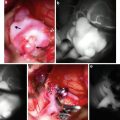
Fig. 1
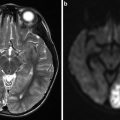
Sagittal T1-WI and SWI images (a, b) showing pulvinar T1 hyperintensity and SWI hypointensity (arrowheads) in a 30-year-old male with Fabry’s disease (Images courtesy of D. Mandell)
Several studies have shown increased white matter diffusivity on diffusion-weighted and diffusion tensor imaging, predating structural changes. A recent MR study comparing white matter lesion load, white matter diffusivity changes, and arterial diameters, but not the T1 hyperintense pulvinar sign, has shown that increased basilar artery diameter is the best discriminator between patients with Fabry’s disease and normal controls [8].
PRES
The term posterior reversible encephalopathy syndrome is a clinical and radiologic syndrome with specific imaging findings, characterized by reversible, mostly posterior territory, vasogenic edema, which is the end point of a myriad of different etiologies.
Other names used to describe the clinical or radiologic syndrome include hypertensive encephalopathy and reversible posterior leukoencephalopathy. Most authors prefer the term posterior reversible encephalopathy because up to 30 % of patients are not hypertensive at presentation, and imaging changes are often also present in the cortex and not limited to the white matter. Symptoms include headaches, decreased alertness, altered mental functioning, seizures, and visual loss occasionally attributable to cortical blindness.
The clinical and imaging findings have been associated with preeclampsia/eclampsia, posttransplant disorders, renal failure, immune suppression, infection and sepsis, autoimmune disease, chemotherapeutic agents, and numerous other miscellaneous conditions.
Pathophysiology
Although several theories of PRES etiology exist, two main hypotheses are likely. The original theory suggests that hypertension leads to autoregulatory vasoconstriction and hypoperfusion, ischemia, and edema. A more popular theory suggests that severe hypertension results in breakthrough of the autoregulatory mechanism leading to edema.
There has been a recent resurgence of interest in the original theory, as a result of several studies showing that about 30 % of patients who develop symptoms and imaging findings compatible with PRES are not hypertensive or demonstrate cerebral hypoperfusion. Further, several of the underlying diseases or agents associated with the development of PRES affect endothelial cell function [9].
Pathology
The white matter and cortical edema tends to concentrate in the parieto-occipital regions. Pathology reports of biopsy specimens have shown findings of white matter vacuolation, consistent with white matter edema often with mild inflammatory reaction and, less commonly, findings of neuronal necrosis and petechial hemorrhages [10]. Autopsy specimens have shown fibrinoid necrosis of arterioles with thrombosis of arterioles and capillaries, as well as parenchymal microinfarcts and petechial hemorrhages thought to be secondary to vascular changes. Occasionally, pathology specimens have revealed evidence of white matter pallor, attributed to demyelination and astrocytosis [11].
More recent pathology reports have revealed evidence of perivascular T-cell trafficking, endothelial activation, endothelial immunoreactivity to tumor necrosis factor-alpha, robust endothelial VEGF mRNA transcription, and VEGF protein expression [9].
Imaging Findings
CT
CT typically demonstrates focal regions of symmetric, predominantly subcortical white matter parieto-occipital hypodensity. The frontal lobes, cerebellum, and inferior temporal-occipital lobes may also be affected. The distribution does not conform to a vascular territory but is often seen in watershed regions. The cortex may be involved.
MRI
MRI shows T2 prolongation in the subcortical white matter and cortex almost always involving the parieto-occipital lobes. Isolation solely to the posterior brain regions is rare, however, and involvement of other brain regions including the frontal lobes, temporal lobes, and cerebellum is often seen. Thalamic, basal ganglia, and brainstem involvement has been described.
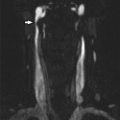
Findings are most commonly associated with hypointensity or isointensity on diffusion-weighted imaging with concomitantly increased ADC values. Foci of predominantly cortical diffusion restriction have been described. These may represent ischemia and irreversible injury. Reversible cortical diffusion restriction has also been described in the setting of seizure activity, presumably related to excitotoxic edema [12] (Fig. 2)

Fig. 2
PRES encephalopathy. (a, b) Hyperintensity is seen on T2-WI images with subcortical and cortical involvement in the parieto-occipital regions (arrowheads). (c, d) DWI is normal due to T2 wash out. (e) ADC map confirms vasogenic edema in the distribution of T2 hyperintensity (black arrow). (f) MRV is normal (Images courtesy of M. Shroff)
Contrast enhancement has been shown in 16–30 %. Petechial and subarachnoid hemorrhages (SAH) occur in 15 % of lesions [10]. FLAIR sulcal hyperintensity may be secondary to SAH but can also occur secondary to proteinaceous exudate. Diffusion tensor imaging has revealed regions of reversible increased anisotropy [13].
Catheter Angiography
Angiographic studies range from normal to findings of vasospasm evidenced by arterial narrowing that can be peripheral, central, or diffuse. Arterial beading has also been described. Peripheral vasospasm is often difficult to characterize on MRA/CTA imaging and is better visualized with catheter angiography [14].
Perfusion Studies
CTP studies, performed in hypertensive patients, have shown increased cerebral blood flow, increased cerebral blood volume, and decreased mean transit time allowing for differentiation between PRES and cerebrovascular accident [15].
Hypoperfusion, however, has been demonstrated using Tc99m-HMPAO SPECT in women with eclampsia, performed within 48 h of delivery [16].
Several recent MR perfusion studies have also shown decreased perfusion in PRES [14]. Findings have generally shown decreased cerebral blood flow and volume in affected regions, with some variability of mean transit time.
The discrepancy of these perfusion findings is not well understood. It may relate to the timing of the perfusion study with respect to disease onset. Animal experiments have shown that vasoconstriction occurs within the limits of autoregulation, at which point there is breakthrough of the autoregulatory mechanism resulting in hyperperfusion and breakdown of the blood-brain barrier. In animal studies, this has been followed by vasodilation and hypoperfusion. Alternatively, authors postulate that abnormal radiopharmaceutical uptake could represent luxury perfusion in the setting of loss of autoregulation and vascular tone, rather than true hyperperfusion.
RCVS
Reversible cerebral vasoconstriction syndrome (RCVS) is defined as a group of disorders that result from prolonged but reversible vasoconstriction of the cerebral arteries. It is usually associated with acute onset, severe headaches and may be accompanied by other neurological signs. Spontaneous resolution of the vasoconstriction is usually seen in 1–3 months [17]. In its broadest definition, the term encompasses disorders of numerous etiologies characterized by reversible vasospasm which do not show histologic hallmarks of vasculitis. RCVS has been known in the literature by multiple names including Call-Fleming syndrome, benign angiopathy of the central nervous system, thunderclap headache with reversible vasospasm, and migrainous angiopathy. Reversible vasospasm in the setting of known etiologic factors such as drug-induced vasculopathy, hypercalcemia, unruptured saccular aneurysm, dissection, postcarotid endarterectomy, and postpartum angiopathy is also included in the umbrella term of RCVS [17]. Known etiologic factors account for up to 25–60 % of cases of RCVS [18].
Epidemiology
The age of onset of RCVS peaks at around 45 years of age and is more common in females, with a 2.6:1 preponderance in a French study to 10:1 in Taiwanese study [18].
Clinical
Presentation is similar to aneurismal rupture and characterized by rapid onset thunderclap headache, peaking in less than 1 min and recurring over 1–2 weeks.
Diagnostic criteria were defined by Calabrese and include: [17]
1.
Transfemoral angiography or indirect (CT or MRI) angiography documenting segmental cerebral artery vasoconstriction.
2.
No evidence of aneurismal subarachnoid hemorrhage.
3.
Normal or near-normal cerebrospinal fluid analysis (protein level <80 mg/dL, white blood cell count <10/μL, normal glucose level).
4.
Severe, acute headache, with or without additional neurological signs or symptoms.
5.
The diagnosis cannot be confirmed until reversibility of the angiographic abnormalities is documented within 12 weeks of onset. If death occurs before the follow-up studies are completed, autopsy should rule out conditions such as vasculitis, intracranial atherosclerosis, and aneurismal subarachnoid hemorrhage, which can also manifest with headache and stroke.
CSF analysis must be performed to rule out subarachnoid hemorrhage in patients presenting with thunderclap headache, with normal imaging findings. CT and MRI are usually normal. Occasionally, there may be a small amount of subarachnoid hemorrhage overlying the cortical surface or in the parenchyma. In 10–39 % of patients, findings may result in ischemic or hemorrhagic parenchymal strokes [19] (Fig. 3a, d). Reversible brain edema, compatible with PRES, is seen in about 10 % of patients [19].
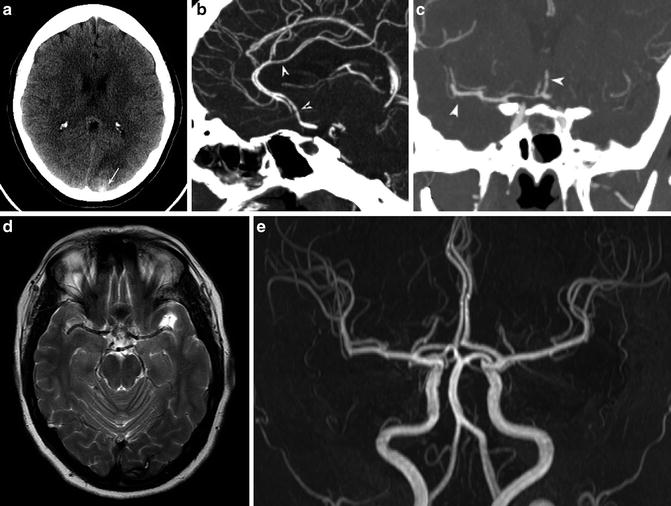

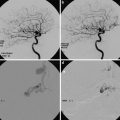
Fig. 3
Reversible cerebral vasoconstriction syndrome. A 36-year-old female presented with an acute thunderclap headache. (a) CT shows occipital parenchymal (arrow) and subarachnoid hemorrhage. (b, c) CTA performed the same day shows beading within middle cerebral artery (MCA) and anterior cerebral artery (ACA) territories (arrowheads). Lumbar puncture and serum biochemistry results revealed no evidence of an inflammatory process. The patient was diagnosed with RCVS, and steroids were withheld. (d) MRI T2-WI study performed 3 months later shows residual hemosiderin deposition in the left occipital lobe. (e) Concurrent MRA shows complete resolution of arterial narrowing
CTA and MRA usually show segmental beading and intermittent dilatation of vessels (string of beads) [17] (Fig. 3b, c). Initial noninvasive angiography may be negative or show distal vessel irregularities. Maximum vessel constriction involving the proximal arterial beds usually occurs 18–22 days following headache onset, often at the time of headache resolution, and may persist for several weeks. This has led some authors to speculate that it is actually the vascular dilation, and not constriction, which is responsible for pain and microvascular rupture resulting in hemorrhage [20]. The gold standard in diagnosis remains catheter angiography, which has shown a sensitivity of 100 % compared to noninvasive methods (MRA and CTA) which showed sensitivities of around 75 % [19].
Recent studies have shown an association between RCVS and vertebral or carotid artery dissection, particularly in postpartum females, and it may be prudent to screen the neck vessels, particularly in patients who present with neck pain [19].
The foremost mimicker of RCVS is primary angiitis of the CNS (PACNS), while the clinical symptoms are usually more gradual in onset in angiitis. Headache is usually subacute and progressive, rather than thunderclap type, and may be accompanied by transient neurological deficits eventually progressing to stroke. Asymptomatic infarcts are often seen on initial MR studies. Angiographic findings may lead to clinical confusion due to their similarities in the two pathologies.
Distinguishing features are that in at least 70 % of cases, MRI will be normal in RCVS, while it will be abnormal in PACNS in up to 90 % of cases showing multiple, deep, or superficial infarcts of different ages. CSF analysis should be normal in RCVS and abnormal in over 95 % of PACNS cases. Irregular, eccentric, and asymmetric occlusions without interspersed dilations are more suggestive of a vasculitis rather than RCVS [18]. However, such subtle angiographic differences may be difficult to appreciate, particularly due to the limits in spatial resolution of noninvasive angiography. Vessel wall imaging, which utilizes a single inversion recovery-prepared fast spin echo black blood T1-WI sequence prior to and following contrast administration, has shown promise in distinguishing the two entities [21]. Vessel wall enhancement is seen with inflammatory processes affecting the vessel lumen and is absent in RCVS.
Moyamoya
Moyamoya syndrome is an occlusive cerebrovascular disease which affects the terminal internal carotid arteries and proximal circle of Willis vessels. Associated engorgement of the lenticulostriate and thalamoperforating leads to the moyamoya or “puff of smoke” appearance on angiography. Moyamoya disease refers to the idiopathic form of the arteriopathy; moyamoya syndrome refers to an association with other pathologic processes including neurofibromatosis, sickle cell anemia, Down syndrome, and prior cranial irradiation.
Epidemiology
Incidence of moyamoya disease in Japan has range up to 0.94/100,000 of the population, with increasing incidence reported in recent years attributed to better diagnostic measures [22]. Rates of moyamoya syndrome in the United States and Europe are generally one tenth those in Japan [23]. The disease has a bimodal distribution with peaks in the first and fourth decades of life [23]. Both symptomatic and asymptomatic forms of the disease have a slight female to male predominance of about 2:1 [22].
Children generally present with ischemic events such as TIAs or strokes, while adult patients more commonly present with hemorrhage. The disease accounts for ≥6 % of strokes in children [24].
Clinical associations with moyamoya include skull base, sellar, and suprasellar tumors and optic nerve gliomas either idiopathically or as a result of radiation treatment, Fanconi anemia, sickle cell anemia and other hemoglobinopathies, neurofibromatosis type I, progeria, inflammatory factors such as anticardiolipin syndrome, protein S elevation, SLE, neuro-Behcet’s, Kawasaki disease; collagen vascular disorders such as Marfan, congenital cardiac disease, renal artery stenosis, infectious diseases such as tuberculosis, leptospirosis, tonsillitis, atherosclerosis, and fibromuscular dysplasia (Fig. 4). These clinical associations can be seen in the setting of unilateral disease.
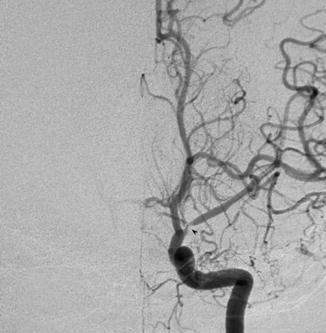

Fig. 4
Conventional angiogram showing left MCA stenosis in a child with neurofibromatosis representing moyamoya syndrome (Courtesy of M. Shroff, G. DeVeber)
Genetics
The familial form of disease accounts for about 15 % of patients.
To date one gene locus has been implicated in moyamoya: the ring finger protein 213 (RNF213) gene located on chromosome 17q has been identified as a susceptibility gene for moyamoya disease. Other gene foci identified by chromosomal linkage analyses include 3p24.2-26, 6q25, 8q23, and 12p12. The inheritance pattern is thought to be autosomal dominant with incomplete penetrance [25].
Clinical Presentation
Children with moyamoya typically present with evidence of TIAs (40 %) or infarction (40 %) [26]. Motor disturbances vary from weakness to paralysis of the extremities. Other presentations include headaches, seizures, and decreasing cognitive function. Paroxysmal symptoms have been seen in the setting of elevated body temperatures, exercise or episodes of crying, and hyperventilation leading to hypocapnia and vasoconstriction.
Forty to 65 % of adults present with hemorrhage usually localized to the basal ganglia and ventricular system. Hemorrhages are often recurrent and more common in females [27].
Hemodynamic studies, discussed below, have shown a decreased cerebral blood flow in children with moyamoya in comparison to age-matched controls and adults with the disease, an increased cerebral blood volume suggesting a chronic state of vasodilation, and an increased oxygen extraction fraction and significantly impaired cerebrovascular reactivity [28]. These findings may help to explain why ischemia is the most common presenting symptom in children.
Pathophysiology
Moyamoya disease is characterized by complex channels of medium- and small-sized muscular arteries branching off the circle of Willis, anterior choroidal arteries, internal carotid arteries, and posterior cerebral arteries and connecting to the distal portions of the anterior and middle cerebral arteries. These channels give rise to numerous, dilated or stenotic, tortuous perforating vessels that correspond to the lenticulostriate and thalamoperforating vessels. Dilated vessels are more common in younger patients, while stenotic vessels in older patients.
Microscopic findings in stenotic vessels are characterized by intimal thickening, thickening and duplication of the elastic lamina, fibrosis of the tunica media, and fibroelastosis of the wall. The media is thinned, and there is a decrease in the external diameter of the vessel [29]. Dilated vessels show fibrosis of the media, attenuation of the wall, and moderate increase in elastic fibers [29]. Lipid deposits have been found, but are thought to relate to atherosclerosis.
Hemorrhage is attributed to rupture of the perforating vessels, aneurismal rupture, or very rarely rupture of dilated collateral arteries on the brain surface. Frequency of intracranial aneurysms is increased in patients with moyamoya. Aneurysms can be saccular or dissecting and occur centrally, most frequently in the basilar arteries or peripherally [29].
Imaging
Angiography
Catheter angiography remains the gold standard in moyamoya diagnosis. While MRA is an acceptable alternative in diagnosing moyamoya, catheter angiography is recommended in adult surgical candidates, with pediatric cases considered on an individual basis [30]. The vascular picture is characterized by stenosis of the supraclinoid distal ICA, the proximal MCA and ACA branches, and the development of dilated striate arteries. Involvement of the posterior cerebral artery (PCA) is seen in about 30 % of patients, usually at the late stage of disease.
Six angiographic stages are described: [31]
1.
Narrowing of the carotid termination.
2.
Dilation of the proximal ACA and MCA with initial moyamoya blush and dilation of the perforating moyamoya vessels.
3.
Stenosis of the ACA and MCA with intensification of the moyamoya blush.
4.
Minimization of the moyamoya blush with extension of internal carotid artery (ICA) occlusion to the junction of the posterior communicating artery (PComm). The constituent vessels are thinned, and there is a concomitant increase in the collateral vessels from the extracranial carotid arteries.
5.
Further reduction of the moyamoya and complete disappearance of the main arteries arising from the ICA. Occlusion of the ICA can reach as far as C2. Persisting increase in ECA collateral circulation.
6.
Disappearance of the moyamoya, with intracranial supply from the ECA and potentially vertebrobasilar system.
Angiography can be used to identify aneurysms, define a specific transdural anastomotic vessel, and, in very rare cases, identify other vascular anomalies. Investigation of the ECA system for stenoses is also recommended and may have implications for treatment success rates [30] (Fig. 5).
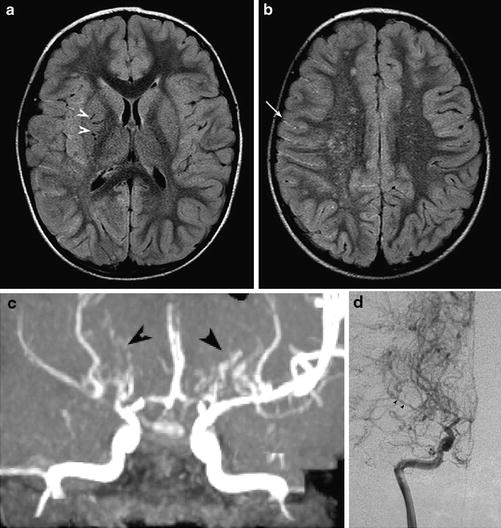

Fig. 5
Moyamoya. (a, b) Axial FLAIR images show multiple flow voids in the basal ganglia (white arrowheads), white matter leukoaraiosis in the watershed regions, and the ivy sign in the right hemispheric sulci (arrow). (c) MRA shows numerous leptomeningeal and lenticulostriate collaterals (black arrowheads), which show retrograde filling of the distal right MCA on conventional angiography, (d) (Courtesy of M. Shroff, G. DeVeber)
CT
Unenhanced CT may be completely normal or show subarachnoid and intraparenchymal hemorrhage or atrophy and infarction in the anterior circulation territory.
CTA
CTA is a powerful tool in the diagnosis of suspected vascular abnormalities in the setting of intracerebral hemorrhage. It can be used for the assessment of intracranial stenoses and the identification of aneurysms and areas of contrast enhancement. It has also been used as a method for neurosurgical planning and assessment of EC-IC bypass.
MRI
MRI findings include atrophy, particularly in the anterior circulation territory. Evidence of prior infarcts or hemorrhage can be seen on FLAIR and gradient-recalled echo imaging. Asymptomatic microbleeds, in the periventricular deep white matter, have been seen in up to 44 % of patients [32].
Diffusion-weighted imaging will detect acute infarcts. The flow voids at the skull base should be carefully scrutinized for evidence of ICA and proximal ACA/MCA diminution. Increased flow voids can also be seen within the basal cisterns, basal ganglia, and around the circle of Willis consistent with increased collateral circulation (Fig. 5a).
Increased intersulcal FLAIR signal corresponding to increased contrast in the subarachnoid space on enhanced T1-WI images has been termed the “ivy sign” and is felt to represent slow flow in dilated pial collaterals (Fig. 5b).
MRA
According to the guidelines for diagnosis and treatment of moyamoya disease, MRA can provide a definitive diagnosis when the following findings are fulfilled on time of flight (TOF) imaging using a scanner of at least 1.5-T.
1.
Stenosis or occlusion of the terminal portion of the intracranial internal carotid artery or proximal portion of the anterior and/or middle cerebral arteries.
2.
Abnormal vascular networks in the basal ganglia. When two or more visible flow voids in the basal ganglia are present at least unilaterally on MRI, they can be deemed as representing an abnormal vascular network.
3.
Bilaterality of findings 1 and 2.
In cases of unilaterality, the guideline authors consider a quasi-moyamoya or moyamoya syndrome [33].
Functional and Hemodynamic Studies
In general, hemodynamic studies show decreased cerebral blood flow, increased mean transit time, and increased cerebral blood volume in the affected hemisphere, coupled with an increased oxygen extraction fraction [30]. The cerebral metabolic rate of oxygen is often decreased, reflecting the influence of limited cerebral blood flow, particularly in ischemic cases [34]. Many perfusion studies are performed at baseline and following a vasodilatory challenge using acetazolamide or end-tidal CO2 manipulation. The use of a vasodilatory challenge allows for the calculation of cerebrovascular reactivity which is defined as a change in blood flow per unit change in stimulus, such as carbon dioxide. In the setting of a downstream stenosis, maintenance of normal cerebral blood flow is possible due to a relaxation of vascular tone, resulting in normal baseline perfusion parameters. Analogous to the myocardial stress test, a cerebrovascular reactivity study uncovers the compensatory vasodilatory state by stressing its limits. The strengths and limitations of the currently utilized functional and hemodynamic studies are discussed below.
PET is the only imaging modality that can measure oxygen extraction fraction and the cerebral metabolic rate of oxygen consumption. Its limitations include the need for an on-site cyclotron due to the rapid decay of 15O (2 min half-life) and a PET scanner, the availability of which is less than MR, CT, or SPECT. The whole body radiation exposure is 0.5–2.0 mSv, depending on the quality of the PET camera and protocol. Increased oxygen extraction fraction is linked to progression of disease and reversed with EC-IC bypass.
CT and MR are relatively easily accessible, but technically difficult in moyamoya patients due to the lack of availability of a reliable arterial input function in the diseased anterior circulation vessels, occasionally necessitating the placement of ROIs in posterior circulation vessels [35]. The presence of multiple diseased vessels precludes the accurate comparison of concentration time curves derived from one major vessel to those derived in the microvascular territories supplied by several different diseased vessels. Flow maps will only be accurate for the territory of the vessel in which the arterial input function was positioned, but not for those tissues fed by other diseased vessels [36]. A recent study of 16 patients showed poor angiographic stage correlation with normalized baseline CT perfusion parameters (with respect to ipsilateral cerebellar hemisphere), but significant correlation with percent changes of CBF during an acetazolamide challenge [37].
SPECT uses the single-pass lipophilic isotopes 99mtechnetium and 123iodine that cross the blood-brain barrier. The use of single-pass isotopes permits an estimation of blood flow, due to first pass deposition through the microcirculation, without providing quantitative measurements. Measurements are weighted to tracer delivery/deposition, but immune to the input function problem of CT and MR perfusion studies. While technetium isotope use alone requires a 24 h interval between baseline and acetazolamide challenge, the combination of technetium and iodine allows the study to be performed in a single sitting using separate windowing for the 99mtechnetium and 123iodine keV [27].
A recently developed MRI technique uses blood oxygenation level-dependent contrast (BOLD) imaging while manipulating the end-tidal CO2 (ETCO2) through a rebreathing circuit [38]. This method does not rely on input functions or pharmacologic agents and provides spatial and quantitative maps of percent BOLD signal change per mm Hg change in ETCO2. It can be performed on all 1.5 and 3-T MRI systems with echoplanar capability. In normal tissues, BOLD signal increases with hypercapnea due to a washout of deoxyhemoglobin. The paramagnetic effects of deoxyhemoglobin normally cause dephasing of protons in local tissues, reducing the returned BOLD signal. This method not only shows patients in whom CVR is exhausted but also those with a paradoxical response or steal phenomenon in patients with uncompensated moyamoya disease. CVR defects may also reverse with EC-IC bypass.
Proton MR spectroscopy has shown reduced levels of choline, creatine, and N-acetylaspartate in the affected brain of moyamoya patients with relative increase 6 months following revascularization surgery [39].
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL)
Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy is a genetic small artery disease that leads to dementia and early death.
Its main clinical features include migraine with aura, ischemic events, mood disturbances, and cognitive impairment. Other presentations such as apathy or seizures have also been described. Dementia and dependence are prominent features of the end stages of the disease [40].
Epidemiology
The prevalence of the disease is reported as 2–4 per 100,000. The mean age of disease onset is in the mid third decade, with earlier discovery of the disease in patients presenting with migraine (mean age 28 years) than those with stroke (mean age 41 years) [41]. The median age of death is reported as 65 years for males and 71 years for females, with 80 % of patients dependent by the time of death [40].
Genetics
CADASIL is a genetic disorder due to mutations in the extracellular domain of the Notch 3 gene located on chromosome 19. The gene contains 33 exons, but only mutations in exons 2–24 occur in CADASIL, 60 % occurring in exons 3 and 4.
Pathology
Macroscopic examination shows diffuse myelin pallor sparing the U-fibers. Lacunar infarcts are seen in the white matter and basal ganglia. Pontine involvement is most common in the brainstem, with relative sparing of the medulla. Microscopic examination reveals a classic non-amyloid granular osmiophilic material in the media extending into the adventitia of small- and medium-sized leptomeningeal arteries.
Fibrosis of the adventitia and sometimes duplication and hyalinosis of the lamina are described. Loss of vascular smooth muscle cells occurs but to a lesser extent than in Binswanger’s dementia [42]. Apoptosis in layers 3 and 5 of the cortex is associated with increased severity of white matter lesions [43].
MRI Imaging
MR imaging shows areas of FLAIR hyperintensity in the white matter, pons, and external capsules (Fig. 6).
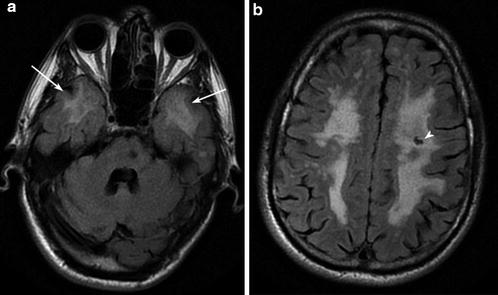

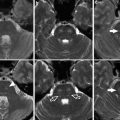
Fig. 6
CADASIL Axial FLAIR images show widespread T2 signal hyperintensity in the periventricular, deep, and subcortical white matter, volume loss, temporal pole involvement (arrows), and subcortical lacunar infarcts (arrowhead)
Microbleeds are seen in 25–69 % of patients and were present most frequently in the thalami, cortical/subcortical regions, white matter, pons, and basal ganglia [44, 45]. Number does not correlate with white matter lesion burden, but is increased with age. Similarly, dilated Virchow-Robin spaces, mainly in the lentiform nuclei and subcortical white matter of the temporal lobes, are seen significantly more frequently in CADASIL patients than age-matched controls and show a correlation with increasing age, not white matter lesion burden [46].
Recent studies have shown a correlation with the disease burden at diagnosis and severity of progression [44].
CARASIL
Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy is a recently recognized single gene disorder caused by mutations in the HTRA1 gene. Its prevalence is currently unknown, with approximately 50 patients described in the literature to date, mostly from Japan with a 3:1 male to female ratio.
Its main clinical manifestations are ischemic stroke, progressive dementia, early alopecia, and spondylosis deformans. Ischemic stroke occurs in about half of patients and the onset of dementia is in the fourth decade (mean 32 years). Alopecia, seen in 90 % of patients, usually presents in the third decade, but may be seen in adolescence. Low back pain is seen in 80 % of patients, with an onset between 20 and 40 years.
CT shows diffuse white matter hypodensity, volume loss, and corticospinal tract Wallerian degeneration. Similarly, MRI studies show T2 hyperintense periventricular and deep white matter lesions, with some sparing of U-fibers. Lesions subsequently extend into basal ganglia, thalami, brainstem, and cerebellum. Temporal lobe involvement may be seen, similar to CADASIL [47].
Vasculitis
PACNS
Primary angiitis of the CNS (PACNS) is a rare, potentially severe, form of vasculitis that involves the small- (200–300 μm in diameter) and medium-sized (500 μm) vessels of the brain and spinal cord. PACNS is also referred to as granulomatous angiitis, granulomatous giant cell angiitis, noninfectious granulomatous angiitis, and isolated angiitis. Its current name reflects the fact that many cases have a non-granulomatous histology.
Epidemiology
PACNS has an estimated incidence of 2.4 per 1,000,000 inhabitants, with a slight female predominance [48, 49]. Although recent large cohort studies state a slight female predominance, caution must be taken in interpreting this data, as several studies include patients with angiographic, but not pathologic, proof of disease and, therefore, may occasionally include patients with benign angiopathy of the CNS, who were once characterized as a subset of PACNS with a more favorable prognosis and have since been reclassified as RCVS. Earlier studies showed either a slight male predominance of 4:3 or no sex predilection. The median age of onset is about 50 years [49].
Clinical Symptoms
Most frequent symptoms include headache, altered cognition, focal neurological manifestations, stroke, and visual symptoms [48]. Onset is usually gradual and progressive.
The largest series includes 101 patients, showing no statistically significant correlation with male gender and increased mortality rate or higher disability scores (modified Rankin scale 4–6). Instead, presentation with a focal neurological deficit rather than headache, cognitive impairment versus headache or constitutional symptoms, cerebral infarction versus no infarction, and large-vessel versus small-vessel involvement significantly correlated with increased mortality.
The disease in children may more often present with seizures [49].
Pathology
Biopsy of the brain or spinal cord is considered the gold standard. Biopsy results are often negative in 25–50 % of cases [48]. Including leptomeninges in the biopsy results in slightly higher diagnostic yields [49].
Three patterns are recognized: granulomatous, lymphocytic, and necrotizing. Findings include inflammatory infiltrates of giant cells, plasma cells, lymphocytes in the vessel wall accompanied by necrosis, or granuloma formation. A sparse perivascular mononuclear cell infiltrate is insufficient for diagnosis as it can be seen in many other conditions. Necrotizing vasculitis is associated with the destruction of the internal elastic lamina.
CSF analysis is abnormal in 80–90 % of cases. ESR and CRP are more likely to be elevated in children [49].
Imaging
CT imaging will show nonspecific findings of vasculitis such as infarcts or hemorrhages and is less sensitive than MRI.
MRI imaging is abnormal in nearly all cases, even those that are angiography or biopsy negative, and most commonly shows cortical and subcortical infarcts, which are frequently multiple and frequently involve both cerebral hemispheres. Infarctions may occur in a large or perforator artery distribution. Intracranial hemorrhage occurs in about one tenth of patients [48]. Contrast enhancement of the meninges, perivascular spaces, and parenchymal mass lesions may occur [48]. Although nonspecific, negative MRI and negative CSF sampling are thought to have a high negative predictive value for the presence of vasculitis. The specificity of both tests is estimated at 36 % (Figs. 7 and 8).
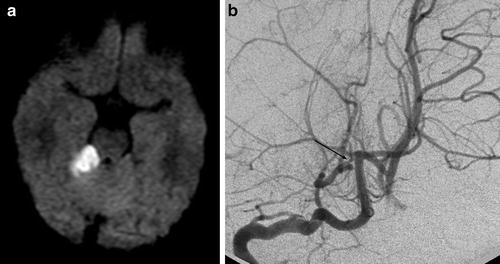
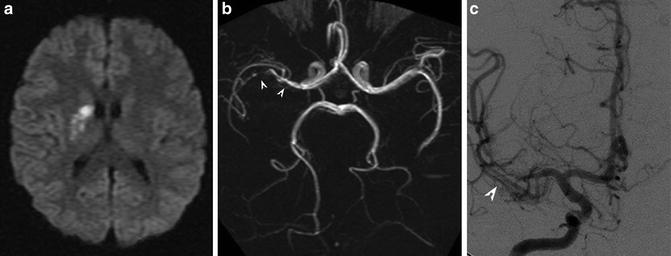

Fig. 7
PACNS. Superior cerebellar artery (SCA) infarct shown on DWI (a) secondary to stenosis of the right SCA shown on the right vertebral artery injection (b) (Courtesy of M. Shroff, G. DeVeber)

Fig. 8
PACNS. (a) DWI showing right MCA lenticulostriate territory infarction. (b, c) MR and digital subtraction angiography show beading and marked stenosis of the right M1 and M2 vessels
High-resolution pre- and postcontrast T1-WI black blood imaging of the vessel walls has been used to distinguish vasculitic processes from atherosclerosis and RCVS (Fig. 9). Although this is a novel technique, preliminary results have shown concentric luminal enhancement in vasculitis, eccentric enhancement in atherosclerosis, and lack of enhancement in RCVS.
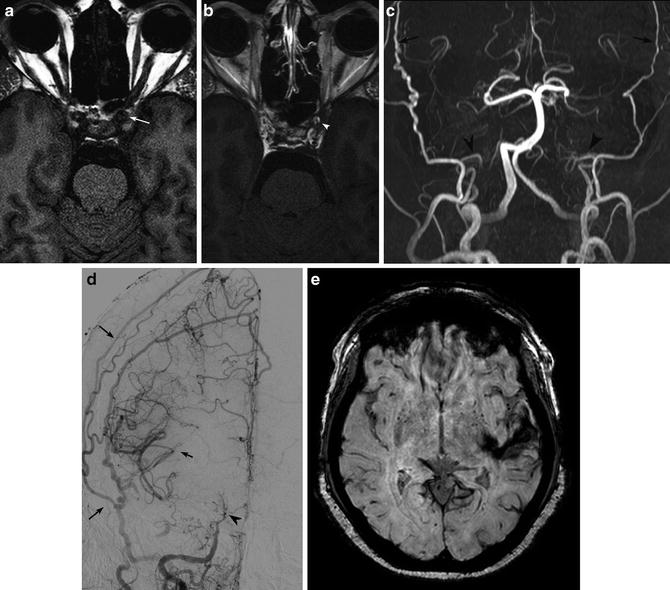

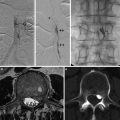
Fig. 9
Vessel wall imaging shows thickening (a, white arrow) and enhancement (b, white arrowhead) of the cavernous ICA walls in a patient with rheumatoid arthritis and SLE. Marked bilateral ICA stenosis (c, d) (black arrowheads) and resultant collateralization through ECA branches (black arrows). The patient presented with left temporal lobe hemorrhage on SWI (e)
Angiography
The classic angiographic signs of vasculitis are alternating areas of stenosis and dilation (beading) involving both large and small vessels. A recent study showed angiographic abnormalities in 90 % of patients with PACNS [48]. MRA and CTA show similar findings, although with a lower sensitivity owing to the limited spatial resolution of 500 μm, below the threshold of detecting small-vessel vasculitides.
Takayasu
Epidemiology – 1–2/1,000,000, with more reports initially in South East Asia, but recent reports also in the western world.
Pathology
This is a large-vessel vasculitis with predominant involvement of the aorta and great vessels. Histologically, findings are those of a chronic large-vessel vasculitis characterized by lymphocytic, NK cell, neutrophil, and macrophage infiltration in the outer thirds of the media and adventitia. Giant cells and granulomas have been described. The elastic fibers are fragmented, and there can be loss of smooth muscle cells, medial weakening, and vascular dilation. Reactive fibrosis and aneurismal formation is seen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree