, enhancement, and endplate edema of the C4 to C5 level (arrows), respectively. There is sparing of the disc.
Spinal Cord
Infection
Spinal meningitis (pyogenic, TB/brucellosis, Cryptococcus, cysticercosis)
Spinal meningitis results in infection of the leptomeninges and subarachnoid space. Also termed infectious arachnoiditis, the most causative etiology is bacterial pathogens. Occurring most commonly in newborns, bacterial meningitis affects infants between 3 and 8 months of age; adults are usually affected in the second and sixth decades of life.
Clinical presentation can vary and depends on the pathogen’s virulence and the host’s response. The infection can spread hematogenously from a remote site, extend directly from adjacent VDO, or may result from direct inoculation. MRI can detect spinal meningitis, because the increased protein within the CSF changes the MR signal intensity, which is particularly noticeable on T1WIs. On the postcontrast images, associated enhancement of the meninges is identified.
Pyogenic leptomeningitis represents the most common intradural spinal infection. In newborns, group B Streptococcus, gram-negative bacilli, and Listeria monocytogenes are the most common causes. In adults, common culprits include Neisseria meningitides, S. aureus, and Streptococcus species. MRI findings can be completely normal in meningitis, so laboratory studies of CSF are most valuable in suspected cases.
In acute pyogenic leptomeningitis, precontrast T1-weighted (T1W) images can be normal appearing or show mildly increased CSF signal, or dural meningeal thickening (51) if there is a secondary myelitis. Other findings include nerve root clumping, adhesions, multiple CSF loculations, and poor visualization of the cord–CSF interface. T2-weighted (T2W) images are less helpful due to the intrinsic hyperintensity of the CSF; however, cord enlargement may be present (51).
Postcontrast T1W images may show inflamed cord–pial surface, dural enhancement and thickening, and abnormal enhancement of the cauda equine nerve roots (Fig. 14.11). Enhancement can be diffuse (smooth), linear, or nodular (51). Enhancement subsides as the infection temporally progresses from the acute to the healing phase.
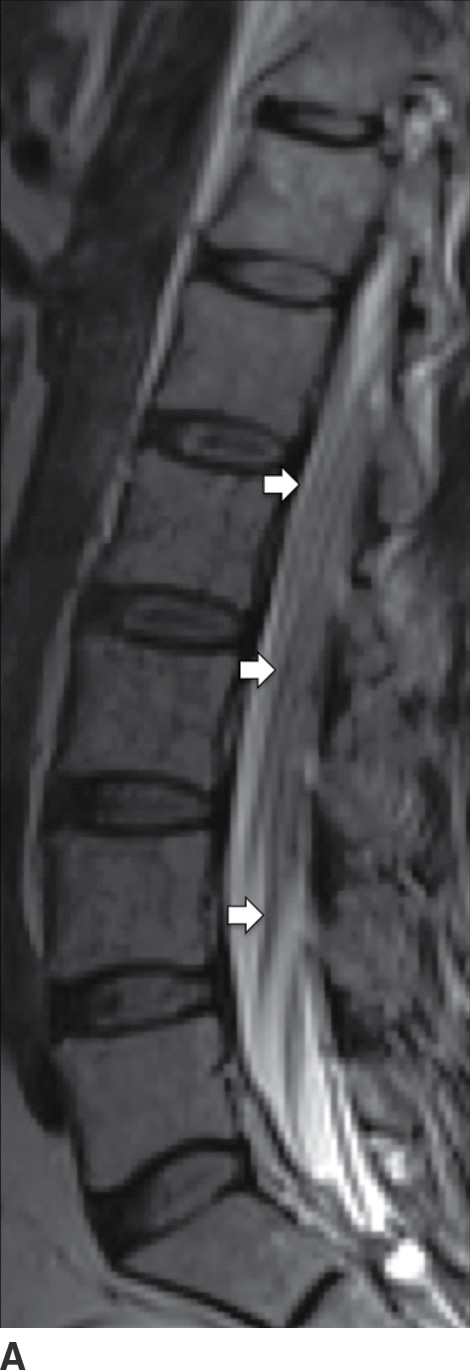
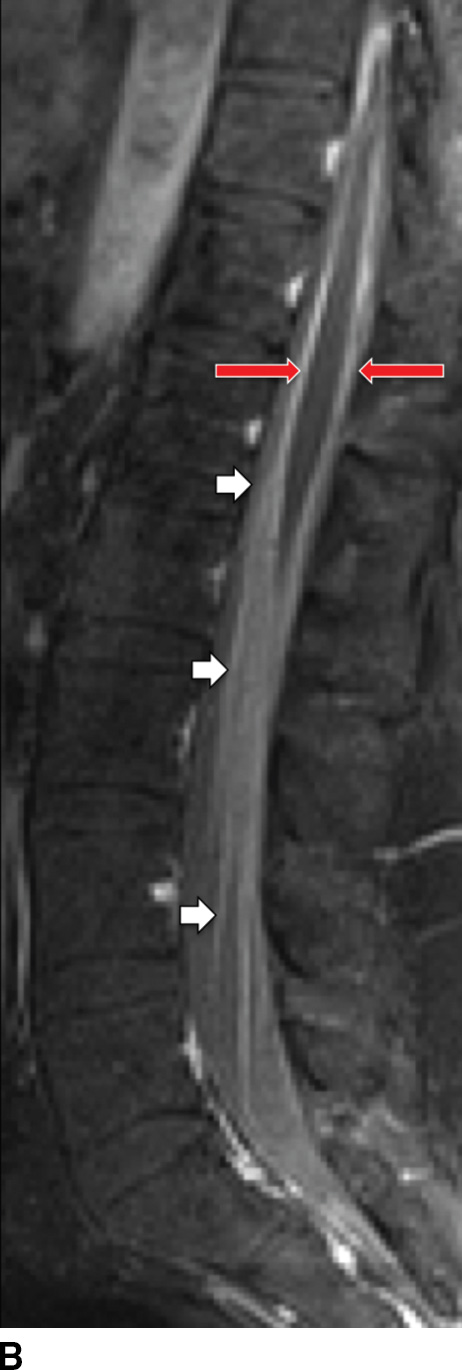
FIG. 14.11 Bacterial leptomeningitis. Sagittal T2-weighted (A) and postcontrast T1-weighted FS (B) MR images show diffuse, smooth thickening, and abnormal enhancement of the cauda equina (white arrows) and leptomeningeal enhancement of the lower thoracic cord (red arrow).
Tuberculous (TB) leptomeningitis is the most common cause of granulomatous meningitis, often occurring in conjunction with TB myelitis and radiculitis (52). The tuberculomas of TB meningitis tend to remodel and invade the cord, making delineation of the disease extent increasingly difficult. MR findings mimic those of pyogenic leptomeningitis (Fig. 14.12), sometimes showing tuberculomas along the inner surface of the dura (53).
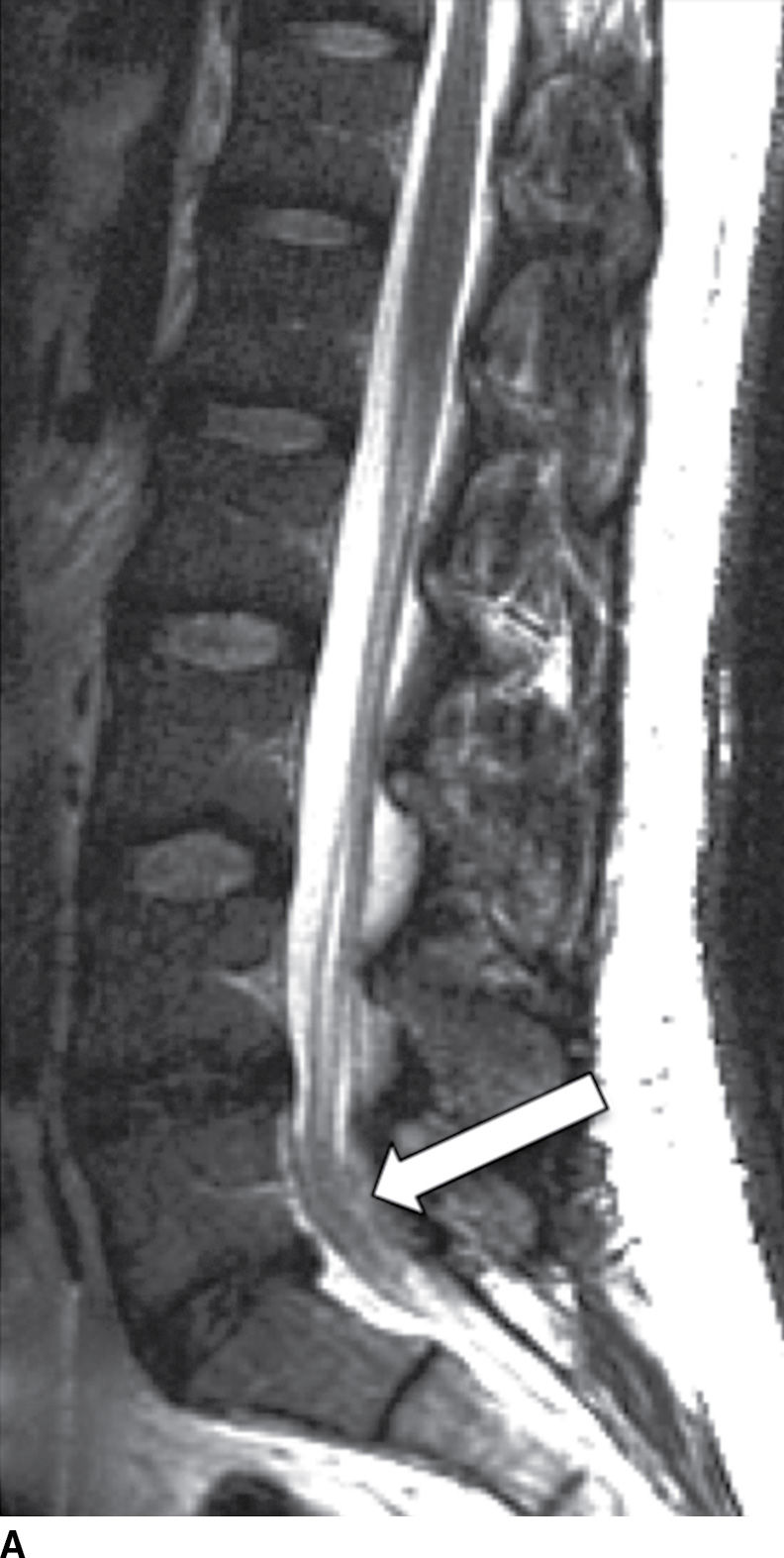
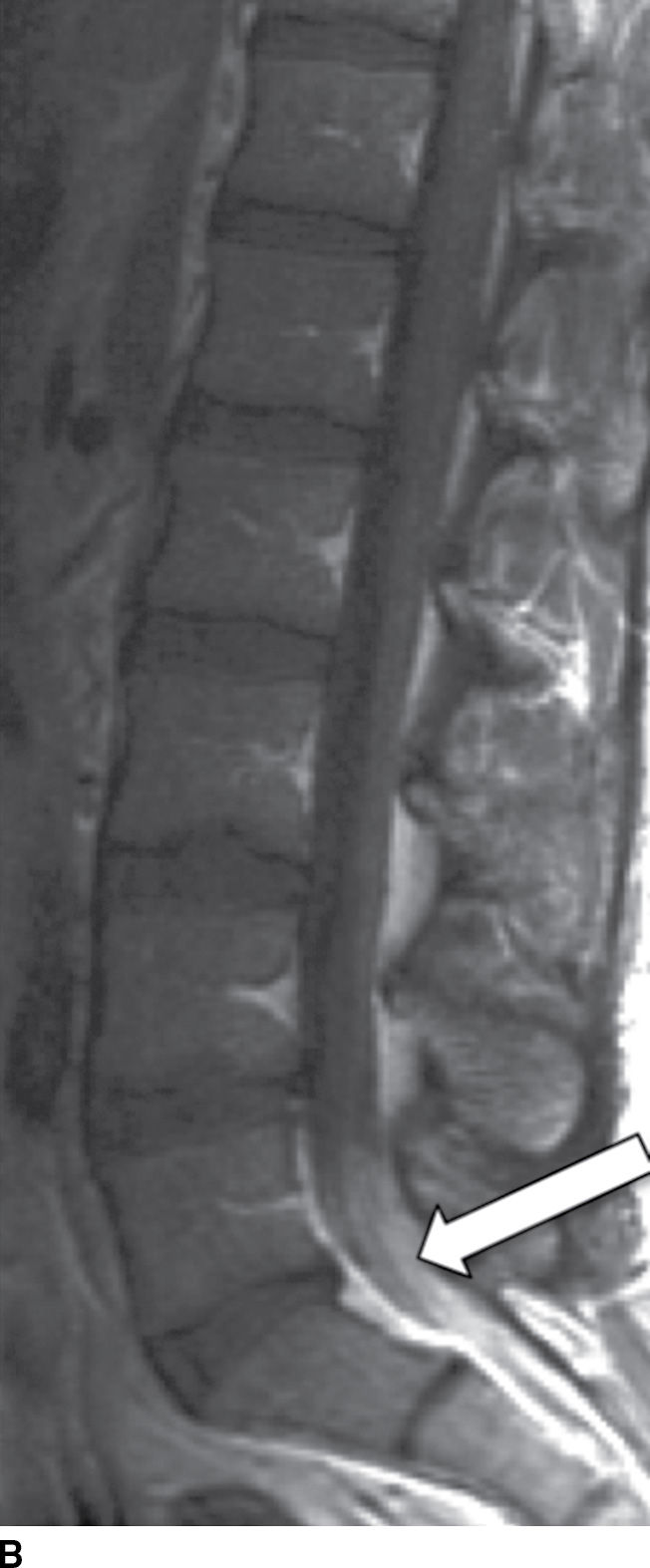
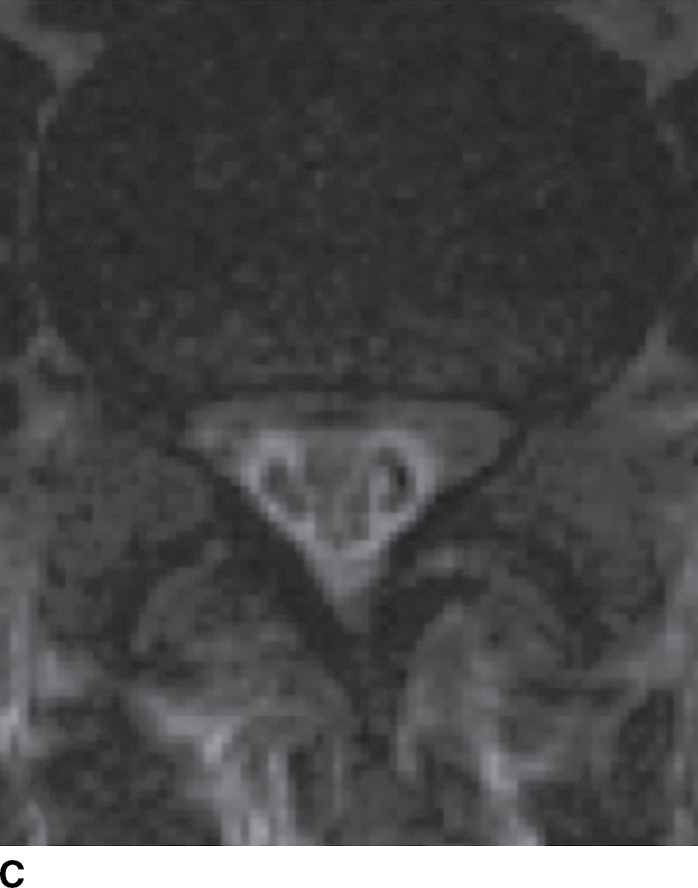
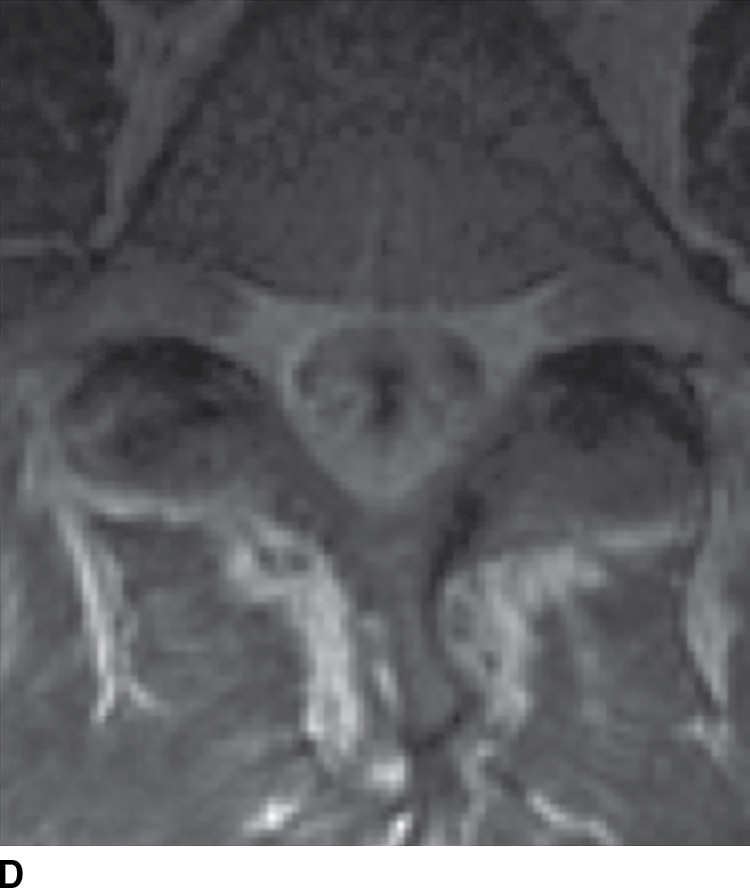
FIG. 14.12 TB leptomeningitis. Sagittal (A) and axial (C) T2-weighted and sagittal (B) and axial postcontrast T1-weighted (D) MR images show ill definition and abnormal enhancement of the cauda equina at the L5 to S1 level (arrows). Cerebrospinal fluid testing revealed TB as the causative agent. (Courtesy of Nafi Aygun, MD, The Johns Hopkins Hospital, Baltimore, MD.)
Brucellosis can also cause granulomatous meningitis, with spinal involvement occurring up to 30% of the time. Spondylitis and meningitis can occur simultaneously during the acute phase of this disease, potentially confounding the clinical presentation (54). The simultaneous infection may be related to inflammatory vasculitis that can occur in brucellosis, enabling the pathogen to enter the CSF space. MRI can show signs of spondylitis and arachnoiditis with variable cord–pial surface enhancement (54).
Spinal cryptococcosis, a fungal disease, is usually seen in patients with acquired immunodeficiency syndrome (AIDS), often manifesting with signs of meningeal irritation and scant MRI findings (minimal meningeal enhancement) (55). The pathogen causes infiltrating granulomas, which expand in both the extradural and intradural extramedullary spaces of the spine.
Leptomeningeal spinal cysticercosis shows many cystic lesions occupying the thecal sac, best demonstrated on T2W sequences. Rim enhancement of the cysts can also be present. Sheetlike homogeneous enhancement overlying the cord surface and the cauda equina can also be evident, indicating diffuse arachnoiditis (56).
Nonviral myelitis/spinal cord abscess
An infected spinal cord can progress to necrosis and intramedullary abscess. Nonviral spinal cord infection (myelitis) is a rare disease, which may start with initial venous infarction, with superimposed bacterial infection (57). Myelitis is most common in AIDS patients with bacterial fungal, viral, and parasitic causes as possibilities (58) (Fig. 14.13).
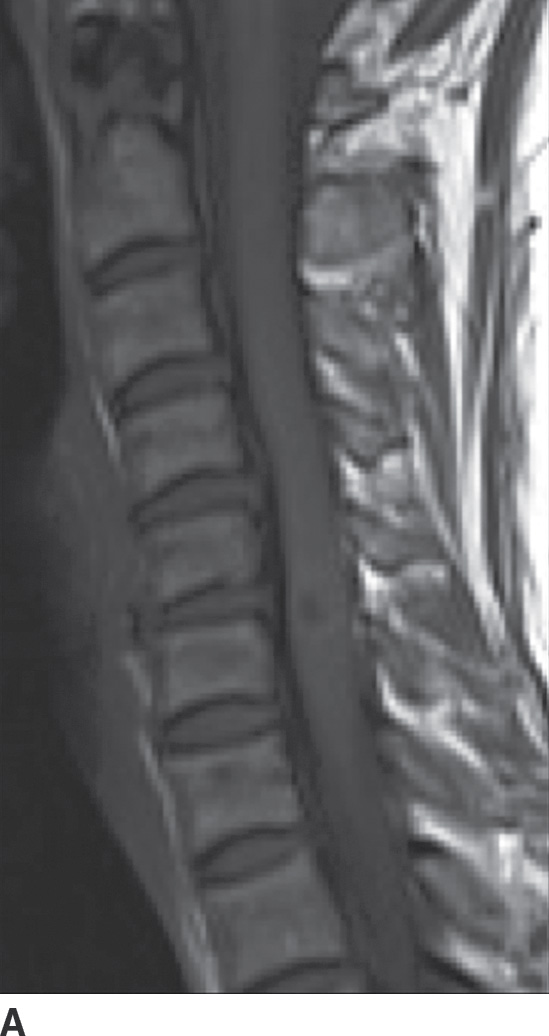
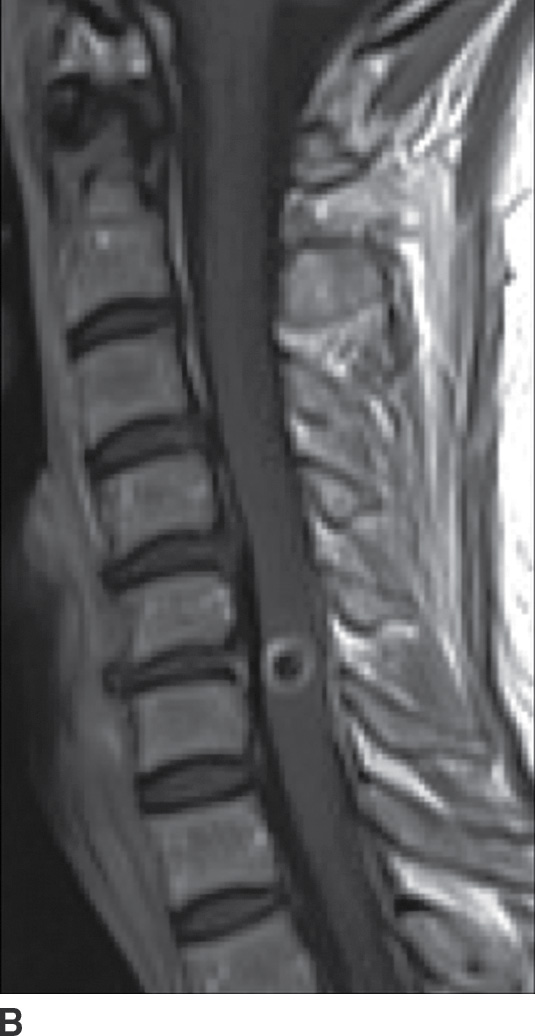
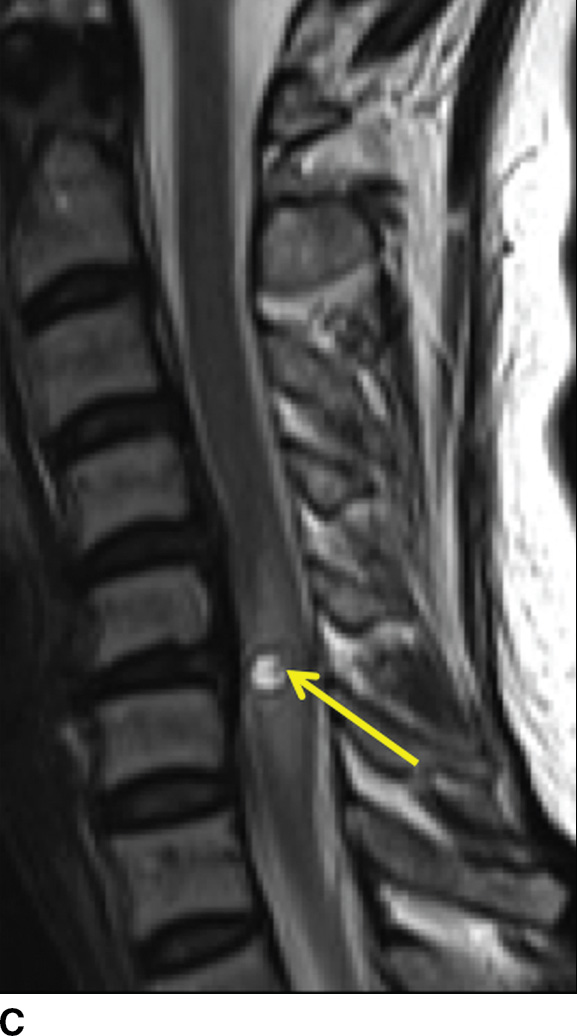
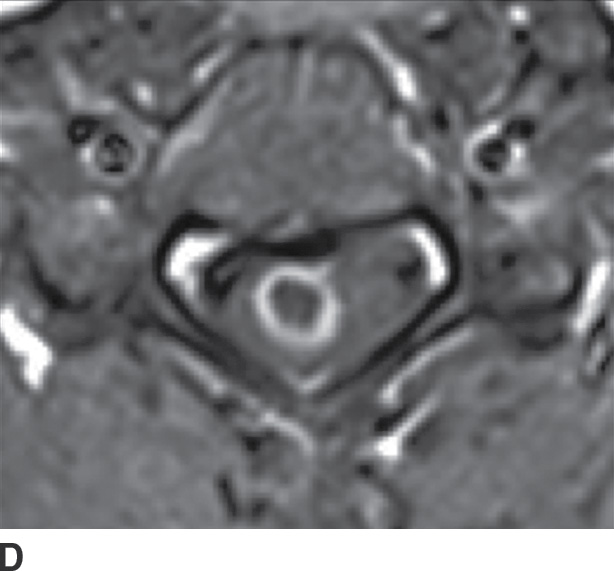
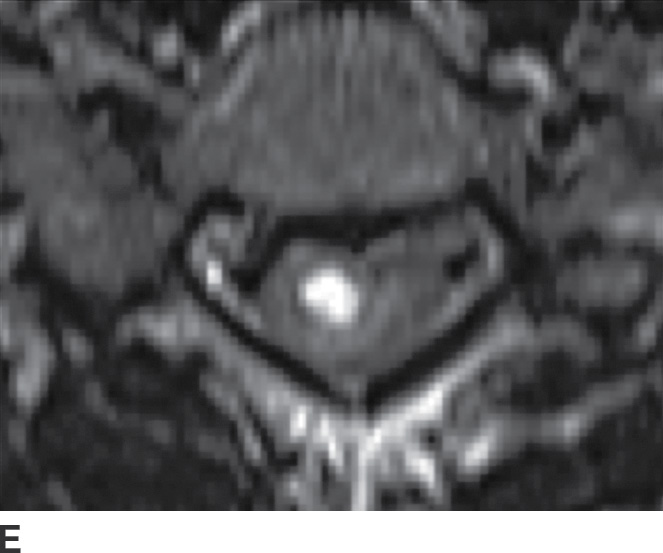
FIG. 14.13 Intramedullary neurocysticercosis. Sagittal T1-weighted (A), postcontrast T1-weighted (B), and T2-weighted (C) MR images show a rim-enhancing cystic lesion in the lower cervical spinal cord. Surrounding cord edema and expansion is present on T2-weighted imaging (C). The yellow arrow highlights a small T2-weighted hypointense structure, representing the scolex of the organism. Axial postcontrast T1-weighted (D) and T2-weighted (E) images show the lesion to be centered in the right paramedian cord gray matter region. Cerebral involvement was also present (not shown). (Courtesy of Nafi Aygun, MD, The Johns Hopkins Hospital, Baltimore, MD.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree