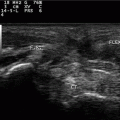
Fig. 12.1
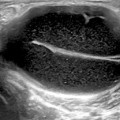
a Longitudinal scan of a normal temporal artery; 12.1b Longitudinal scan of a temporal artery from a patient with temporal arteritis. Notice the halo sign
US Technique to Examine the Two Branches of the Temporal Arteries
To perform temporal US, the patient lies supine with the head towards the opposite side of the artery that the sonographer examines. The sonographer can sit in front or behind the patient. Place the probe longitudinally in front of the ear. After detecting the common superficial temporal artery, the probe is moved continuously forward to the parietal branch. Then the probe is placed transversely to examine the parietal branch and the common superficial temporal artery in short axis. From the bifurcation, the frontal branch is followed longitudinally and transversely [4, 16] .
The sonographer needs to avoid putting too much pressure, to avoid compressing the temporal arteries, and more gel should be applied in areas with hair. The frequency of the linear probe should be as high as possible, preferably 15 MHz or higher. Adjust the color gain, because if it is too low, only the center of the lumen will show color signals leaving an anechoic rim between the lumen and the wall, mimicking the pathological halo sign; if the color gain is too high, the inflamed area may be covered and disease may be missed. The pulse repletion frequency should be set at about 2.5 kHz. The sonographer uses CD mode and angles the box so that the blood flow is not completely parallel to the probe. Color should cover the artery lumen completely, but should not continue over the artery wall. Both sides should be examined. Temporal arteries should be investigated as completely as possible [17].
The facial arteries are mainly involved in patients with jaw claudication. In patients presenting with retro-auricular pain, consider to examine the occipital arteries, which are exclusively involved in some patients [18].
As mentioned before, in this pathological condition, stenosis of short segments tend to occur. The CD shows a blurring mixture of colors (aliasing) together with persistent blood flow in the diastole. Pulsed-wave Doppler then displays at least a twofold increase in maximum systolic blood-flow velocity in the stenosis, compared with the blood-flow velocity in the area before or behind the stenosis [17] .
Many studies have been performed comparing temporal artery US with histology and clinical diagnosis of temporal arteritis. Three meta-analyses have been published [15, 19, 20]. The sensitivity of temporal artery duplex US was 87 % with regard to clinical diagnosis, and specificity was 96 % in one of the meta-analyses [20]. As the halo of the temporal arteries disappears quickly and recurs only in severe flares, routine US examinations are not necessary. Despite this, biopsy of the temporal artery is still considered the gold standard in the diagnosis of GCA [21] .
Large-Vessel Giant-Cell Arteritis
Patients with LV-GCA may present as the aforementioned classical cranial temporal arteritis, pure PMR, intermittent arm claudication, paresthesias and Raynaud’s phenomenon or pyrexia of unknown origin [3, 4] . Extracranial arterial involvement is much more common than previously assumed. US facilitated the examination of extracranial arteries, such as the femoral and popliteal arteries, the aorta as well as the proximal arm arteries. Clinically, in addition to auscultation of the axillary region, peripheral pulses should be palpated and blood pressure measured bilaterally; if the pulses are not palpable, the arteries of both arms and legs should be examined.
When comparing patients with classical cranial temporal arteritis with patients with proximal arm artery involvement in LV-GCA, the first patients’ cohort tend to be older (72 vs. 66 years), and there are lesser females (66 vs. 83 %). Time between onset of symptoms and diagnosis was longer in LV-GCA (7 vs. 2 months) [3]. Patients with LV-GCA less commonly develop anterior ischemic optic neuropathy [10, 22], more frequently complain of PMR, and often lack typical cranial symptoms [23]. Critical limb ischemia does not usually occur [22]. The axillary arteries are more commonly involved than the subclavian and brachial arteries [4]. Bilateral involvement is usually present [4, 24]. In contrast with GCA, in LV-GCA, the wall swelling remains much longer and the dark appearance of the wall becomes more echogenic because of less edema and increasing fibrosis [4] (Table 12.1).
Table 12.1
Comparison of patients with extracranial and cranial GCA. (Results from 110 patients with established diagnosis of GCA. Adapted from Czihal et al. 2012) [23]
Variable | Extracranial GCA (n = 59) | Cranial GCA (n = 51) |
|---|---|---|
Age (years) | 65 ± 7.6 | 73.7 ± 7.0 |
Time to diagnosis | 28.7 ± 25.1 | 6.5 ± 6.5 |
Cranial symptoms | 32 (54.2) | 51 (100) |
Headache | 19 (32.2) | 40 (78.4) |
Jaw claudication | 12 (20.3) | 35 (68.6) |
Persistent visual impairment | 3 (5.1) | 27 (52.9) |
Polymyalgia rheumatic | 29 (49.2) | 14 (27.5) |
Constitutional symptoms | 40 (67.8) | 21(42) |
Temporal artery abnormality | 9 (15.3) | 35 (68.6) |
US in LV-GCA
About 50 % of newly diagnosed GCA patients have axillary artery involvement [4, 25]. Only 60 % of the patients with LV-GCA have temporal artery involvement .
To examine the axillary arteries, place the probe longitudinally in the axilla along the humeral head and neck, like the scan for detecting glenohumeral joint effusions [16, 26]. The axillary artery is located at the level of the humerus or 1–2 cm medial to it, and proximal to the circumflexa humeri artery. The color box needs to be steered to avoid a blood flow perpendicular to the sound waves. Like evaluating the temporal artery wall, the grayscale image probe and vessel should be as parallel as possible. A normal vessel shows an intima–media complex of less than 1 mm. A bright line represents the interface between artery lumen and vessel wall, followed by a dark line representing wall tissue, and another bright line representing the interface between media and adventitia (Fig. 12.2).
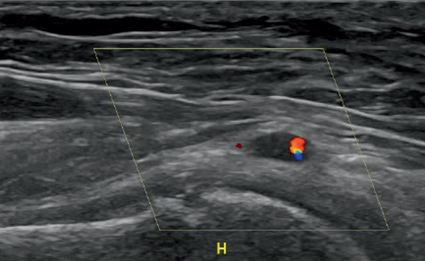

Fig. 12.2
Color Doppler image of the axillary artery of a woman aged 66 years, with temporal arteritis and no palpable radial pulse in the right arm
In the large-vessel vasculitis, the artery wall is thickened, usually more than 1 mm. A homogeneous wall swelling, preferably circumferential, of 1.5 mm or more is pathognomonic for the diagnosis of axillary vasculitis [3]. The edematous wall swelling, hypoechoic in untreated patients, frequently persists at follow-up and becomes brighter with treatment, because of fibrosis.
Other arteries like subclavian, common carotid and vertebral arteries can also be examined by US. However, these arteries are rarely affected in GCA, without the involvement of either the temporal or the axillary arteries .
Takayasu’s Arteritis
In contrast to LV-GCA patients in which the age of onset is usually more than 50 years, Takayasu’s arteritis usually occurs before the age of 40 years. Arteries involved include, the subclavian arteries which are the most commonly involved (93 %), followed by the aorta (65 %), and the common carotid arteries (58 %) [17]. Aortitis results in markedly increased risk for developing thoracic aortic aneurysms and dissections [27]. Characteristically, temporal arteries are never affected in Takayasu’s arteritis. Though the arterial involvement pattern may be similar for both LV-GCA and Takayasu’s arteritis, in this last one, arteritis affection is usually less symmetrical, affecting more frequently left subclavian and common carotid arteries and less frequently the axillary arteries [24].
Ultrasound in Takayasu’s Arteritis
In Takayasu’s arteritis, US can be very useful in early diagnosis, to monitor disease progression and the effect of therapy . In general, the US findings in Takayasu’s arteritis are similar to those in GCA. CD assessment reveals hypoechoic, concentric wall thickening, brighter than in temporal arteritis, sometimes termed “macaroni sign”. The reason for this difference in echogenic pattern lies, probably, in the more chronic course of Takayasu’s arteritis and less wall edema [14]. In suspected cases, it is advisable to examine the carotid, subclavian, vertebral arteries and abdominal aorta. The renal arteries should also be examined in case of arterial hypertension. Vasculitis can be easily differentiated from arteriosclerotic lesions, which are heterogeneous and irregular with calcifications.
However, US and angiography are considered complementary in the evaluation of Takayasu’s arteritis patients and, unfortunately, in most cases, the diagnosis of Takayasu’s arteritis is not established before stenosis occurs .
Isolated Aortitis
This vasculitis is listed as an SOV in the new Chapel Hill nomenclature [2]. Clinically, many patients present with pyrexia of unknown origin. If US findings are not clear, other imaging techniques can be performed like MRI, MR angiography (MRA), positron emission tomography (PET), computerized tomography angiography (CTA), or thoracic and abdominal CT scan .
Polymyalgia Rheumatica
PMR is a common inflammatory condition involving the shoulders, neck and pelvic girdle, of elderly people . The etiology of PMR is currently unknown although genetic and environmental factors are thought to be contributing factors [28]. Symptoms may also involve the proximal upper and lower extremities. Pain usually limits active and passive range of motion, and the stiffness is marked in the morning, causing patients difficulty in getting out of bed and performing activities of daily living, such as washing and dressing. Pain often interferes with sleep at night and results in significant daytime fatigue. The shoulders are more often involved than the hips, and symptoms may be unilateral early but tend to become bilateral and symmetrical. Distal musculoskeletal manifestations, which are present in up to 50 % of PMR patients, may include carpal tunnel syndrome, swelling of hands and feet with pitting edema and nonerosive peripheral arthritis. About 40 % of these patients can also experience weight loss, fatigue, and low-grade fever.
PMR shares the same pattern of age and sex distribution as GCA. The diagnosis is usually based in clinical and laboratory findings. Synovitis, vasculitis, and bursitis have all been demonstrated in PMR [29], and some authors say that PMR and GCA are different facets of the same disease. About 40–60 % of patients with GCA have PMR symptoms, and about 9–20 % of patients with PMR also have GCA. However, patients with PMR associated with GCA often have more severe disease, with significant abnormalities in most laboratory variables, including higher ESR and platelet counts, and lower hemoglobin values, than patients with isolated PMR. Patients with PMR should be carefully interviewed and examined to detect features suggestive of GCA [5].
Radiographs of the affected joints in PMR are often normal and are only useful in excluding other conditions like degenerative or inflammatory joint diseases or crystal arthropathies. The presence of bony erosions on radiographs is more consistent with rheumatoid arthritis (RA) [30] .
US in Polymyalgia Rheumatica
Shoulder and hip US increase the specificity for diagnosing PMR ; therefore, musculoskeletal US has been incorporated into the new European League against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria [31]. Based on US and MRI findings, PMR predominantly affects periarticular structures. The findings of bilateral subacromial/subdeltoid bursitis (Fig. 12.3), tendinosis of the long head of the biceps (Fig. 12.4), and bilateral trochanteric bursitis are particularly consistent. Glenohumeral and hip joint effusions and synovitis can also be seen, but are less specific [32]. Entheseal involvement and effusions of the knees, elbows, and radiocarpal joints also have been described [14]. An intra- and inter-reader exercise, performed as a basis for the ACR/EULAR PMR classification criteria study, showed 87 % overall agreement .
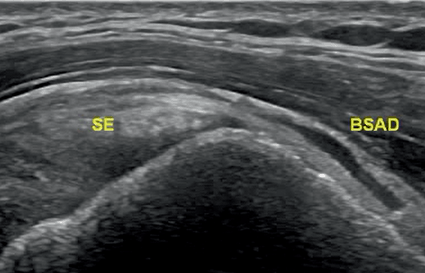
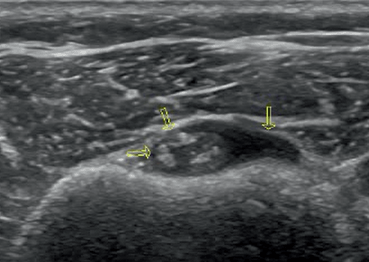

Fig. 12.3
Longitudinal scan of the right shoulder showing a subacromial/subdeltoid bursitis (BSAD) in a patient with PMR. SE—supraspinatus tendon

Fig. 12.4
Transverse scan of the long head of the biceps tendon showing a tenosynovitis in a patient with PMR
A score of 4 or more is categorized as PMR in the algorithm without US and a score of 5 or more is categorized as PMR in the algorithm with US (Table 12.2). In the EULAR/ACR classification study, US had high specificity in discriminating PMR from other shoulder conditions (89 %), but not in discriminating PMR from RA (70 %) [31] .
Table 12.2
PMR classification criteria scoring algorithm-required criteria: age ≥ 50 years; bilateral shoulder aching; abnormal CRP and/or ESR. (Adapted from 2012 provisional classification criteria for polymyalgia rheumatic: a EULAR/ACR collaborative initiative) [31]
Points without US (0–6) | Points with US (0–8) | |
|---|---|---|
Morning stiffness > 45 min | 2 | 2 |
Hip pain or limited range of motion | 1 | 1 |
Absence of RF or ACPA | 2 | 2 |
Absence of other joint involvement | 1 | 1 |
If at least one shoulder with subdeltoid bursitis and/or biceps tenosynovitis and/or glenohumeral synovitis (posterior or axillary) and at least one hip with synovitis or trochanteric synovitis | 1 | |
Both shoulders with subdeltoid bursitis, biceps tenosynovitis or glenohumeral synovitis | 1 |
The clinical manifestations of GCA and PMR respond usually within 12–48 h of starting corticosteroid treatment. The initial dose for GCA is oral prednisolone or equivalent of 40–60 mg/day, and for patients with PMR a dosage of 15–20 mg/day is usually sufficient. A prolonged monitoring is necessary, and corticosteroids are gradually tapered, guided by regular clinical evaluation and ESR and/or CRP measurement. In PMR, the initial dose is usually maintained for 2–4 weeks. Subsequently, the dose is decreased by 2.5 mg every 2–4 weeks until the patient is at 10 mg daily; following this, the dose is tapered by 1 mg a month [33, 34]. Methotrexate may be useful as corticosteroid-sparing agent in GCA and PMR in patients with corticosteroid-related toxicity or patients with frequent disease relapses [32]. Retrospective studies favor aspirin (acetylsalicylic acid) as an effective adjuvant treatment for reducing the ischemic complications of GCA [5]. Recent anecdotal reports suggest interleukin (IL)-6 may be a helpful therapeutic target in PMR and large-vessel vasculitis, but additional studies are needed [32] .
Inflammatory Myopathies
MRI and US are regarded as the tools of choice for imaging muscle trauma and disease, bearing in mind that each one has its own specific advantages . MRI is considered the gold standard for muscle diseases imaging. On the other hand, the morphology of normal muscles can be clearly depicted on US, a technique with a superior accessibility and ability to obtain real-time dynamic images.
Sono-anatomy studies revealed that individual muscle fibers are surrounded by a thin fascial layer called endomysium (which cannot be seen with US). The fibers are packed together in bundles (fascicles) which are surrounded by perimysium, and the fascicles are packed together to form muscles, which in turn are surrounded by a thicker epimysium. Muscle fascicles appear relatively hypoechoic compared to the hyperechoic encasing linear septations and sheath. In longitudinal scanning, these hypoechoic fascicles are separated by multiple hyperechoic long lines which represent perimysium or fibroadipose septa. This is called pennate appearance. On short-axis images, muscle septations appear as white dots on a hypoechoic background (Figs. 12.5, 12.6). Within the muscle itself, the muscle fibres attach to a fibrous aponeurosis that eventually becomes a tendon [35].
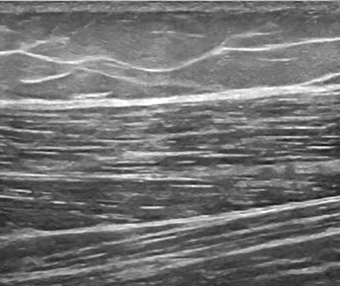
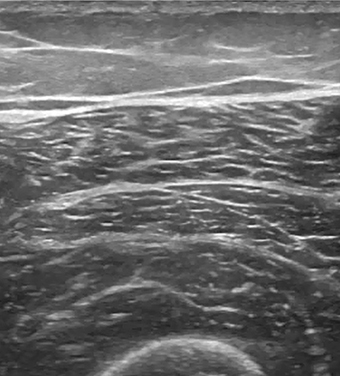

Fig. 12.5
Longitudinal scan of the muscles of the thigh showing the normal pennate appearance

Fig. 12.6
Transverse scan of the normal muscles of the thigh
Muscle tissue inflammation can arise secondary to infection, trauma, vasculitis or autoimmune diseases, including RA, lupus, syndromes, and scleroderma. Polymyositis, dermatomyositis, and inclusion-body myositis are the major entities of a group of skeletal muscle diseases called idiopathic inflammatory myopathies .
MRI permits the identification of muscle edema in fat-suppressed T2-weighted MRI, and allows a detailed examination of a large anatomic area, which is very useful as symptoms are usually difficult to localize to one muscle group. In T1-weighted MRI images, fatty atrophy of the musculature can be seen reflecting the chronic phase of the disease [36]. By using MRI, it is possible to identify the best area to do a muscle biopsy.
US in Inflammatory Myopathies
In rheumatology, US has been mainly used for inflammatory muscle conditions . Before performing a US muscle examination, a clinical history and physical examination are essential. Linear transducers or curved-array transducers (which are preferable especially for obese or very muscular patients, particularly to examine gluteal region or the proximal thigh) can be used. With this technique, it is possible and preferable to do a dynamic and real-time comparison of the contralateral region. To demonstrate a pathology, it is useful to use, if possible, a panoramic or extended view. In early acute stage of myositis, the muscle belly becomes edematous, resulting in diffuse enlargement and relative decrease in overall echogenicity; therefore, US features of acute myositis include normal or increased muscle size, lower echogenicity and enhanced perfusion of the affected muscles [37] (Fig. 12.7). In chronic disease stage, muscle size and perfusion are reduced (Fig. 12.8). The increased Doppler activity within the muscle may be more sensitive to change than grayscale and have a predictive potential [35]. A study based on contrast-enhanced US scanning showed significantly higher blood-flow velocity, blood flow, and blood volume in patients with acute myositis than in normal volunteers [38]. Calcifications can easily be detected because of high echo intensity and acoustic shadowing on US imaging. Being widely available and cheap, muscular US became a useful tool for studying and monitoring patients with myositis. Both MRI and US are also able to identify fluid collections, adipose infiltration, atrophy, and fibrosis.
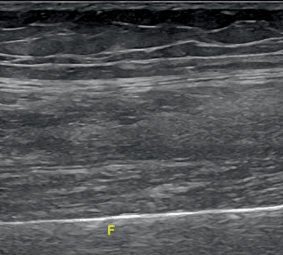
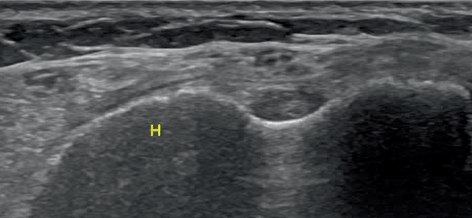

Fig. 12.7
Longitudinal scan of the muscles of the thigh in a patient with acute myositis F femoral bone

Fig. 12.8
Transverse scan of the upper part of the shoulder from a patient with chronic evolution of an inflammatory myopathy. Notice the abnormal deltoid pattern and echogenicity, H humeral bone
Ultrasonic elastography (sonoelastography) measures tissue deformation as a response to an external force, assuming that the deformation is lower in rigid tissues, compared to elastic soft tissues. This method is based on comparing the radiofrequency of ultrasonic waves obtained before and after an easy made compression with a conventional transducer, using a free hand technique. As muscular elasticity is affected, hence modified in myopathies, sonoelastography became a useful method for its assessment. A study involving 24 patients with muscular pathology, namely polymyositis, dermatomyositis, mixed connective tissue disease, systemic lupus erythematosus (SLE) and RA myopathy, analyzed the utility of US in the assessment of inflammatory myopathies, and of sonoelastography in the assessment of the elasticity of skeletal muscle in myositis. Despite all the limitations, the authors concluded that analysis of the color information from elastography could be a reliable method for the management of patients with idiopathic inflammatory myopathies [39] .
Fibromyalgia
FM is a common form of noninflammatory rheumatism , with symptoms often mimicking those of arthritis or muscle disorders, and a widespread pain of idiopathic origin. Because of its very wide range of symptoms, including fatigue, headache, irritable bowel syndrome, paresthesias, Raynaud’s syndrome, muscle weakness, bladder dysfunction, sleep disorders, decreased cognitive functioning, depression, and anxiety, it can be confused with other rheumatic and non-rheumatic diseases.
Its prevalence increases with age and affects women more than men. According to the 1990 ACR diagnostic criteria, the diagnosis of FM requires the presence of chronic widespread pain and tenderness in at least 11 out of 18 tender points (TP) when applying a pressure of 5 kg. However, in 2010, a new set of criteria has been proposed by the ACR that does not require a TP examination but includes a subjective measure of the number of painful body regions and a somatic symptom severity scale .
Ultrasound in Fibromyalgia
Arthralgic symptoms in the hip region are commonly mentioned by FM patients. A study about sonographic assessment of the hip in FM patients failed to demonstrate significant pathological US abnormalities in the majority of the patients [40] .
A study used sono-myography (SM) and sonomyoelastography (SME) to analyse morphology, stiffness and blood flow in the TP of a group of FM patients, in comparison to a healthy control group. There was no significant difference in stiffness, nor number of localized hypoechoic areas at TP. Similarly, there was no significant difference between FM women and healthy female controls using SME. Furthermore, local blood-flow assessment did not appear to reveal any differences between both the patients and control groups [41].
In another study, which included ten FM patients as well as ten control subjects, using contrast-enhanced US, results revealed reduced muscular vascularization in FM patients when compared with controls [42] .
Regional Pain Syndrome
Regional pain is one of the most common complaints encountered in the rheumatology clinic. Various conditions manifest as regional pain, including ligamentous and tendinous structure abnormalities, nerve entrapments, and tumors, muscles and bone pathologies. Clinical examination may be sufficient for the diagnosis; however, imaging modalities are useful in unclear cases. Reproducing patient’s symptoms by movements or palpation while scanning (sonopalpation), make ultrasonography one of the most useful imaging modalities for differential diagnosis of regional pain [43].
Some of the conditions leading to regional pain were described in detail in other parts of this book. The aim of this section is to help understand the concept of scanning in regional pain syndromes and present less common or less familiar conditions which may manifest as regional pain.
In general, clinical examination should always precede US scanning. Differential diagnosis of potential causes of pain should then be systematically revised during US examination, starting with most common pathologies and easy-to-assess structures, followed by less common causes of pain and more profound assessment. In the majority of cases, pathological changes result in decreased echogenicity, thickening of involved structures, and/or irregular outlines (enthesitis, tendinopathy, stenosing tenosynovitis, nerve entrapment; Figs. 12.9, 12.10).
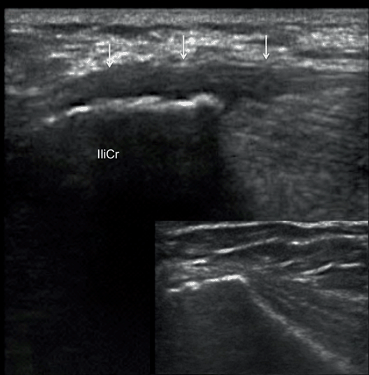
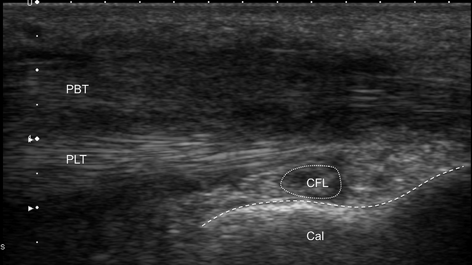

Fig. 12.9
Longitudinal scan of the iliac crest (IliCr) of the patient with spondyloarthopathy and left iliac crest pain. Thickening and decreased echogenicity of the fascia insertion (arrows), and irregular outline of the iliac crest reflect enthesitis. The lesion is clearly distinguishable by its low echogenicity in comparison with the normal isoechoic entheses in adjacent areas (inset)

Fig. 12.10
Longitudinal scan of the peroneus tendons below the lateral malleolus. The patient was complaining of pain behind the lateral ankle exacerbated by activities. Ultrasound examination shows normal structure of peroneus longus tendon (PLT) and hypoechoic, blurred structure of the peroneus brevis tendon (PBT). Diagnosis: peroneus brevis tendonitis. CFL calcaneofibular ligament, Cal calcaneus
Detection of focal lesions, compressible (i.e. bursae) or not (benign and malignant neoplasms, or ganglion cysts), usually does not present difficulties. In smaller lesions, a comparison to the contralateral side may be necessary. Sonopalpation helps to correlate the ultrasound findings to the patient’s symptoms. This is confirmed on direct compression of the localized lesion which would elicit the anticipated type of pain. Dynamic examination may reveal abnormalities not apparent on static images, like adhesions following previous inflammation, ligamentous laxity, tendon subluxation, entrapment, impingement, and snapping syndromes (Figs. 12.11, 12.12).
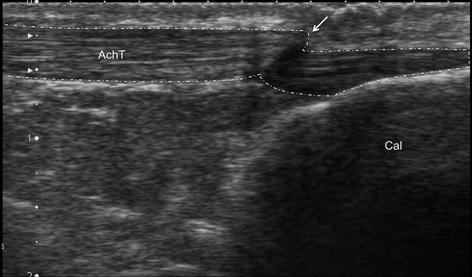
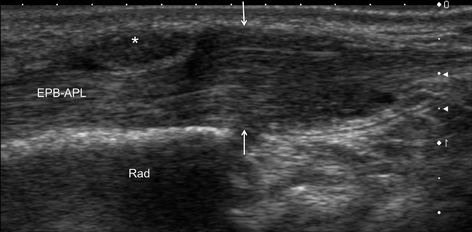

Fig. 12.11
Longitudinal scan of Achilles tendon (AchT) of patient complaining of chronic Achilles tendon pain. Thickness and echostructure of Achilles do not showed abnormality. On plantar flexion, apparent folding of Achilles (arrow) revealing adhesions within retrocalcaneal bursa as the result of previous bursitis, Cal calcaneus

Fig. 12.12
Longitudinal scan on the radial side of the wrist of patient with de Quervain’s disease. Thickening of the retinaculum (*) causing entrapment of the tendons. During passive abduction of the thumb, ultrasound revealed crowding of the tendons before the retinaculum (arrows). The patient was not able to actively abduct the thumb because of tight stenosis of the first extensor’s compartment. EPB–APL extensor pollicis brevis and abductor pollicis longus tendons, Rad radius
Unexpected findings may be occasionally seen, confirming the value of US as the rheumatology bedside procedure (Figs. 12.13, 12.14).
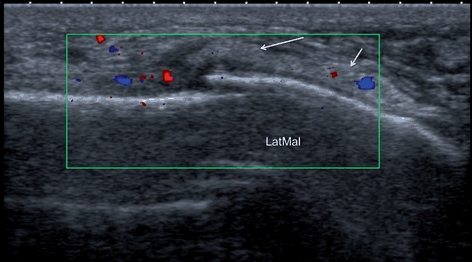
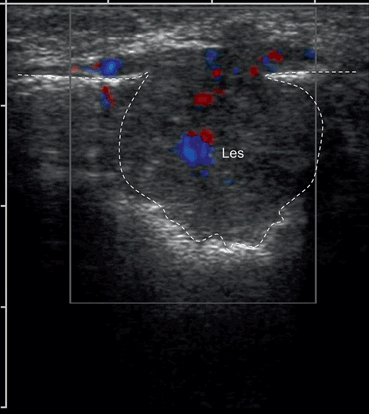

Fig. 12.13
Longitudinal scan of the lateral ankle of the patient with rheumatoid arthritis, who complained of ankle pain on weight bearing, ankle swelling, and restricted range of movements. The pain did not respond to blind steroid injection to tibiotalar joint. Ultrasound scan showed fractures of lateral (LatMal) and medial malleolus. Cortical discontinuity, thickening of periosteum (arrows), swelling of subcuteneous tissue, and hypervascularity

Fig. 12.14
Ultrasound scan of the patella of patient complaining of the knee pain on movements. Ultrasound showed the destruction of the patella by the pathological lesion (Les). Patient presented also with small subcutaneous nodules, hyperechoic on ultrasound scan. Biopsy confirmed the diagnosis of angiosarcoma
Retinacula
Normal tendon retinacula appear as fibrous bands surrounding the tendons and attach to the underlying bone [44] . Normal thickness of retinacula of the ankle ranges between 0.9 and 1.5 mm [45]. Chronic overuse in the presence of additional intrinsic factors (i.e., diabetes) may lead to degeneration and thickening of the retinaculum, restraining the tendons’ sliding (retinaculum impingement) [46]. Morphologically, thickening of the retinaculum was found to be the result of fibrosis, hyaline degeneration and vascular proliferation [47]. Thickening of the tendon sheath and fibrosis of the tendon are commonly associated findings [48]. Stenosing tenosynovitis of finger flexors and de Quervain’s disease are common examples of retinaculum impingement (Figs. 12.12, 12.15) [49].
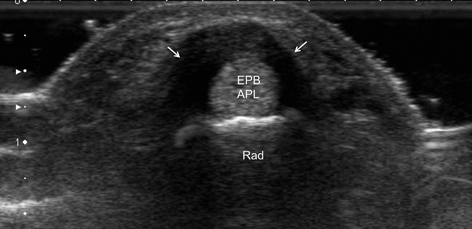

Fig. 12.15
Transverse scan of the radial side of the wrist. Patient presented with pain and swelling on the radial side of the wrist exacerbated by adduction of the thumb. Thickening of the retinaculum (arrows) over first extensors compartment (extensor pollicis brevis—EPB, abductor pollicis longus—APL) in keeping with the diagnosis of de Quervain’s disease. Rad radial bone
Muscle Anatomic Variants
Muscle anatomic variants may appear as swelling and cause pain or discomfort during activities . Accessory muscles can be easily mistaken with bursitis, tenosynovitis, tendonitis or neoplasm, and may be a cause of nerve compression [50].
reversed, digastric or duplicated palmaris longus (prevalence 7 %)—may be confused with flexor tenosynovitis and may cause median nerve compression
Extensor digitorum brevis manus (2–3 %)—may be confused with extensor tenosynovitis (Fig. 12.16)
Anconeus epitrochlearis (11 %)—may cause ulnar nerve entrapment in cubital tunnel
Accessory abductor digiti minimi (24 %)—may cause ulnar nerve compression in Guyon’s canal
Accessory soleus muscle (0.7–5.5 %)—may cause localized compartment syndrome and be confused with soft tissue tumor
Flexor digitorum accessorius longus (6–8 %)—may cause tarsal tunnel syndrome
Peroneus quartus (13–26 %)—may cause ankle pain and instability
If not anticipated, additional muscles may cause confusion during US examination (Fig. 12.16.). Awareness of such anatomical variation type helps to avoid unnecessary therapeutic actions .
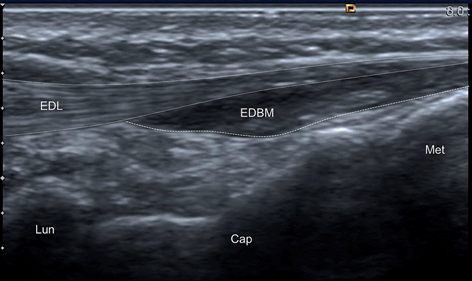

Fig. 12.16
Longitudinal scan of the wrist. Patient presented with swelling on the dorsum of the hand and pain on activities. Ultrasound scan shows hypoechoic, poorly compressible area adjacent to the extensor tendons. Extensor digitorum brevis manus (EDBM) may be easily confused with tenosynovitis of extensor digitorum longus (EDL). Lun lunate, Cap capitate, Met metacarpal bones
Nerve Entrapments
Entrapment neuropathies typically occur within osteofibrous tunnels. Sonographically, swelling and decrease echogenicity of the nerve, blurring of internal structure, thickening and increased echogenicity of perineurium may be seen proximally and distally to the site of compression [54] . Simple principle may be accommodated while diagnosing entrapment neuropathies: swollen, easy to spot and homogenously hypoechoic nerve often indicate nerve pathology (Fig. 12.17).
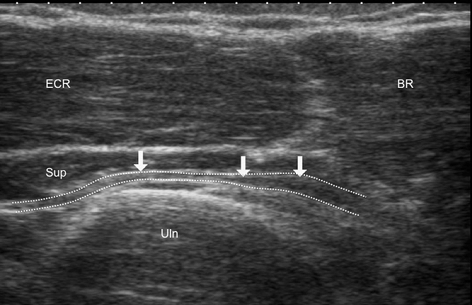

Fig. 12.17
Oblique scan of upper lateral forearm. Patient complained of pain at and below the lateral epicondyle exacerbated by activity. Ultrasound scan shows swelling and decreased echogenicity of deep branch of the radial nerve (thick arrows) proximally to its compression within supinator (Sup) muscle. ECR extensor carpi radialis, BR brachoradialis muscle, Uln ulna
Nerve Tumors
On US, nerve tumors usually present a fusiform shape with slightly tapered ends, oriented longitudinally in the nerve axis. Neurofibromas develop centrally, whereas schwannomas are located eccentrically in relation to the nerve (Fig. 12.18) . Most lesions appear as hypoechoic masses and have well-defined margins. Schwannomas commonly are heterogeneous tumors, well vascularized, and may contain internal cystic changes [54].
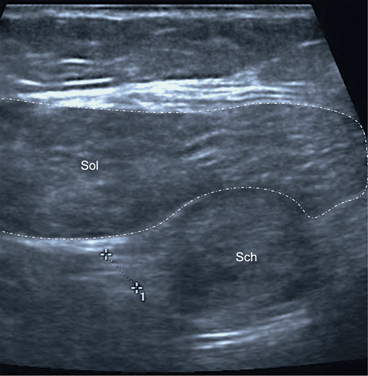

Fig. 12.18
Transverse scan of the calf in its distal third. Patient with anxiety and fibromyalgia presented with pain and paresthesia of the right lower leg. Ultrasound scan at the point of greatest tenderness showed ovoid mass adjacent to the tibial nerve (measure), located below the soleus muscle (Sol). MRI scan confirmed the diagnosis of schwannoma (Sch). MRI magnetic resonance imaging
Neuromas are commonly formed following nerve injury as the result of the reparative process and fibrosis (Fig. 12.19




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree