Fig. 1
Right temporal lobe cavernous malformation. (a) T1-weighted MR. (b) Postcontrast T1-weighted MR. (c) FLAIR image. (d) Gradient-echo (GRE) T2*-weighted MR. (e) Diffusion-weighted MR. The lesion presents the characteristic “popcorn” magnetic resonance appearance, secondary to blood components in different stages of degradation (a–c). A hypointense rim is well demonstrated on FLAIR images (c). A subtle contrast enhancement can be seen after intravenous gadolinium administration (b). GRE T2* weighted confirms the hemorrhagic component of the lesion. Subacute hemorrhagic foci within the lesion can demonstrate restricted diffusion, due to the presence of methemoglobin (e)
CCMs are considered one of the four most common types of vascular malformations, accounting for approximately 8–16 % of all cerebrovascular malformations. Their estimated prevalence on the basis of MR imaging findings is up to 0.4–0.5 % of the population [6]. Two forms have been described: a sporadic form, in which patients usually have an isolated lesion, and a familial form, usually characterized by multiple lesions, with an autosomal dominant pattern of inheritance [7]. Typically, patients with a single lesion have a sporadic form of the disease, while those with multiple lesions (10–30 % of all cases) often have an autosomal dominant form localizable to the CCM1/KRIT1, CCM2/MGC4607, or CCM3/PDCD10 gene loci. Genetic linkage analyses mapped three CCM loci to chromosome 7q (CCM1), 7p (CCM2), and 3q (CCM3) [8]. The hallmark of familial CCM is the presence of multifocal lesions throughout the brain with the appearance of new lesions over time (Fig. 2). However, in about 20 % of individuals with multiple lesions, no mutation is observed [8]. The sporadic form of CCM is often characterized by a solitary lesion (or a cluster of lesions). There is a high association between CCM and DVA (Fig. 3). This association with a DVA is more likely to define the nongenetic familial form of the disease. Familial CCMs are unlikely to be associated with DVA, while sporadic forms have a high association rate with this vascular malformation [9]. Even though the multiplicity of lesions is characteristic of the familial form, it has been reported in 10 % to up to 33 % of supposed sporadic cases. Additionally, they are commonly seen after therapeutic irradiation of the brain (Fig. 4), along with capillary telangiectasias in the radiotherapy bed [10].
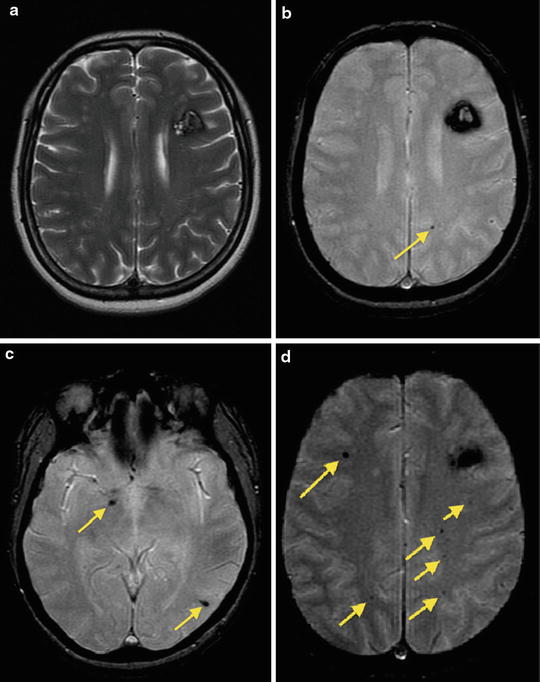
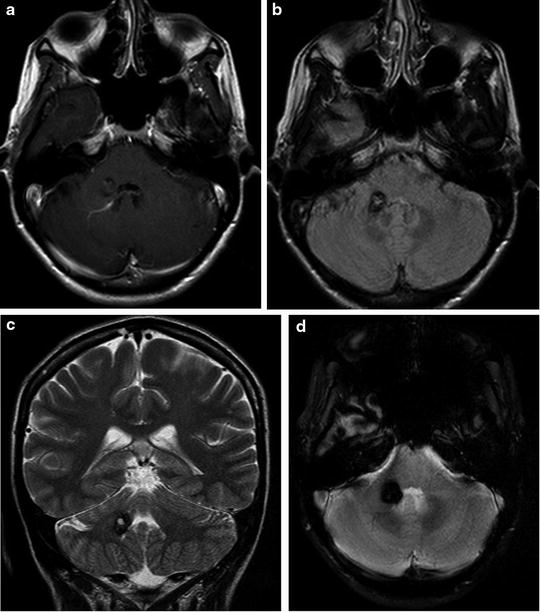
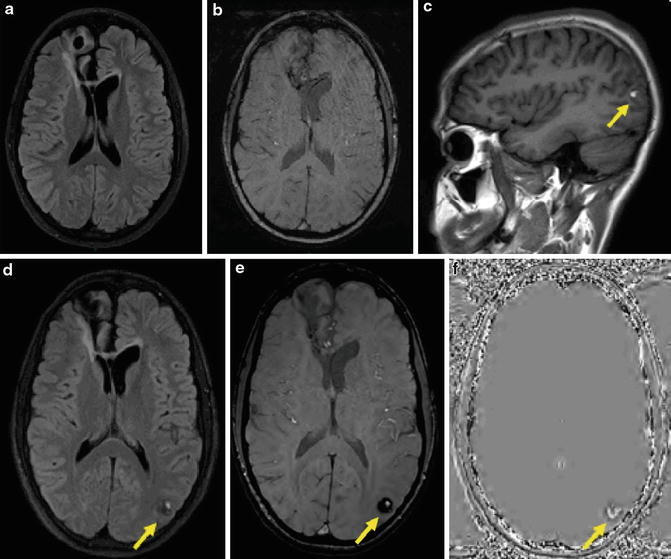

Fig. 2
Multiple cavernous malformations in a patient with familial form. Axial T2-weighted (a) and GRE T2*-weighted (b) images demonstrate a CCM lesion in the left frontal lobe. Note multiple small foci hypointense lesions on GRE T2*-weighted images (b–d, arrows) consisting with small cavernous angiomas

Fig. 3
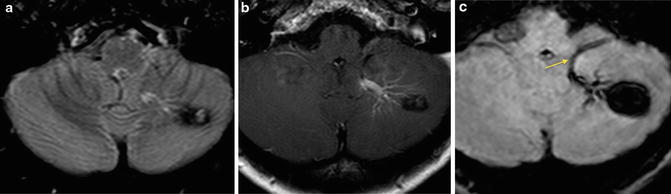
Cavernous malformation with associated developmental venous anomaly. Axial postcontrast T1-weighted (a) and FLAIR images (b) and coronal T2-weighted (c) image demonstrate an heterogeneous signal intensity lesion, hypointense on GRE T2* weighted, without mass effect, in the right cerebellum peduncle, adjacent to the fourth ventricle. There is a developmental venous anomaly adjacent to the CCM (a)

Fig. 4
A 15-year-old male with germinoma was treated with surgery followed by radiation therapy and chemotherapy. FLAIR (a) and SWI (b) obtained 2 years after the treatment show postsurgical changes in the right frontal lobe, and no lesion is seen in the left occipital lobe. Sagittal T1-weighted (c), axial FLAIR (d), SWI (e), and SWI phase (f) images obtained 4 years after the radiation therapy show a round lesion of mixed signal intensity with peripheral hypointense rim in the left occipital lobe, suggestive of cavernous angioma
Although CCM can occur in virtually all parts of the central nervous system, they are much more common in the cerebral hemispheres, followed by the infratentorial compartment. A superficial cerebral hemisphere location with proximity to the subarachnoid space (Fig. 5) and the ventricle is very common. Infratentorial involvement corresponds roughly to one fourth of the cases. Although the brain stem and the cerebellum hemispheres are equally affected, the pons is the most common location of the brain stem (Fig. 6). CCM can also rarely occur in the spinal cord (Fig. 7), where it frequently coexists with multiple brain lesions (Fig. 8) [11, 12].
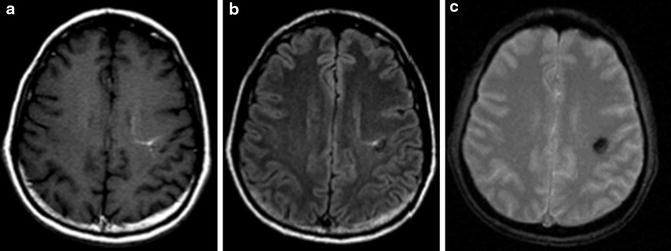
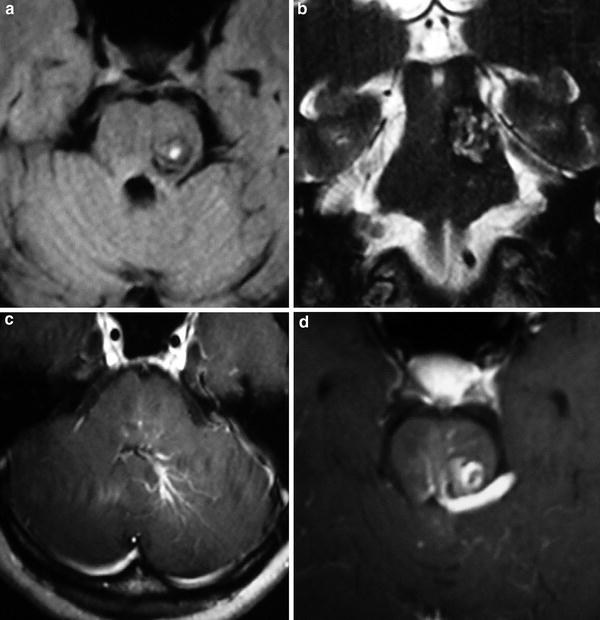
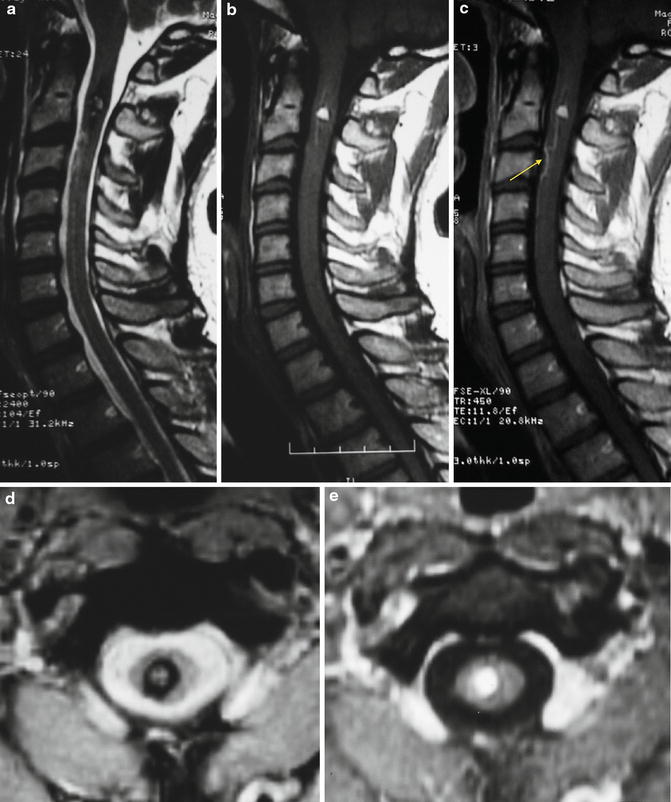
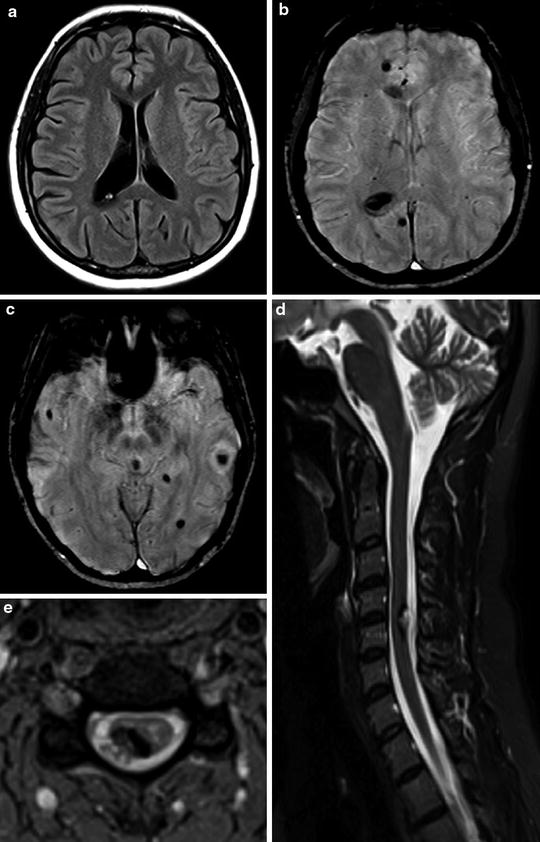

Fig. 5
Cavernous malformation with associated developmental venous anomaly located at the subcortical region of the left frontal lobe. (a) Postcontrast T1-weighted MR. (b) FLAIR MR. (c) GRE T2*-weighted MR. The lesion demonstrates the heterogeneous signal characteristics of hemorrhage in various stages of resolution. A developmental venous anomaly is seen after the intravenous contrast administration

Fig. 6
Cavernous malformation with associated developmental venous anomaly located in the left aspect of the pons. Axial FLAIR (a) and coronal T2-weighted MR (b) images show a round heterogeneous lesion, with a hypointense rim. On the axial postcontrast T1-weighted MR (c, d) images, a developmental venous anomaly is identified with a drainage vein at the inferior aspect of the fourth ventricle, the left ambiens cistern, and the quadrigeminal cistern

Fig. 7
Cavernous malformation in the cervical spine. A heterogeneous cervical spine lesion with peripheral hypointense rim on the T2-weighted (a) and GRE T2*-weighted (d) images and spontaneous hyperintense on T1-weighted image (b) is seen. On T1-weighted MR after intravenous contrast administration (c), a venous structure is demonstrated inferiorly to the described cavernoma (arrow)

Fig. 8
Multiple cavernous malformations. Axial FLAIR image (a) demonstrates a subependymal CCM in the atrium of the right lateral ventricle. On susceptibility-weighted images (SWI), multiple cavernomas can be detected as hypointensity lesions (b, c). Sagittal T2-weighted (d) and axial GRE T2*-weighted (e) images also demonstrate a cavernoma in the posterior and right aspect of the cervical spine, with an exophytic component (d, e)
Extra-axial lesions are extremely atypical and most lesions arise from the leptomeninges or the cranial nerves (Fig. 9). They have been described in the cerebellopontine angle, intraventricularly, in the pituitary fossa, optic chiasm, and cavernous sinus. Extra-axial cavernous lesions usually demonstrate atypical findings on cross-sectional imaging, including hyperintense signal intensity on T2-weighted sequences and intense homogenous enhancement. Dural-based cavernomas in association with dural sinuses have also been described [13–15].
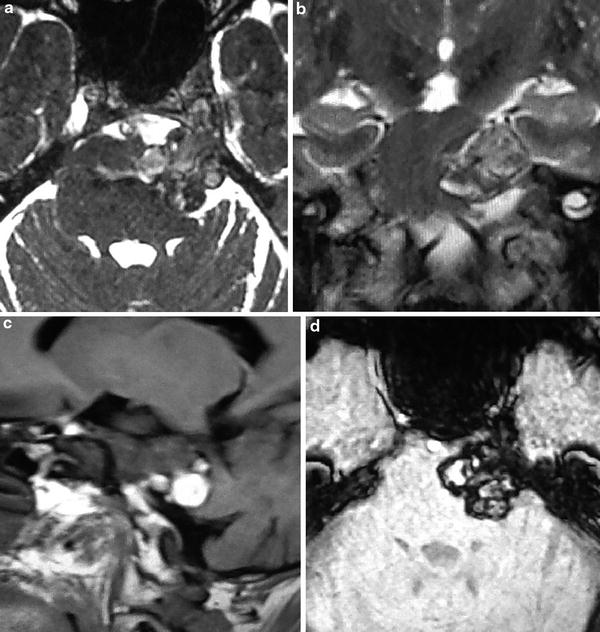

Fig. 9
Cavernous malformation of the left trigeminal nerve. An expansive, extra-axial lesion of mixed signal intensity on both T2-weighted (a, b) and T1-weighted (c) images, involving the cisternal component of the left fifth cranial nerve, invading the ipsilateral Meckel cave. On GRE T2*-weighted (d) image, the lesion presents areas of low signal intensity, as well as a hypointense rim
Clinical Manifestations
Most lesions are found incidentally in neuroimaging studies and may remain asymptomatic throughout life. They can be discovered by an episode of symptomatic hemorrhage or gradual and nonspecific symptoms can be seen. Usually, patients clinically present with CCMs between the fourth and sixth decades of life, but symptoms can start early in life. Common clinical manifestations are seizures, focal neurological deficits, and hemorrhage (Fig. 10) [11, 12]. Symptoms related to mass effect and headache can also occur.
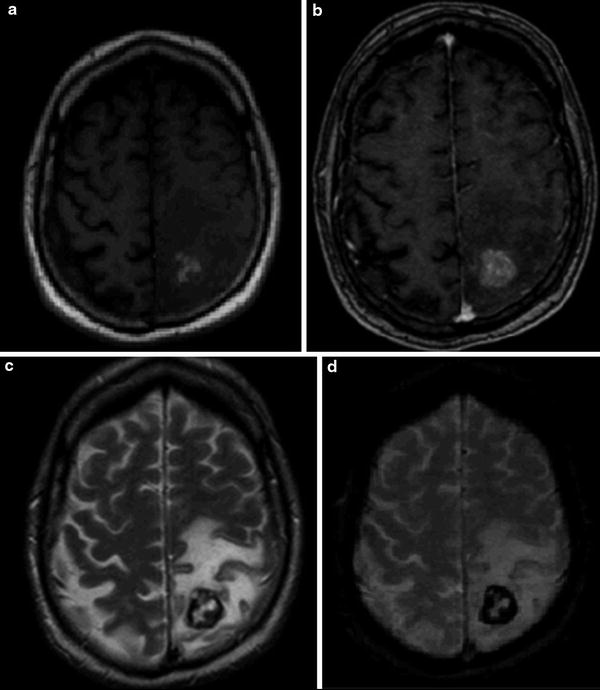

Fig. 10
Supratentorial cavernous malformation with a recent hemorrhage. An 80-year-old man with mental confusion, seizures, and right hemiparesis. Axial T1-weighted (a) and T2-weighted (c) images demonstrate an expansive, round, and hemorrhagic lesion in the left postcentral gyrus, surrounded by moderate amount of edema, which also involves the precentral gyrus. The postcontrast T1-weighted image (b) shows mild contrast enhancement. The susceptibility artifacts generated by the blood products can easily be seen on GRE T2*-weighted image (d)
When clinically apparent, seizures are reported as the most common symptom, occurring in 40–60 % of the symptomatic patients, and are the most frequent manifestation of the supratentorial lesions. Seizures are most often focal, can be refractory to treatment, and are thought to be related to cortical involvement associated with irritative effect of hemorrhage and the inflammatory reaction, as well as to some degree of cortex compression. A long-standing seizure without any acute episode of hemorrhage can be frequently observed.
Focal neurologic deficits can occur in 10–45 % of the symptomatic patients. It is a rare condition, mostly seen in infratentorial involvement. Progressive deficits are more related to lesion growth and less commonly to an episode of hemorrhage. The enlargement of the lesion is more likely to be caused by chronic or recurrent extravasation of blood and thrombosis.
It is well known that small and subclinical hemorrhages are a common occurrence. Symptomatic hemorrhage is a rare condition. The risk of a hemorrhage from a CCM is thought to range from 0.1 % to 0.7 % per year and can reach up to 25 % in patients with a prior history of hemorrhage. The risk of bleeding is increased in young and female patients with infratentorial lesions. Seizures and acute neurological deficits are the most frequent symptoms reported. Extension of hemorrhage to the subarachnoid space and to the ventricles is uncommon, as well as superficial siderosis, secondary to multiple hemorrhagic episodes [11, 12, 16].
CCMs can exhibit a wide range of behaviors, including enlargement, regression, and de novo formation. These lesions have also been described to be associated with prior brain radiotherapy. CCMs take about 1–26 years to develop after radiation [10]. The diagnosis of cavernoma should be considered when a new hemorrhagic lesion appears in the radiation bed, particularly if the radiation therapy was performed in childhood (Fig. 4).
Histological Findings
By gross pathology, CCM presents as a well-circumscribed, red-colored spherical or lobulated mass lesion, with a grapelike configuration, containing multiple thin-walled vascular structures. A rim of gliotic brain tissue usually delimits the periphery of the CCM with a variable degree of extralesional hemosiderin pigment from prior hemorrhages. There is no capsule involving the lesion [4, 5].
Upon microscopic examination, CCM has a classic appearance of “popcorn” or a “mulberry-like” cluster of sinusoidal blood cavities surrounded by a single layer of endothelium, with little or no intervening neural tissue. The vascular structures are filled with blood, thrombi in different stages, and organized connective tissue. Adjacent brain parenchyma is usually gliotic. Almost all CCMs have peripheral deposition of hemosiderin, suggesting the occurrence of multiple episodes of asymptomatic bleeding. Calcifications and cholesterol deposit are common findings within them. Arteriovenous shunting is absent, and irrigation, as well as drainage, is performed by normal vasculature. Different from arteriovenous malformations, no muscular or elastic tissue is present in vessel walls, no matter their thickness [4, 5, 17].
Although the exact mechanism underlying the pathogenesis of CCMs is unknown, the importance of the mechanism involved in the proliferation and differentiation of angiogenic precursors and of members of the apoptotic mechanism has been pointed out as a key regulator of the pathogenesis of CCM [6].
Neuroimaging
Approximately 80 % of the CCM cases are supratentorial intra-axial lesions. However, they can be found anywhere in the central nervous system, including the brain stem and spinal cord. Extra-axial lesions have also been described. They are usually solitary, although up to one third of patients with sporadic lesions have more than one lesion. In the familial form, multiple lesions are usually seen in supratentorial and infratentorial compartments. Lesions vary in size from few millimeters to several centimeters across, with an average size of around 1.5 cm. They usually lack mass effect, unless there have been recent episodes of bleeding. Typical lesions show little or no enhancement [7, 11, 12, 14].
Computed tomography (CT) can detect CCM as a spontaneous high-attenuation lesion, with a variable presence of calcification. However, CT can be normal in around 30–50 % of the cases. Although CCM may be diagnosed by using CT scans, MR imaging is much more sensitive for demonstrating these lesions. Owing to the blood stagnation phenomenon, or chronic microhemorrhage, CCM contains large amounts of deoxyhemoglobin or hemosiderin, which generate the susceptibility effect, causing marked signal loss in specific MR sequences, including susceptibility-weighted imaging (SWI) and GRE sequences, particularly a T2*-weighted GRE sequence. These sequences are highly sensitive for the susceptibility artifact, which make them especially useful in the detection of smaller and concomitant cavernous lesions that may not be detected with traditional sequences.
Thus, the sensitivity of MRI to flowing blood and blood products of varying ages, combined with the greater contrast resolution of MRIs, greatly increases the specificity of MRI compared to any other modality and has largely eliminated misdiagnosis of CCM. MRI is also used in the follow-up monitoring of patients with known CCM and for the assessment of family members in whom similar lesions are suspected. In addition, it is extremely helpful in presurgical planning to assess the extent of the lesion, define borders, and plan the surgical approach and exposure.
Computed Tomography: CT scanning is not sensitive for the diagnosis of CCM and can be normal in 30–50 % of patients. When this lesion is detected on CT scans, it usually appears as a rounded slightly hyperdense lesion that may or may not have interspersed calcifications. If there has been a recent episode of hemorrhage, then it is more conspicuous and may be surrounded by a mantle of edema. Although most lesions do not enhance, faint enhancement can occasionally be seen. CTA does not bring additional information, unless there are associated malformations, such as DVAs.
Magnetic Resonance Imaging: MRI is the method of choice, demonstrating a characteristic “popcorn” or “mulberry” appearance with a rim of signal loss due to hemosiderin, better depicted on susceptibility-weighted sequences [12, 14]. Recently, more advanced imaging techniques such as high-field and susceptibility-weighted magnetic resonance imaging (SWI) have been employed for the evaluation of CCMs. Furthermore, diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI) have been applied to the preoperative and intraoperative management of these lesions.
T1-weighted sequences can demonstrate a round heterogeneous lesion, with a “popcorn” or “mulberry” appearance, containing blood components in different stages of degradation. Deoxyhemoglobin related to acute bleeding has intermediate signal intensity on T1W images, whereas methemoglobin related to subacute bleeding shows hyperintense signal. Hypointense areas correspond to hemosiderin deposition, related to chronic hemorrhage.
T2-weighted sequences reveal a heterogeneous lesion surrounded by a marked hypointense rim, reflecting hemosiderin deposition.
FLAIR sequences can demonstrate perilesional edema in cases with recent episodes of bleeding.
Since cavernomas contain large amounts of deoxyhemoglobin (blood stagnation) or hemosiderin (chronic microhemorrhage), sequences that are highly sensitive for susceptibility artifact such as SWI and GRE T2*-weighted sequences are extremely useful for detecting marked signal loss within the lesions (Fig. 11). Especially for multiple lesions, frequently associated with the familial form of the disease, these techniques are of great use. SWI seems to be more sensitive than T2* to detect cavernomas (Fig. 12) [18]. The use of the blood pool agent ferumoxytol in susceptibility-weighted MRI has been recently discussed and seems to enhance the visibility of CCM, among other vascular malformations [19]. Since ferumoxytol has a good biocompatibility profile, has no known long-term toxicity, and, in contrast to the gadolinium-based contrast agents, can be safely given to patients with decreased renal function, it seems to be a promising tool for the diagnosis and follow-up of cavernomas. However, further studies are necessary to support the practical use of this agent.
Diffusion-weighted images (DWI) usually do not demonstrate restricted diffusion unless there is subacute hemorrhage (Fig. 1).
Contrast-enhanced T1-weighted sequences can demonstrate minimal or absent gadolinium enhancement. Associated DVAs can also be detected in T1-weighted postcontrast images.
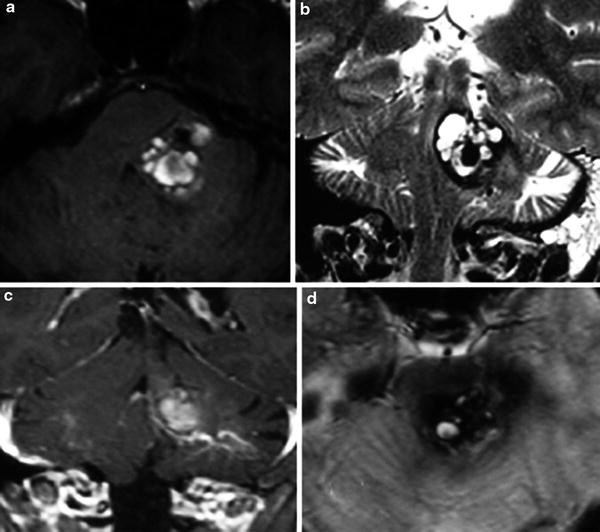
Fig. 11
Cavernous malformation associated to a developmental venous anomaly. (a) Axial T1-weighted MR. (b) Coronal T2-weighted MR. (c) Coronal postcontrast T1-weighted MR. Axial GRE T2*-weighted MR. A left pontine and middle cerebellar peduncle CCM with the classic “popcorn” MRI appearance, surrounded by a hypointense rim. The lesion causes compression of the fourth ventricle. After the intravenous contrast administration, a DVA is observed adjacent to the inferior aspect of the CCM
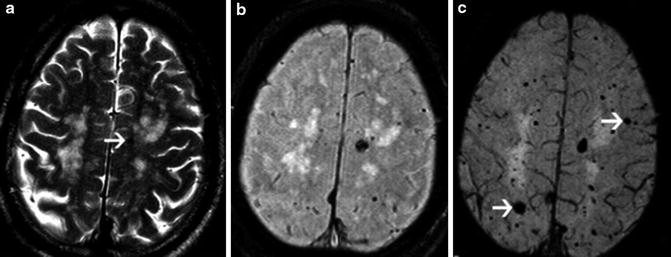
Fig. 12
A 74-year-old male patient with familial CCM. Axial T2-weighted image (a) shows areas of white matter high signal intensity and a small focus of low signal intensity on the left (arrow). T2-weighted GRE image (b) demonstrates multiple foci of low signal intensity on the frontoparietal regions. SWI (c) shows a higher number of lesions and confirms the presence of larger lesions (arrows), which are not seen on the T2-weighted GRE image (Reprinted with permission from Ref. [18])
Digital Subtraction Angiography: On DSA, most CCMs are angiographically occult and do not demonstrate arteriovenous shunting. When they are evident on angiograms, the findings are nonspecific and include minimal vascular blush in the late venous phase. When they occur in combination with other complex vascular malformations, angiography can be helpful in defining other lesions.
A classification system based on imaging and pathologic features has been reported to stratify these lesions [20]. Type I lesions are characterized by hyperintensity on both T1WI and T2WI, which is consistent with subacute hemorrhage. In Type II, loculated regions of hemorrhage are surrounded by gliosis and hemosiderin-stained brain parenchyma. These CCMs exhibit a mixed signal intensity core on both T1WI and T2WI, with a well-circumscribed hypointense rim on T2WI, and are the classic CCM with a “popcorn” appearance. Type III lesions demonstrate a core that is iso- or hypointense on T1WI and hypointense on T2WI, surrounded by a hypointense rim on T2WI, consistent with chronic resolved hemorrhage or hemosiderin within and surrounding the lesion. Type IV corresponds to tiny lesions often seen as punctate hypointense foci on susceptibility sequences [21].
Differential Diagnosis
Although cavernomas show a classic aspect on MRI, some conditions can be misdiagnosed as the multiple familial forms, giant cavernomas, and CCM associated with brain parenchyma hemorrhage. Multiple foci lesions with hypointense lesions on susceptibility sequences can be a variety of pathologic conditions that may mimic CCMs and have to be considered in the differential diagnosis. This variety of conditions include residual hyperintense hemorrhage; infections, specially treated toxoplasmosis, and fungus infection, like aspergillosis and candidiasis; prior radiation therapy; disseminated intravascular coagulopathy; amyloid angiopathy; diffuse axonal injury and metastatic lesions secondary to melanoma and choriocarcinoma; and metastasis from the lung, kidney, and thyroid neoplasms (Fig. 13) [22].
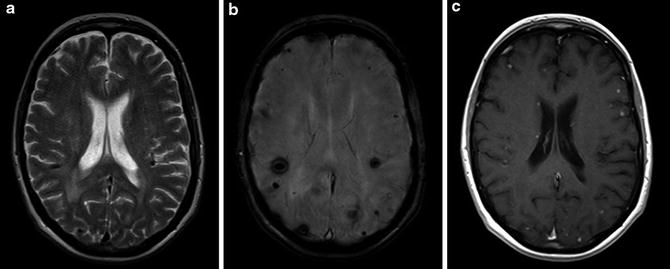

Fig. 13
A 77-year-old woman with lung cancer. Multiple hemorrhagic metastatic lesions are better demonstrated on SWI (b) image than on T2-weighted image (a). Postcontrast axial T1-weighted image (c) shows multiple enhancing small lesions in the cortical-subcortical junction of the hemispheres and at the subependyma of the right lateral ventricle
The distinction of CCM from hemorrhagic neoplasm may be difficult (Fig. 14). Some imaging features can be useful and can allow differentiation from a neoplasm. Some imaging signs are more likely to be observed in CCMs, such as absence or little surrounding edema, absence of identifiable nonhemorrhagic neoplasm, complete hemosiderin ring in the adjacent parenchyma, and expected temporal evolution of the hematoma. The presence of T1 hyperintensity in the perilesional edema around a hemorrhagic lesion can be able to differentiate CCM from other hemorrhagic masses, like hemorrhagic tumors and intracerebral hemorrhages (Fig. 15) [23].
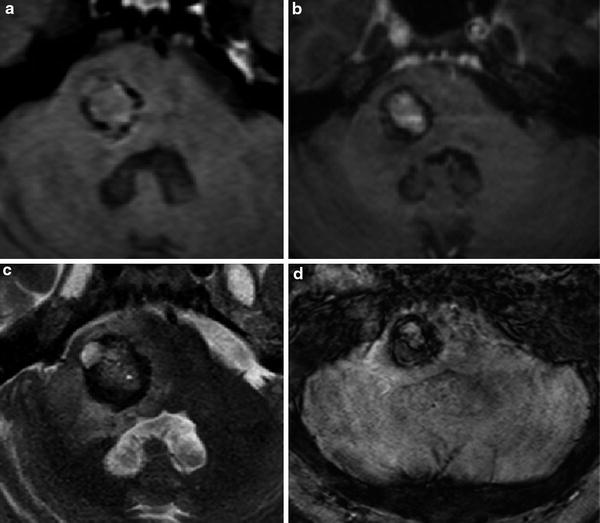
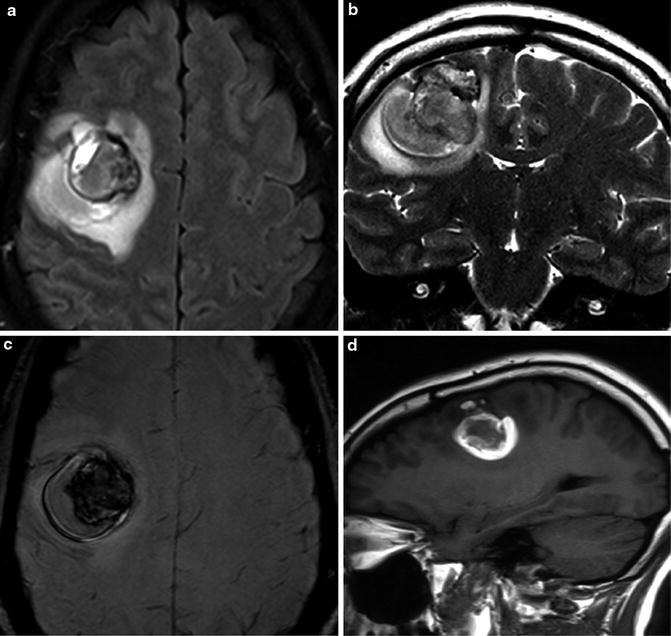

Fig. 14
A solitary breast metastatic lesion on the right lateral portion of the pons in a 65-year-old woman can be confused with CCM. Axial T1-weighted (a), T2-weighted (c), and GRE T2*-weighted (d) images demonstrate an expansive mixed signal intensity lesion, with hemorrhagic component, associated with a hypointense rim, surrounded by edema. The lesion causes compression to the anterior and lateral portion of the fourth ventricle. Enhancement of the central portion of the lesion was seen after gadolinium administration on T1-weighted image (b). The histological diagnosis was made after biopsy

Fig. 15
Axial FLAIR (a), coronal T2-weighted (b), and SWI (c) images demonstrate a hemorrhagic mass with heterogeneous signal intensity, surrounded by edema in the right frontal lobe. Sagittal T1-weighted image (d) demonstrates hyperintensity within the vasogenic edema. Note the centripetal pattern of the T1 hyperintense perilesional signal intensity sign, observed only at the deep area around the hemorrhagic lesion, but not observed at the peripheral margin. The histological diagnosis of CCM was confirmed after surgical excision
Treatment and Final Considerations
Although most CCMs can simply be followed over time, surgical removal is an option in lesions causing significant morbidity related to mass effect, epileptic activity, or repeated hemorrhage. Since cavernomas are well circumscribed and surrounded by a gliotic rim, surgical removal is relatively simple. Control of hemorrhage is usually easier because blood flow through the lesions is slower than that expected in more highly vascularized lesions. The association with DVA should be considered before performing a surgical removal, due to the risk of causing a venous infarct in the surrounding parenchyma. Multiple sequence MRIs play an important role in presurgical planning to assess the extent of the lesion, define borders, and plan the surgical approach and exposure.
Cerebral Cavernous Malformation
General features | Imaging modalities | Neuroimaging characteristics | Hot spot |
|---|---|---|---|
Common cerebral vascular malformation with classic appearance on MRI | MRI is the modality of choice | Characteristic “popcorn” or “mulberry” appearance on MRI with heterogeneous signal intensity within the lesion and a rim of signal loss due to hemosiderin, better depicted on susceptibility-weighted sequences (SWI and GRE T2*) | Look for rounded areas of marked signal loss in susceptibility-weighted MR sequences for detection of smaller and concomitant cavernous lesions that may not be detected with traditional sequences |
Isolated or multiple lesions. In the latter type, they are often familial, with autosomal dominant transmission | CT scans can be normal in 30–50 % of cases | Lack of mass effect, unless there has been recent bleeding | |
Occur in all parts of the central nervous system but most commonly in the cerebral hemispheres | Angiographically occult | Little or no enhancement |
Developmental Venous Anomaly
Developmental venous anomaly (DVA), also known as venous angioma or venous vascular malformation, is the most frequently encountered cerebral vascular malformation. Usually asymptomatic, it is frequently reported as a fortuitous finding in imaging studies. DVAs represent a purely venous entity, with no arterial component. They are mostly considered extreme anatomical variations of the transmedullary veins that are necessary for the drainage of normal brain tissue. Although DVA was thought to be rare before the advent of CT and MR imaging, it is now considered the most common anomaly of the intracranial vasculature, accounting for 63 % of vascular malformations, with an overall incidence of around 2.5 % [24].
DVAs are believed to be adaptations to accidents occurring during embryogenesis between the fourth and seventh stages of embryologic development, resulting in occlusion or maldevelopment of either the superficial or deep veins. Due to the plasticity of the vascular system at this stage, DVAs are formed as compensatory pathways, recruiting and dilating preexisting transmedullary veins [25, 26]. Since the involved vessels are not abnormally formed, but apparently merely dilated without any proliferative potential, the term DVA seems to be more appropriate than “venous angiomas” or “venous vascular malformations” [26].
Although DVAs can be seen anywhere in infra- or supratentorial compartments, draining either to superficial or deep vein systems, the most common locations are the frontoparietal regions, accounting for 36–64 %, usually draining toward the frontal horn of the lateral ventricle. The cerebellar hemispheres are also a very common location, estimated to represent 14–27 % of the cases, draining toward the fourth ventricle [27]. They may be isolated, draining one territory, or may compose a complex venous draining system of a whole cerebral or cerebellar hemisphere.
DVAs frequently coexist with other types of CNS vascular malformations such as arteriovenous malformation, capillary telangiectasia, and, especially, cavernous angiomas (15–30 % of patients) (Fig. 16) [28]. Indeed, the association between DVAs and cavernous malformations is so common that the presence of a DVA on an imaging study should promptly demand a search for a cavernoma, which is more clinically important. They are also associated with head and neck venous malformations (Fig. 17) and hemangiomas and, more rarely, with cortical dysplasia, since both occur at the same developmental stage. DVA is thought to be a manifestation of the underlying abnormal neuronal migration and not its cause [29]. In a prior publication, the frequency of DVAs in patients with cervicofacial venous malformations was described as around 20 %. Most of these cervicofacial malformations were superficial and extensive [30].