Fig. 1
Overall survival (Kaplan–Meier method) of the 185 included patients; the 6 month, 1- and 2-year overall survival were 60, 46 and 23 %, respectively (Zanzonico and Divgi 2008) (figure reproduced with permission from the original publisher Elsevier)
5.4 Patient-Specific Radiation Dosimetry for Radionuclide Therapy of Liver Tumours with Intra-Hepatic Artery Rhenium-188 Lipiodol
As a part of the IAEA’s multicentre study, a practical and clinician friendly algorithm has been developed by Zanzonico and Divgi (2008) for planning treatment of liver cancer by hepatic artery administration of Rhenium-188-labelled Lipiodol. This algorithm is based on the “maximum tolerated-activity” paradigm for radionuclide therapy. As per the protocol, a small “scout” activity of Rhenium-188-labelled Lipiodol is administered to the patient prior to the actual therapeutic administration. At ~ 3 h post-administration, the activities in the normal liver, liver tumours, lungs and total body are measured by gamma camera imaging using the conjugate-view method, with first-order corrections for attenuation (using a Rhenium-188 transmission scan) and scatter (using the “dual-window” method). At the same time, peripheral blood samples are counted and the activity concentrations in whole blood calculated. The blood activity concentrations are then converted into red marrow activity concentrations and then the total red marrow activity using anatomic data from Standard Man anthropomorphic models. Next, the cumulated activities in the normal liver, liver tumours, lungs, red marrow and total body are calculated using the measured activities in the respective source regions and assuming elimination of activity only by physical decay in situ. The absorbed doses to the therapy-limiting normal tissues, liver, lung and red marrow, are then calculated using the MIRD schema, adjusting the pertinent S factors for differences in total body and organ masses between the patient and the anthropomorphic model and including the dose contribution from the liver tumours. Finally, based on maximum tolerated absorbed doses of 3,000, 1,200 and 1,500 rad (cGy) to liver, lung and red marrow, the respective absorbed doses per unit administered activity are used to calculate the therapy activity. Although not required for treatment planning, tumour absorbed dose may also be estimated. This algorithm has been automated using an EXCEL spreadsheet.
The above cited dosimetry methodology was employed in the IAEA sponsored multicentre study in all 185 patients treated with Re-188 HDD Lipiodol. However, dosimetry parameters were fully recorded in only 109 patients. Most patients were in good general status: 54 had KPS of 90 or more and 55 had KPS of 70 or 80. Among these patients 40 had no cirrhosis, 26 were Child A and 23 Child B (20 missing data). Eighty-nine patients had 1 tumour, 11 had 2, 2 had 3 tumours and 7 had 4 nodules. The largest tumour size ranged from 1 to 19 cm (mean 8.85 cm, SD: 4.1), 13 patients had portal vein thrombosis. CLIP score was 0 in 6 patients, 1 in 13, 2 in 25, 3 in 23 and 4 in 3 (39 missing data), AFP value was more than 5 times the local upper value in 63 patients and less than this value in 44 (2 missing data).
The mean injected activity in the scout dose was 5.3 mCi (0.2 GBq) (SD: 2.4 mCi) and in the therapeutic dose it was 102 mCi (3.8 GBq) (SD: 57.9; extremes: 18–363 mCi); then the mean total injected activity was 107.5 mCi (4 GBq) (SD: 57.7). The estimated tumour dose was 63.4 Gy (SD: 59.9) ranging from 1 to 305 Gy.
Initial side effects (pain and fever) were not related to the injected activity or to the tumour dose. The study did not reveal any significant relationship between liver toxicity (regarding AST, ALT or bilirubin) and injected activity or tumour dose.
In this population there were 2 complete responses, 12 partial, 41 with stable disease and 11 with progressive disease (43 missing data). There was no significant relationship between tumour dose and tumour response (Fig. 2) (Bernal et al. 2008).
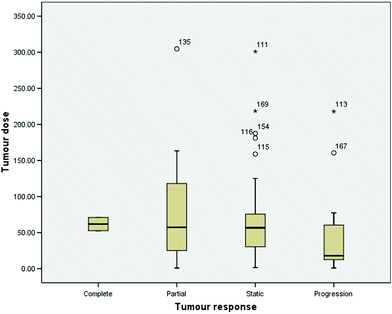

Fig. 2
Boxplot expression of tumour dose in patients who experienced complete response (n = 2), partial response (n = 12), stable disease (n = 41) or progression (n = 11). The bottom and top hinges of the box represent the first and third quartile. The distance between the upper and lower hinges is called the inter quartile range (IQR).The median is indicated by a thick black line inside the box. There was no significant difference between these groups (figure reproduced with permission from the original publisher Elsevier)
There existed a significant difference (p = 0.006) in overall survival (studied by the Kaplan–Meier method and compared by the log-rank test) between patients who received less than 30 Gy versus those who received more than 30 Gy (Fig. 3) (Bernal et al. 2008). Median survival was 471 days among those who received more than 30 Gy versus 146 days for the others. This association between tumour dose and survival, controlled for the tumour size and country, analysed using the Cox proportional hazards model was also significant (p = 0.015).
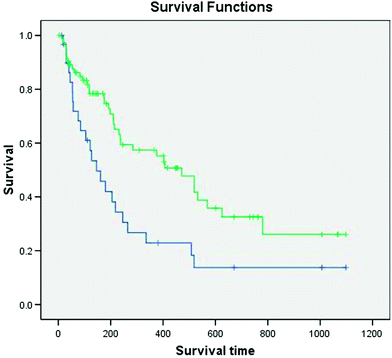

Fig. 3
Overall survival (Kaplan–Meier method; comparison using the log-rank test) was significantly better (p = 0.006) among patients whose tumor dose was greater than 30 Gy (n = 76) than among those who received less than 30 Gy (n = 33) (figure reproduced with permission from the original publisher Elsevier)
5.5 Prognostic Factors Influencing the Efficacy of Re-188 HDD Lipiodol Therapy and Patient Survival
It has been shown that Cirrhosis was a significant negative prognostic factor in the treatment of HCC with Re-188 Lipiodol. In the IAEA trial the observed median survival for cirrhotic patients was 218 days versus 246 for non-cirrhotic patients. As expected, among cirrhotic patients, Child score had statistically significant effect on survival, whereby patients with score A had better survival (median 625 days) than patients with score B (median 161 days). In the same trial survival was also related to CLIP score (Cox regression, adjusting for country: p = 0.015), survival decreasing with increasing score. Tumour control and biochemical response were also associated with improved survival.
Principal tumour dose during first treatment is extremely important and has shown to significantly influence survival. In the IAEA study, the therapeutic control of tumour size (one of the main therapeutic objectives in a palliative setting) could be achieved in more than three-quarters of the patients. This tumour control was clearly related to the treatment: a tumour radiation absorbed dose less than 30 Gy (obtained with the first treatment) corresponded to a 4-fold increase in the progression rate compared to patients whose tumours received >30 Gy. This relationship between tumour delivered dose and response rate has been hypothesised in another study using I-131-Lipiodol; which showed an inverse relationship between tumour size and intratumoral retention of Lipiodol and a direct relationship between intratumoral retention and response rate (Raoul et al. 2003).
6 Results of Other Studies Using Re-188 Lipiodol in the Treatment of HCC
Besides the IAEA sponsored multicentre study, a few other small-scale clinical studies, mostly single centre studies have been carried out using Re-188 Lipiodol, with varying results. Boschi et al. using the Re-188 N-DEDC Lipiodol radio-conjugate reported good results on a limited number of six patients in a pilot study with a follow-up period of over 2 years. It was possible to achieve stable disease in all six, with objective partial response in one patient. Repeated treatments were given on two to three occasions in three patients without evident toxicity. An additional patient given 6 GBq of Re-188 Lipiodol demonstrated myelo-suppression, which recovered with granulocyte colony-stimulating factor (GCSF) and platelet support (Boschi et al. 2004).
Another group from India, Kumar et al. have reported excellent results of Re-188 HDD Lipiodol therapy in two patients with recurrent HCC; in one case after radiofrequency ablation (Kumar et al. 2006a) and the other after post-surgical resection (Kumar et al. 2006b). Complete ablation of the recurrent tumours was reported in both instances.
An interesting biologic dosimetry study comparing the efficacy of Re-188 HDD Lipiodol and I-31 Lipiodol has shown that, in comparison with I-131 Lipiodol, Re-188 Lipiodol yielded a smaller cytotoxic effect and a lower radiation exposure for an expected higher tumour-killing effect. This particular study had concluded the superiority of Re-188 Lipiodol over I-31 Lipiodol for intraarterial radioembolisation and recommended a dose escalation study for Re-188 Lipiodol in the treatment of HCC (De Ruyck et al. 2004).
7 Re-88 Lipiodol: A Case for the Developing Countries
HCC is a disease which is highly prevalent in the developing countries of the world, where the resources are meagre and need for palliative treatment very high. In a palliative setting for HCC, only chemoembolisation has been demonstrated as better than best supportive care, in two randomised controlled trials; unfortunately side effects are frequent and could need intensive cares for several days. In another randomised trial, I-131 Lipiodol radio-conjugate therapy has been demonstrated to be efficient and better tolerated than chemoembolisation. But this treatment requires patient isolation for several days for radiation protection purposes. Besides, when it comes to radionuclide therapy of HCC, the cost of commercially available radiopharmaceuticals like I-131 Lipiodol, Y-90 SIR spheres and Y-90 Therasphere is prohibitively high by developing country standards. As a result hundreds of thousands of needy and deserving patients of HCC are deprived of this treatment option. Development of Rhenium 188 Lipiodol has been a big step forward in meeting the demand for making available a low cost and effective radio-conjugate for therapy of inoperable HCC. There are several advantages of the use of intra-arterial Re-188 Lipiodol injection as compared to other intra-arterial treatments. It is easier to deliver compared to chemoembolisation (injection within the common hepatic artery is possible). In fact, in the IAEA study the technique was feasible in all the participating countries after a short learning period. Good tolerance of Re-188 Lipiodol therapy by the patients avoids a long hospitalisation period and expensive care. It is cheaper than I-131-Lipiodol and Y-90 glass-based or resin-based microspheres, which are now available commercially. Re-188 is available on-site from an in-house generator system, on demand; one generator system can be effectively used for over 6 months. The treatment procedure does not require patient isolation and finally the multimodality applications of Rhenium-188 ensures optimal and cost-effective utilisation of the radionuclide in a variety of other clinical conditions like rheumatoid arthritis, haemophilic bleeding joints (Radiosynovectomy), metastatic bone pain, intravascular radionuclide therapy to prevent restenosis of coronary artery following revascularisation, etc. If appropriately planned and implemented, like Tc-99 m in diagnostic nuclear medicine, Re-188 could become the workhorse of therapeutic nuclear medicine in the future (Bal and Kumar 2008).
It may be noted that the multicentre study organised by the IAEA was the first multicentre study on treatment of liver cancer built and conducted in developing countries. It included very distant and different countries with consequent considerable heterogeneity in patients and thus results. This great heterogeneity between the different countries seems to be related to the disease as well as to care-giver access. In some countries, only patients with good general status were included, but in others, the majority of patients had severe underlying liver disease. These parameters are powerful prognostic factors and could explain the large discrepancy in survival from one country to another. Nevertheless, the IAEA study demonstrated that it is possible to conduct a scientific medical study of this nature and magnitude in developing countries.
8 Conclusions
It has been demonstrated that Re-188 Lipiodol therapy for HCC is well tolerated, particularly in patients with well-compensated liver cirrhosis and good general status. The IAEA’s multicentre study on a reasonably large patient population has provided some significant data to conclude that this treatment is efficient. The objective response rate and the survival data are clearly in accordance with those obtained in several other previous studies with chemoembolisation and I-131 Lipiodol. The study also confirmed that intra-arterial injection of Re-188 Lipiodol is feasible without significant difficulties in developing countries. Re-188 Lipiodol is valuable alternative to I-131 Lipiodol, because it yields lower radiation exposure for higher tumour killing effect and it does not require stringent radiation protection measures and patient isolation like in the case of I-131. It is extremely important to carry on with the next logical step—a Phase III study. It is hoped that some radiopharmaceutical industry would take up this challenge and capitalise on the gains of the IAEA study to consolidate the role of Re-188 Lipiodol therapy in the treatment of HCC.
Annexure-1
Protocol for Re-188 Lipiodol Therapy of HCC (Used in the IAEA’s Multi-Centre Study)
Eligibility Criteria
1.
Histopathology diagnosis of HCC or association of hyper-vascularised liver Tumor(s) in cirrhotic/fibrotic liver.
2.




Patients must have bi-dimensionally measurable disease by conventional imaging methods including radiography, ultrasound, computer tomography, or other anatomic imaging modalities. These modalities must demonstrate a solitary lesion > 5 cm in greatest diameter, OR < 3 lesions < 3 cm in greatest diameter, OR an inoperable solitary lesion > 5 cm in greatest diameter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








