Fig. 1
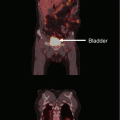
(a, b) Magnetic resonance imaging (MRI) scans at initial presentation with skeletal metastases from prostate cancer in a 66-year-old man, previously treated with radical prostatectomy (a). The white arrow indicates a large, painful pubic bone metastasis. Due to public holidays in December 2008, treatment with a standard regimen of 30 Gy in 10 fractions was difficult to organize. Therefore, the patient was irradiated with 7 fractions of 4 Gy. Complete pain relief was obtained. In March 2010, increasing pain in the irradiated region and the lower back resulted in repeat MRI. A large new metastasis in the sacral bone with considerable soft tissue extension was found (b, white arrow). Taking into account performance status (ECOG 1) and life expectancy (more than 6 months), the patient was treated with two conventionally simulated fields encompassing both the previously irradiated and the new lesion (30 Gy in 10 fractions). Excellent pain response was obtained again

Fig. 2
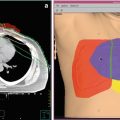
Computed tomography scans in a 76-year-old man with known metastatic prostate cancer, treated with endocrine therapy and zoledronic acid. In November 2009, the disease was considered castration resistant. Palliative radiotherapy (8 Gy single fraction) was administered to the sacroiliac joints and os sacrum. Complete pain response was achieved. Chemotherapy was initiated, but later discontinued when disease progression occurred in June 2010. The patient was referred for local reirradiation to the same painful region. He received another single fraction (8 Gy) with complete response
2.3.1 Retreatment Rates in Randomized Trials
Among the randomized trials comparing single versus multiple fractions for painful bone metastases, retreatment rates were consistently higher after the single-dose schedules. Percentages varied from 11 to 42 % after a single fraction to 0–24 % after multiple fractions (Table 1). It has been proposed that because of the higher necessity for retreatment, the single-fraction regimen was not as effective as more protracted regimens when looking at durability of response. On the other hand, the retreatment percentages in the trials were most likely biased, since retreatment was done at the discretion of the treating radiation oncologists and patients, neither of whom were blinded to the initial treatment. Disbelief in the effectiveness of a single fraction, or the awareness that still more radiation was possible, may contribute to these percentages. The study protocols did not prescribe a minimum time interval between initial and retreatment, or a minimum pain score requirement, and the dose for retreatment was also not defined. In addition many patients with recurrent pain or poor response to initial radiation may have been lost to follow-up or may not be referred back to their radiation oncologist for consideration of retreatment. A reanalysis of the original database of the Dutch trial was completed using the consensus criteria for response with a closer look at specific reasons to retreat (Chow et al. 2002; van der Linden et al. 2004a, b). After 8 Gy single fraction, 24 % of the patients were retreated versus only 6 % after 24 Gy in six fractions (p < 0.001). Response to initial treatment without the effect of retreatment was 71 % after SF vs. 73 % after MF (p = 0.84). Retreatment raised the response rate to 75 % for SF; MF response rates remained unaltered (p = 0.54). The response status after initial treatment did not predict for retreatment: 35 % SF vs. 8 % MF nonresponders, and 22 % SF vs. 10 % MF patients with progressive pain were retreated. After progressive pain was observed, mean time to retreatment was 7 weeks in SF patients versus 10 weeks in MF patients. The preceding mean pain score was 7.5 in SF patients and 7.8 in MF patients. In conclusion, it appeared that physicians were more reluctant to retreat initial MF patients than initial SF patients.
Table 1
Retreatment percentages in randomized trials on dose fractionation schedules for bone metastases
Publication year | Number of randomized patients | Randomization arms | Retreatment (%) | p-value | ||
|---|---|---|---|---|---|---|
Low dose | High dose | |||||
Trials comparing single-fraction versus multiple-fraction regimens | ||||||
RTOG | 2005 | 898 | 8 Gy vs. 30 Gy/10# | 18 % | 9 % | <0.001 |
Foro Arnolot | 2008 | 160 | 8 Gy vs. 30 Gy/10# | 28 % | 2 % | a |
Kaasa | 2006 | 376 | 8 Gy vs. 30 Gy/10# | 27 % | 9 % | 0.002 |
BPTWP | 1999 | 761 | 8 Gy vs. 20 Gy/5# | 23 % | 10 % | <0.001 |
DBMS | 1999 | 1157 | 8 Gy vs. 24 Gy/6# | 24 % | 7 % | <0.001 |
Nielsen | 1998 | 241 | 8 Gy vs. 20 Gy/5# | 20 % | 2 % | a |
Cole | 1989 | 29 | 8 Gy vs. 24 Gy/6# | 25 % | 0 % | a |
Price | 1986 | 288 | 8 Gy vs. 30 Gy/10# | 11 % | 3 % | a |
Trials comparing different single-fraction regimens | ||||||
Jeremic | 1998 | 327 | 4 Gy vs. 6 Gy vs. 8 Gy | 42 % | 38 % | NS |
Hoskin | 1992 | 270 | 4 Gy vs. 8 Gy | 20 % | 9 % | a |
Trials comparing different multiple-fraction regimens | ||||||
Niewald | 1996 | 100 | 20 Gy/5# vs. 30 Gy/15# | 2 % | 2 % | NS |
Tong | 1982 | 750 | Single metastases 20 Gy/5# vs. 40 Gy/15# Multiple metastases 15 Gy/5# vs. 20 y/5# vs. 30 Gy/10# | 24 % 23 % | 11 % 12 % | a |
2.3.2 Effectiveness
Until 2014, responses to retreatment have been reported only in retrospective and nonrandomized prospective studies. Price et al. reported on seven patients who, after failure to respond to the initial single 4 Gy fraction, were given a second radiotherapy within 8 weeks (Price et al. 1986). Four of them received a single 8 Gy and the other three a fractionated course with a higher total dose. No significant pain relief was achieved by the second radiotherapy treatment in any of these seven patients (Price et al. 1988). Cole reported in 42 patients that retreatment of patients after initial single- or multiple-fraction treatment was not successful in most patients, and 50 % needed supplementary stronger analgesics (Cole 1989). Hoskin et al. randomized patients in a prospective study to either 4 or 8 Gy single dose for the initial treatment of metastatic bone pain. During the 12-week study period, 28 patients randomized to 4 Gy were retreated with radiotherapy to the same site compared to 12 randomized to 8 Gy. 12/17 (71 %) evaluable patients responded to retreatment in the 4 Gy arm and 4/9 (44 %) responded in the 8 Gy arm (Hoskin et al. 1992). Uppelschoten et al. reported that after long intervals from initial single 6 Gy of radiation, retreatment with another 6 Gy was able to reduce pain in 13 out of 18 patients (72 %) (Uppelschoten et al. 1995). Mithal et al. published a retrospective analysis of 105 consecutive patients treated with palliative radiotherapy for painful bone metastases. A total of 280 individual treatment sites were identified, of which 57 were retreated once and eight were retreated twice. The overall response rate to initial treatment was 84 % for pain relief, and to retreatment the response rate was 87 %. 7/8 (88 %) patients retreated for a second time also achieved pain relief. No relation to radiation dose, primary tumor type, or site was observed (Mithal et al. 1994). Jeremic et al. investigated the effectiveness of a single fraction of 4 Gy given for retreatment of bone metastasis after initial single-fraction radiotherapy. Of 135 patients retreated, 109 patients were retreated because of pain relapsing, and 80 (74 %) patients responded (complete response (CR) = 31 %, partial response (PR) = 42 %). Among the 26 patients that initially did not respond, 46 % responded. The authors concluded that the lack of response to an initial single fraction should not deter repeat irradiation. The same group also reported the efficacy of a second single 4 Gy retreatment for painful bone metastases following two previous single fractions. The overall response rate of 25 patients (19 responders and six nonresponders) was 80 %, with both complete response and partial response being 40 % (Jeremic et al. 2002). In Radiation Therapy Oncology Group (RTOG) studies that involved wide-field or hemibody radiotherapy, patients who relapsed after wide-field irradiation were reported to tolerate local irradiation within that field with success (Quasim 1981; Salazar et al. 1986). Van der Linden et al. studied 173 patients who were retreated within the randomized Dutch Bone Metastasis Study (n = 1157) (van der Linden et al. 2004a, b). In 137 retreated SF patients, 33 % received a second SF and 67 % received MFs. In 36 retreated initial MF patients, 25 % received second MF and 75 % received a SF. To evaluate whether response to initial treatment influenced the choice for the second treatment schedule, the response status before retreatment was studied. There was no correlation between the initial response status and the retreatment schedule. In total, 63 % of retreated patients responded to retreatment: 66 % of retreated SF patients responded compared with 46 % of MF patients (p = 0.12, HR 1.6 (0.9–3.0)). After retreatment, time to response was not different for initial SF and MF patients, but the mean duration of remission was substantially longer in initial SF patients, 16 weeks vs. only 8 weeks in initial MF patients. For SF patients, response to retreatment was irrespective of the initial response: 66 %, 67 %, and 70 % of initial nonresponders, responders, and progressive patients, respectively, responded to retreatment. Although more initial SF than MF nonresponding patients responded to retreatment, due to small numbers, this difference was not significant (66 vs. 33 %, respectively, p = 0.13, HR 3.0 (0.7–12.7)). Of nonresponders to an initial SF, 88 % responded to a second SF and 53 % to MF. Of the latter, 10 % of responses were due to a decrease in analgesic intake. Overall, no major differences in mean time to response after retreatment were reported. Mean duration of remission ranged from 4 weeks in initial MF nonresponders to 25 weeks in initial SF nonresponders. Patients with prostate cancer had the lowest success rate: 20 % of initial nonresponding patients responded, and only 19 % of patients with progression responded again. Patients with breast cancer had the highest response percentages for nonresponders and progressive patients (82 % and 89 %, respectively). Mean duration of remission was longest in initial nonresponding breast cancer patients (23 weeks). Van Helvoirt et al. actively contacted 298 consecutive patients 4 weeks after initial single-fraction therapy to evaluate response and, if necessary, to offer retreatment (van Helvoirt and Bratelli 2008). Seventy-five percent of the patients were satisfied with their pain response, and 75 % of the non-satisfied patients requested retreatment. Of these, 87 % responded after a second single fraction. In 37 patients with recurrent pain, 83 % responded to a second single fraction. In 2012, Huisman et al. performed a systematic review on ten articles and a meta-analysis on seven (Huisman et al. 2012). Of the 2,694 patients initially treated for metastatic bone pain, 527 (20 %) patients underwent reirradiation. Overall, a pain response after reirradiation was achieved in 58 % of patients (pooled overall response rate 0.58, 95 % confidence interval = 0.49–0.67). There was a substantial between-study heterogeneity (I 2 = 63.3 %, p = 0.01) because of clinical and methodological differences between studies. In 2014, the results from the international randomized trial on retreatment in painful bone metastases were published (Chow et al. 2014a, b). A total of 850 patients were randomized between a single fraction of 8 or 20 Gy in 5 fractions for retreatment. In the intention-to-treat population, 118 (28 %) patients allocated to 8 Gy and 135 (32 %) allocated to 20 Gy had an overall pain response to treatment (p = 0.21, response difference of 4 · 00 % [upper limit of the 95 % CI 9 · 2, less than the prespecified non-inferiority margin of 10 %]). In the per-protocol population, 116 (45 %) of 258 patients and 134 (51 %) of 263 patients, respectively, had an overall pain response to treatment (p = 0.17, response difference 6 · 00 % [upper limit of the 95 % CI 13 · 2, greater than the prespecified non-inferiority margin of 10 %]). They concluded that for retreatment, 8 Gy in a single fraction seemed to be non-inferior and less toxic than 20 Gy in multiple fractions.
In patients with longer life expectancies, a second or even third retreatment may be considered if earlier radiotherapy treatments have given satisfactory pain responses. Cumulative total doses should be calculated taking into account nearness of critical radiosensitive organs, such as the spinal cord, kidney, bowel, or skin. No clear-cut answer is yet available to guide which dose schedules should be used for a first retreatment, second retreatment, or even third retreatment.
3 Radiotherapy for Bone Metastases: Spinal Cord Compression (SCC)
3.1 Initial Treatment for Spinal Cord Compression
There is no standard fractionation schedule for the treatment of spinal cord compression. Rades et al. have published several papers based on a large retrospective database (Rades et al. 2002, Rades et al. 2005a, 2006, 2008a, b, 2009, 2010a, b, c). In general, radiotherapy is most effective in improving motor function when neurological complaints have developed over a longer period of time (>14 days), in patients with a longer interval from primary diagnosis to SCC, who are ambulatory before radiotherapy, with a good performance status, or with favorable histologies like myeloma and lymphoma (Rades et al. 2005a). In their series they showed that both short-course (single fraction of 8 or 20 Gy in 5 fractions) and long-course treatments (30–40 Gy in 10–20 fractions) resulted in the same functional outcome in patients with breast cancer (n = 335), prostate cancer (n = 281), non-small cell lung cancer (n = 252), or renal cell carcinoma (n = 87). An exception was the treatment of myeloma patients (n = 172). Functionally, they fared better with long-course radiotherapy with improvements of motor function reported at 12 months in 74 versus 40 % after short-course radiotherapy (p = 0.003) (Rades et al. 2006).
In addition to functional outcome, however, significantly more in-field recurrences appeared after short-course radiotherapy than after long-course schedules (at 1 year, 18 vs. 5 % recurrence, p < 0.001) (Rades et al. 2005a, b). This effect was most prevalent in patients with breast cancer (1-year local control 96 % after long course vs. 84 % after short course) and prostate cancer (94 vs. 77 %). If patients have an expected good prognosis, a long-course schedule should therefore be considered.
Three randomized trials have been published on the relative effectiveness of different schedules for SCC in patients with a life expectancy of less than 6 months. The first compared in 276 patients 16 Gy in 2 fractions with a split-course treatment of 15 Gy in 3 fractions followed after an interval by 15 Gy in 5 fractions (Maranzano et al. 2005). After treatment, 68 and 71 % were able to walk with no difference between both arms. Median survival was 4 months and median duration of improvement was 3.5 months for both arms. In the second trial by the same group, 303 patients were randomized between 16 Gy in 2 fractions and a single fraction of 8 Gy (Maranzano et al. 2009). No difference in response was found between the two RT schedules adopted. Median duration of response was 5 and 4.5 months for short-course and single-dose RT (p = 0.4), respectively. The median overall survival was 4 months for all cases. The more recent randomized SCORE 2 trial has shown that 20 Gy in 5 fractions is equivalent to 30 Gy in 10 fractions for patients with intermediate and poor life expectancy using motor function and ambulatory status as end points (Rades et al. 2016). Currently in the UK, the randomized SCORAD trial is studying the effectiveness of 8 Gy single fraction versus 20 Gy in 5 fractions in patients with spinal cord compression and a minimum life expectancy of 2 months.
In order to make an appropriate decision for treatment in individual patients with SCC, a number of scoring systems can be used, all based upon prognostic factors for survival and outcome (Chow et al. 2006; Rades et al. 2008a, b). Important prognostic factors are the performance status of the patient, the number of spinal metastases, the presence of visceral metastases, and the type of primary tumor.
In conclusion, based on the available literature, the common practice in most Western countries is to deliver 20 Gy in 5 fractions in patients with a minimum life expectancy of 3 months or 30 Gy in 10 fractions in patients with a more prolonged life expectancy (minimum 1 year). A shorter schedule of 1 or 2 × 8 Gy is perhaps the optimal schedule in patients with a prognosis less than 3 months.
Oral dexamethasone is usually combined with radiotherapy to diminish any surrounding edema and should be started as soon as possible, preferably before the first radiation fraction can be delivered. The appropriate dose of dexamethasone is still a matter of debate. High doses of dexamethasone (96–100 mg/day) seem more effective than low-dose dexamethasone (10–16 mg/day), but are associated with significantly more serious adverse effects (Vecht et al. 1989; Heimdal et al. 1992; Sorensen et al. 1994). Moderate doses of dexamethasone (16–32 mg/day) have proven to be effective and safe and are therefore advocated. If the neurological situation improves after palliative radiotherapy, a reducing schedule of dexamethasone should be started. If the neurological situation deteriorates despite adequate doses of dexamethasone and radiotherapy, and no surgical intervention is possible, or if paraplegia persists, a reduction schedule should be started to prevent disabling side effects from long-term use of high-dose steroids.
3.2 Retreatment for Spinal Cord Compression
Responses to retreatment in patients with spinal cord compression have been reported so far only in retrospective studies (Rades et al. 2005b, 2008c). Outcome after retreatment with 1 ×x 8 Gy (n = 48), 5 × 3 Gy (n = 29), 5 × 4 Gy (n = 30), 7 × 3 Gy (n = 3), 10–12 × 2 Gy (n = 11), or 17 × 1.8 Gy (n = 3) was published in a series of 124 patients. Cumulative biologically effective dose (BED) (first course of RT plus re-RT) ranged from 77.5–142.6 Gy2 and was ≤120 Gy2 in 114 (92 %) patients. Motor function improved in 45 (36 %) patients, was stable in another 62 (50 %) patients, and deteriorated in 17 (14 %) patients, with no difference between the radiation doses. Multivariate analysis found the effect of retreatment on motor function was significantly associated with the response to the first course of radiotherapy (p = 0.048), performance status (p = 0.020), time to development of motor deficits before retreatment (p = 0.002), and visceral metastases (p < 0.001). Within the limitations of these retrospective study and the relatively short follow-up after retreatment, spinal retreatment appeared to be effective and safe when the cumulative BED was ≤120 Gy2.
4 Toxicity
4.1 Acute and Late Toxicity
If repeated radiotherapy for bone metastases is considered, toxicity will depend on the previously applied dose, time between initial and second treatment, and localization within the body in relation to sensitive organs in the vicinity. Toxicity in the study by Jeremic et al. using 4 Gy as retreatment dose was low and only gastrointestinal (Jeremic et al. 1999). Grade 1 or 2 diarrhea (RTOG acute toxicity criteria) was observed in 25/135 (19 %) patients. No acute toxicity > grade 3 was reported. Pathological fractures were reported in 3/135 (2 %) patients and spinal cord compression in 3/135 (2 %) patients in their series. In 25 patients retreated with a second retreatment dose of 4 Gy from the same group, no acute or late high-grade toxicity (>3) was observed (Jeremic et al. 2002). No pathological fractures or spinal cord compression were seen in any of these patients during the follow-up. Within the Dutch Bone Metastasis Study, toxicity 1 month after retreatment was scored in approximately 73 % of the 173 retreated patients (van der Linden et al. 2004a, b). No major differences in nausea, vomiting, itching, painful skin, or tiredness were reported between initial SF or MF patients. Most SF and MF patients reported none or only mild nausea and vomiting. Nausea score 4 (very bad) was reported in 12 % of MF patients vs. 6 % of SF patients (p = 0.39). Vomiting score 4 (very bad) was reported in one MF patient and two SF patients (p = 0.49). Significant skin reaction defined by itching score 4 (very bad) was seen in two SF patients. One SF patient reported a painful skin score 4 (very bad). Severe tiredness was reported in 18 % of SF patients and 27 % of MF patients (p = 0.41). After retreatment in the study by Rades et al. in 124 patients with SCC, acute toxicity was mild, and late toxicity, such as radiation myelopathy, was not observed (Rades et al. 2008c).
4.2 Risk of Myelopathy
Radiation-induced myelopathy is a late complication after radiotherapy and can occur months to years after treatment. The exact pathogenesis of myelopathy remains obscure. Although significant recovery of the spinal cord after elective small doses is possible (Ang et al. 2001; Kirkpatrick et al. 2010), when applying higher radiotherapy doses, larger doses per fraction, and when previous exposure to radiation is the case, a higher probability of developing radiation-induced myelopathy will be present. Experimental data indicate that the total dose of the first and second radiotherapy, interval to retreatment, length of the irradiated spinal cord, and age of the treated animals influence the risk of radiation-induced myelopathy. An experimental study based upon the available literature concluded that the (extrapolated) probability of myelopathy at 45 Gy is 0.03 % and at 50 Gy, 0.2 % (Schultheiss 2008). The dose for a 5 % myelopathy rate is 59.3 Gy. Graphical analysis indicates that the sensitivity of the thoracic cord is less than that of the cervical cord.
There is extensive literature on the use of single fractions of 8 or 10 Gy for uncomplicated spinal metastases, none of which has identified a detectable risk of myelopathy. A retrospective analysis of 465 patients treated for spinal cord compression identified only one possible case of myelopathy in a patient receiving 16 Gy in 2 fractions, becoming symptomatic 19 months after initial presentation (Maranzano et al. 2001). In addition the estimated risk of radiation myelopathy from palliative radiotherapy for non-small cell lung cancer was calculated using over 1000 patients taking part in a series of Medical Research Council (MRC) trials (Macbeth et al. 1996). These patients will have had similar doses of radiotherapy to the spinal cord. Only five patients were reported as having radiation myelopathy, two who had received 17 Gy in 2 fractions and three who had 39 Gy in 13 fractions; there were no cases in patients who received a single fraction of 10 Gy. The overall cumulative risk was estimated as 0.8 % at 1 year and 1.5 % at 2 years. The risk of radiation myelopathy therefore appears negligible after a single fraction. Nieder et al. studied the cumulative doses in 78 patients (Nieder et al. 2005, 2006). On the basis of the literature data included in their analysis, the risk of myelopathy appeared small after a cumulative biologically equivalent dose of 135.5 Gy2 when the interval was not shorter than 6 months and the dose of each course was <98 Gy2. In more detail, risk points were given to cumulative BED (0 = <120 Gy2, 1 = 120–130 Gy2, 2 = 130–140 Gy2, 3 = 140–150 Gy2, 4 = 150–160 Gy2, 5 = 160–170 Gy2, 6 = 170–180 Gy2, 7 = 180–190 Gy2, 8 = 190–200 Gy2, 9= > 200 Gy2), highest BED of all treatment series in a particular individual (if one course > = 102 Gy2 = 4.5 points), and interval between initial and second treatment (if <6 months, 4.5 points). In low-risk patients (≤3 points), chance of radiation-induced myelopathy was 3 %, in intermediate-risk patients (4–6 points) 25 %, and in high-risk patients (>6 points) even 90 %. Stereotactic body radiotherapy (SBRT) in spinal metastases, if given at least 5 months after conventional palliative radiotherapy with a reirradiation thecal sac maximum dose of 20–25 Gy2, appears to be safe provided the total maximum dose does not exceed approximately 70 Gy2, and the SBRT thecal sac maximum dose comprises no more than approximately 50 % of the total dose in Gy2 (Sahgal et al. 2012).
5 Radiotherapy Techniques
5.1 Dose Planning, Patient Positioning Verification
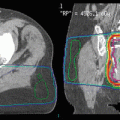
At the simulation procedure, usually, there is no need of fixation of the affected limb or, in case of spinal metastases, the torso of the patient. For lesions in the skull or head and neck, a mask can be made. Preferably, a computed tomography (CT) scan is used to optimally review the area at which the patient points out the origin of the pain (Haddad et al. 2006). Markers can be placed on the skin. Then, the painful area of the patient is scanned widely. Ideally, patients can wait after the simulation procedure for a short while and receive the radiotherapy treatment at the same visit, a so-called one-stop treatment. For bone metastases in long bones, where no vital organs are in the way, usually a straightforward planning technique using two parallel-opposed fields is used, and optimal target coverage is readily achieved. For metastases in the spine, especially in lower thoracic and lumbar vertebra with bowel, stomach, and/or kidneys in the proximity of the painful vertebra, there is an increased risk of toxicity when these organs at risk receive a large dose of radiation, especially in single-dose regimens. Most vertebrae lie virtually in the midbody, and therefore, the radiotherapeutic option with the most optimal dose coverage is a two-field parallel-opposed technique (Barton et al. 2002). Unfortunately, with this approach a large dose is applied to the ventral lying organs at risk, and a single posterior field may therefore be preferred prescribed at a dose depth to cover the vertebral body with the 80 % isodose at the ventral side of the vertebra. Six to ten MV beams should be used to reduce the area of high dose in the subcutaneous tissues dorsally and/or bowel ventrally. When large fields are used for extensive lesions in the bone, prophylactic antiemetic drugs and steroids should be given 15–30 min before treatment to prevent toxicity (Sykes et al. 1997; Kirkbride et al. 2000b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree