Another aspect of the evaluation of brain masses is to determine whether a lesion is solitary or multiple. Brain metastases can be multiple or single, and sometimes, differentiation of a single brain metastasis from a primary brain tumor is difficult. Some brain tumors can also be multicentric such as multicentric meningiomas, true multicentric gliomas, or multicentric primary CNS lymphoma (PCNSL). Some syndromes such as neurofibromatoses 1 and 2 predispose patients to the development of multiple brain tumors.
CT density and MR signal intensity
The CT attenuation and MR signal intensity of brain tumors depend on many factors, such as the type of tumor histology, cellularity, and presence of cysts, hemorrhage, necrosis, and/or calcification. Many brain tumors have surrounding edema, which manifests as low density on CT and hyperintensity on FLAIR and T2-weighted MRI sequences. Tumors with high cell density and a large nuclear-to-cytoplasm ratio have a relatively higher CT attenuation and lower signal on FLAIR and T2-weighted MRI sequences. Conversely, cystic portions of brain masses demonstrate low density unless proteinaceous or hemorrhagic contents within the cyst increase their CT attenuation. Calcification and blood products can also contribute to the higher density of some brain tumors. Similarly, proteinaceous or hemorrhagic contents of cysts can alter their MR signal intensity.
Contrast enhancement
Contrast enhancement in a brain lesion is due to breakdown of the blood–brain barrier with leakage of intravascular contrast medium. Therefore, neoplasms that show leakage and/or increased vascularity will demonstrate contrast enhancement. Accordingly, enhancement is very common in high-grade neoplasms and metastatic lesions of the brain. Extra-axial tumors that do not have a blood–brain barrier also enhance, such as meningiomas, schwannomas, and choroid plexus tumors. However, contrast enhancement is not specific for high-grade neoplasms, and there are a number of primary intra-axial low-grade tumors, such as pilocytic astrocytomas, gangliogliomas, and many subependymal giant cell astrocytomas, that typically demonstrate contrast enhancement as well. Use of corticosteroids may decrease contrast enhancement in brain tumors; therefore, knowledge of the patient’s recent use of steroids may be helpful in the interpretation of images.
Advanced MR imaging techniques
Diffusion MR imaging is commonly performed for assessment of cerebral ischemia. Diffusion MR imaging is also helpful in the evaluation of brain masses, both for differentiation of neoplasms versus tumor mimics and for more detailed characterization of brain tumors. Highly cellular neoplasms with small compact cells and high nuclear-to-cytoplasm ratios, such as CNS lymphoma and medulloblastoma, typically show reduced (restricted) diffusion with high signal on trace DWI and dark signal on apparent diffusion coefficient (ADC) maps. Some solid portions of glioblastomas can also show reduced diffusion (Fig. 4.1). The central necrotic portions of abscesses usually show restricted diffusion, which may be helpful in distinguishing them from rim-enhancing necrotic neoplasms with central increased diffusion. One important caveat to consider is that the presence of gross hemorrhage may complicate and hinder the accurate interpretation of diffusion imaging.
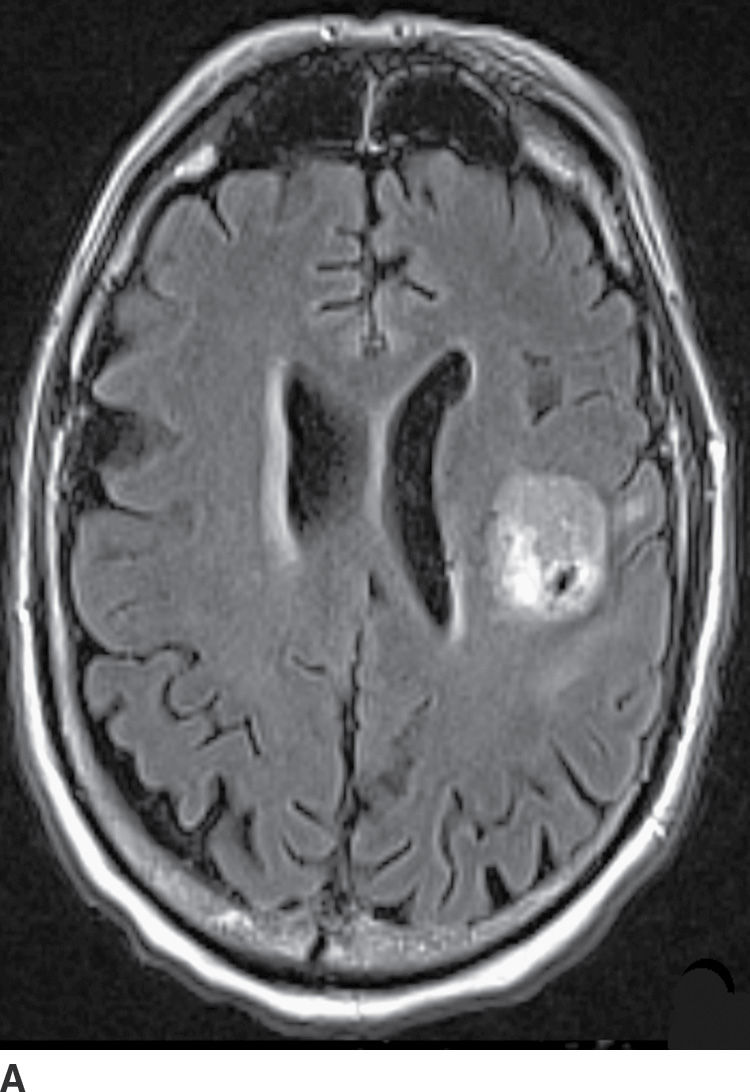
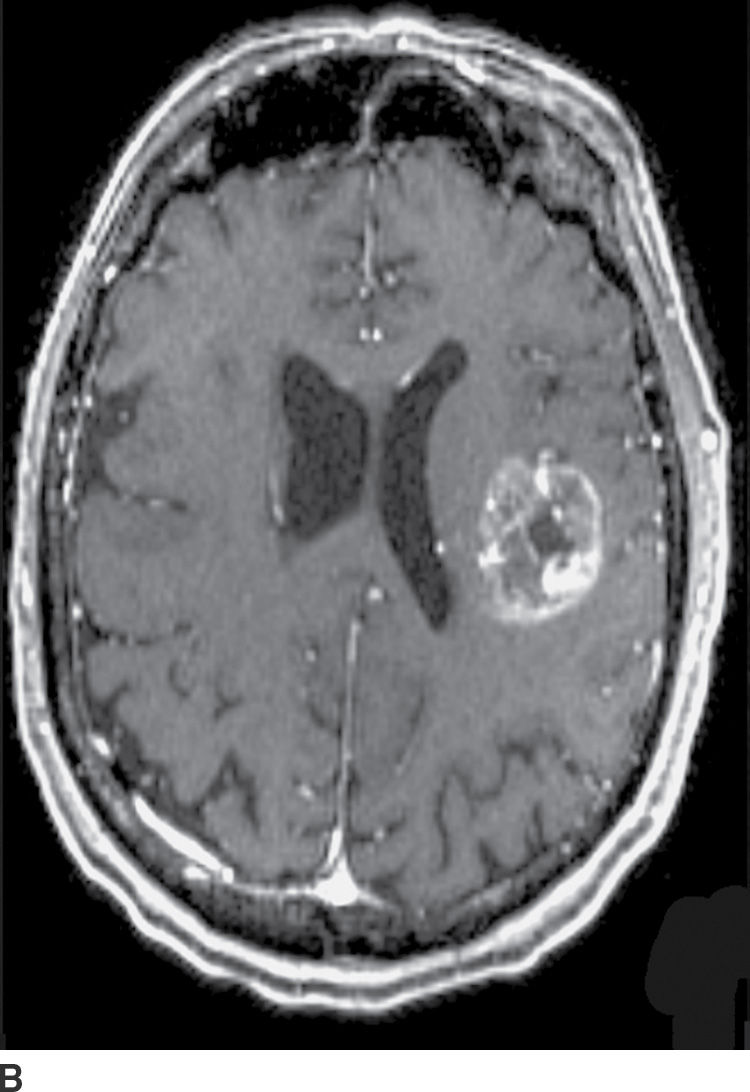
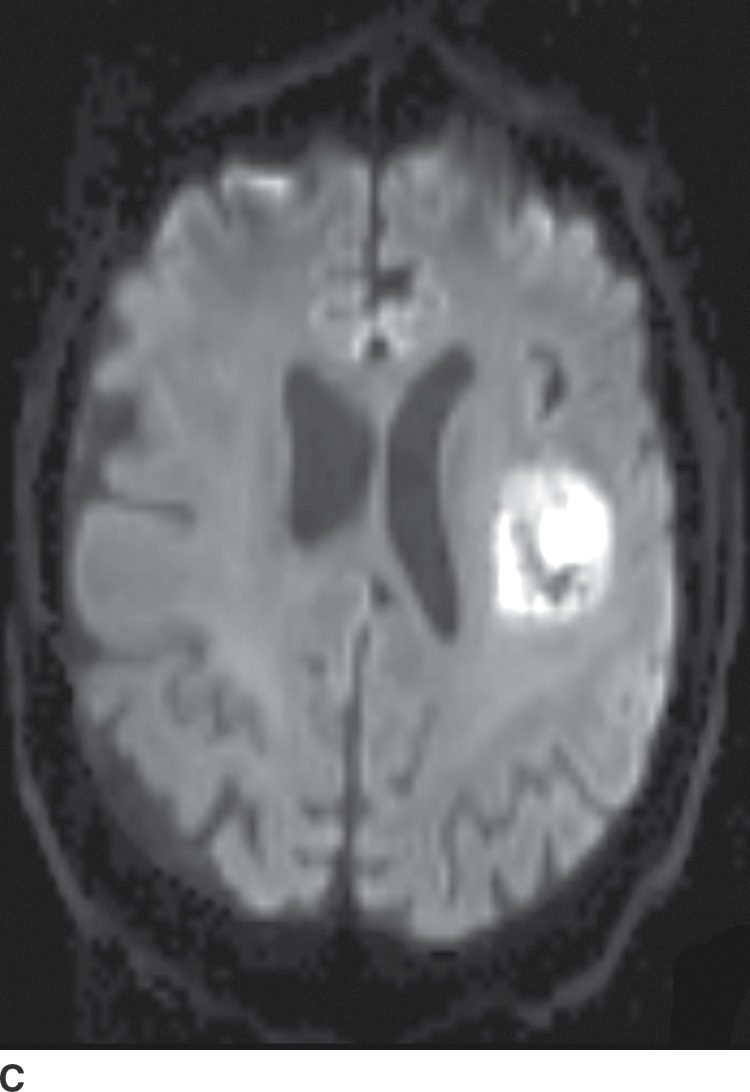
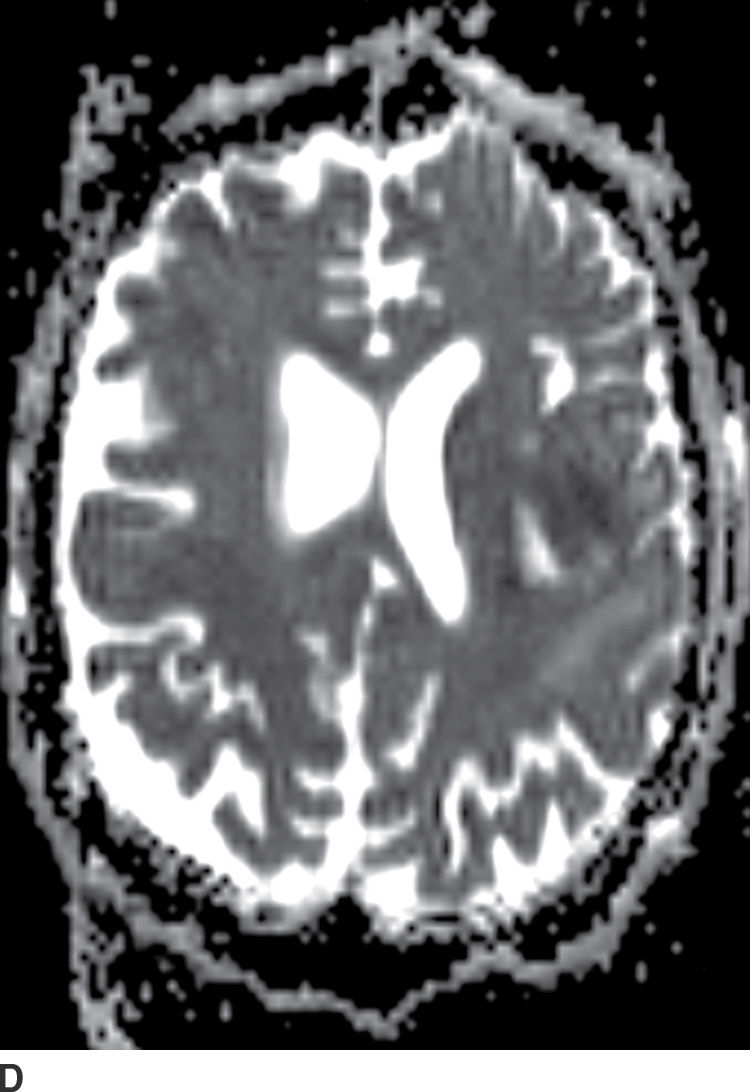
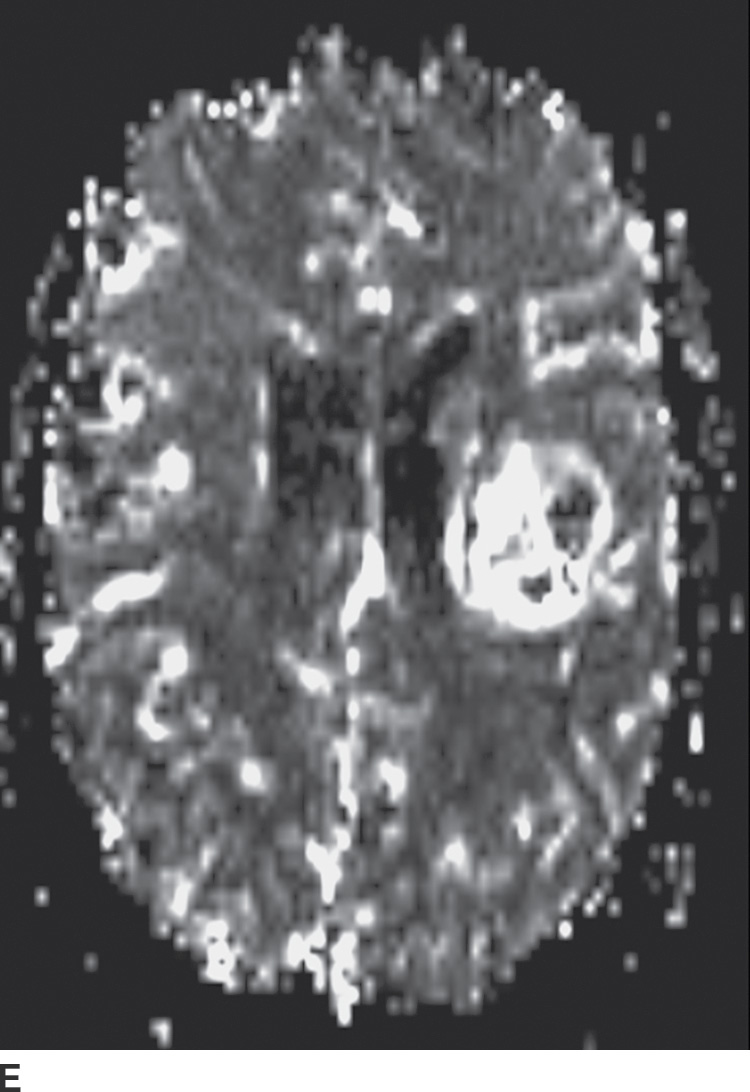
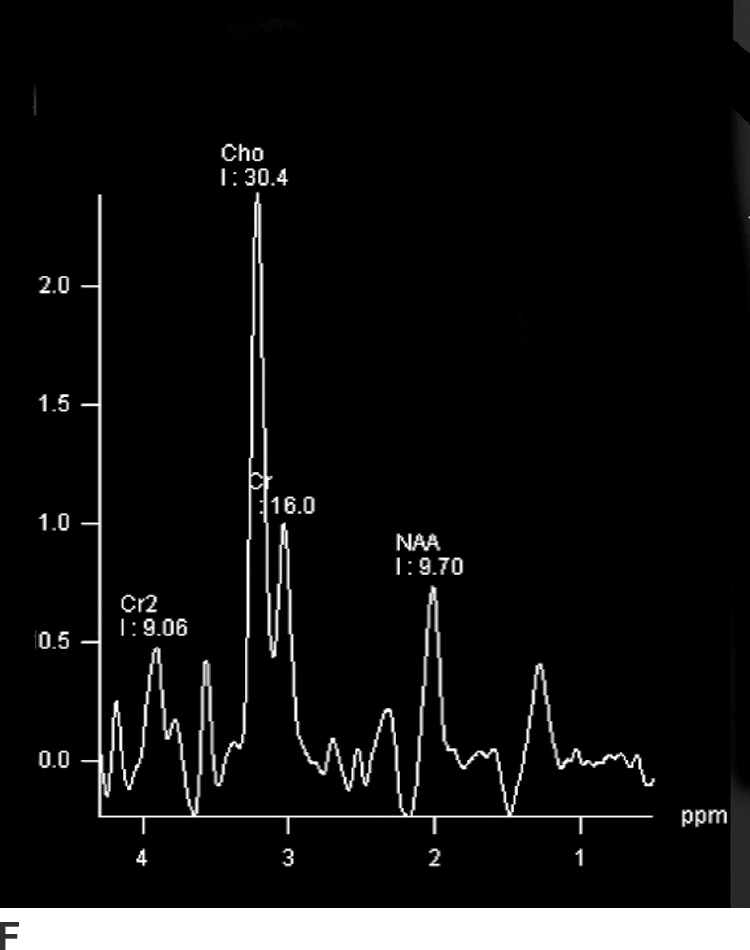
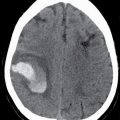
FIG. 4.1 Glioblastoma. Use of advanced imaging techniques in conjunction with conventional MRI in a 59-year-old patient. A FLAIR image (A) demonstrates a heterogenous signal mass in the left frontal lobe, which shows heterogenous contrast enhancement and central nonenhancing necrosis on postcontrast T1WI (B). Axial diffusion trace DWI (C) and apparent diffusion coefficient (ADC) images (D) show restricted diffusion, which is typically due to high cellularity in the enhancing solid portions of this high-grade tumor. Cerebral blood volume (CBV) map (E) from dynamic susceptibility contrast perfusion imaging shows increased relative CBV in the tumor, which is typical for adult high-grade gliomas with increased vascularity. MR spectroscopy (F) of the tumor shows markedly elevated choline (Cho) and abnormally decreased NAA.
Perfusion imaging for the evaluation of brain neoplasms is typically done following a bolus of gadolinium contrast material and derivation of various perfusion parameters. The most commonly used parameter is relative cerebral blood volume (rCBV), which compares the tumor cerebral blood volume (CBV) with the contralateral normal white matter. Typically, adult high-grade gliomas have elevated CBV, whereas low-grade gliomas and their nonneoplastic mimickers have CBV that is similar to or lower than that of normal brain parenchyma (Fig. 4.1). There are some exceptions to this general rule; for example, grade II oligodendrogliomas can show elevated CBV despite being low grade. Perfusion imaging can also sometimes help in distinguishing tumor progression (high rCBV) from radiation necrosis (low rCBV), which can otherwise have a similar appearance.
Magnetic resonance spectroscopy (MRS) has also been used in the evaluation of brain neoplasms and lesions that mimic neoplasms, as well as for presurgical assessment of tumor grade (3,4). Elevation of choline (Cho), a marker of cell membrane turnover, along with decreased N-acetyl aspartate (NAA), a neuronal marker, is commonly seen in neoplasms. These changes are often more pronounced in higher-grade tumors (Fig. 4.1). Nevertheless, many of these findings are not entirely specific for tumors, and MRS should always be used in the context of other conventional and advanced MR imaging findings.
Classification of brain tumors
Traditionally, CNS neoplasms have been classified based on histopathology of tumor cells. More than a 100 different types of primary CNS tumors have been described. Although there are many classification systems, the most commonly used system is the WHO classification, the most recent edition of which was released in 2007 (fourth edition) (5). New tumors have been continuously added to this classification with each new edition (6). Although the current WHO classification system is mainly based on histomorphologic criteria, a number of genetic and molecular markers, which can have diagnostic and therapeutic significance, are being increasingly recognized.
CNS neoplasms are divided into two general categories of primary and secondary (metastatic) neoplasms. Primary CNS neoplasms are further subdivided into six categories based on histology, which include neuroepithelial tumors, meningeal tumors, cranial/spinal nerve tumors, germ cell tumors, sellar region tumors, and lymphoma/hematopoietic tumors. Detailed discussion of all primary and secondary brain neoplasms is well beyond the scope of this book, and interested readers are referred to more in-depth reviews (4,7–12). In this chapter, we will briefly describe the most commonly encountered primary and metastatic brain tumors. While various tumors can occur in multiple different areas of the intracranial cavity, throughout this chapter, we will discuss the different neoplasms based on both their histology and their common locations.
Glial (Neuroepithelial) Tumors (Gliomas)
These tumors arise from the supporting non-neuronal cells (glial tissue) of the brain. They constitute the largest category of primary brain neoplasms and are further subdivided into several subcategories based on cell origin (astrocytes, oligodendrocytes, ependymal cells, and choroid plexus), though pluripotential neural stem cells are also considered to be the origin of some primary CNS neoplasms. These cells have a high rate of proliferation and therefore are prone to genetic errors, which can modify them into tumoral stem cells (13).
Astrocytomas
Astrocytomas are the largest group of primary CNS neoplasms, which are further subdivided into different types based on histology. According to the WHO classification, astrocytomas range from grade I (benign) to IV (highly malignant). A subset of astrocytomas such as pilocytic astrocytomas and subependymal giant cell astrocytomas is relatively localized and slow growing and is considered grade I. Diffusely infiltrative tumors with cytologic atypia but without frank anaplasia and high mitotic activity are classified as grade II. Presence of anaplasia and high mitotic activity designate a tumor as grade III, and microvascular proliferation and necrosis are generally indicative of a grade IV neoplasm. In addition to traditional histologic features, there are a number of immunohistochemical markers such as MIB-1 (Ki-67) indices that are frequently used as markers of cell proliferation.
Patient age is an important factor in predicting grade and location of astrocytomas. Pilocytic astrocytomas of the posterior fossa and suprasellar region usually occur in children, as do subependymal giant cell astrocytomas. Diffusely infiltrating grade II astrocytomas and pleomorphic xanthoastrocytomas (PXAs) have a peak incidence in older children and young adults. In contrast, in older adults, astrocytomas more commonly involve the supratentorial brain and are primarily high grade.
Pilocytic astrocytoma
This grade I neoplasm is more common in the posterior fossa and suprasellar regions and will be discussed in those sections, respectively.
Subependymal giant cell astrocytoma
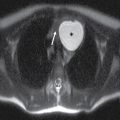
This tumor is a well-circumscribed WHO grade I tumor that almost invariably occurs in young patients with tuberous sclerosis (TS). These tumors arise in the lateral ventricles adjacent to the foramina of Monro and may cause obstructive hydrocephalus (Fig. 4.2). The MR signal is variable and they can demonstrate contrast enhancement. The most helpful imaging finding in the differentiation of a subependymal giant cell astrocytoma from subependymal nodules of TS is demonstration of progressive enlargement, although lesions larger than 10 to 12 mm are usually considered to be subependymal giant cell astrocytomas (14).
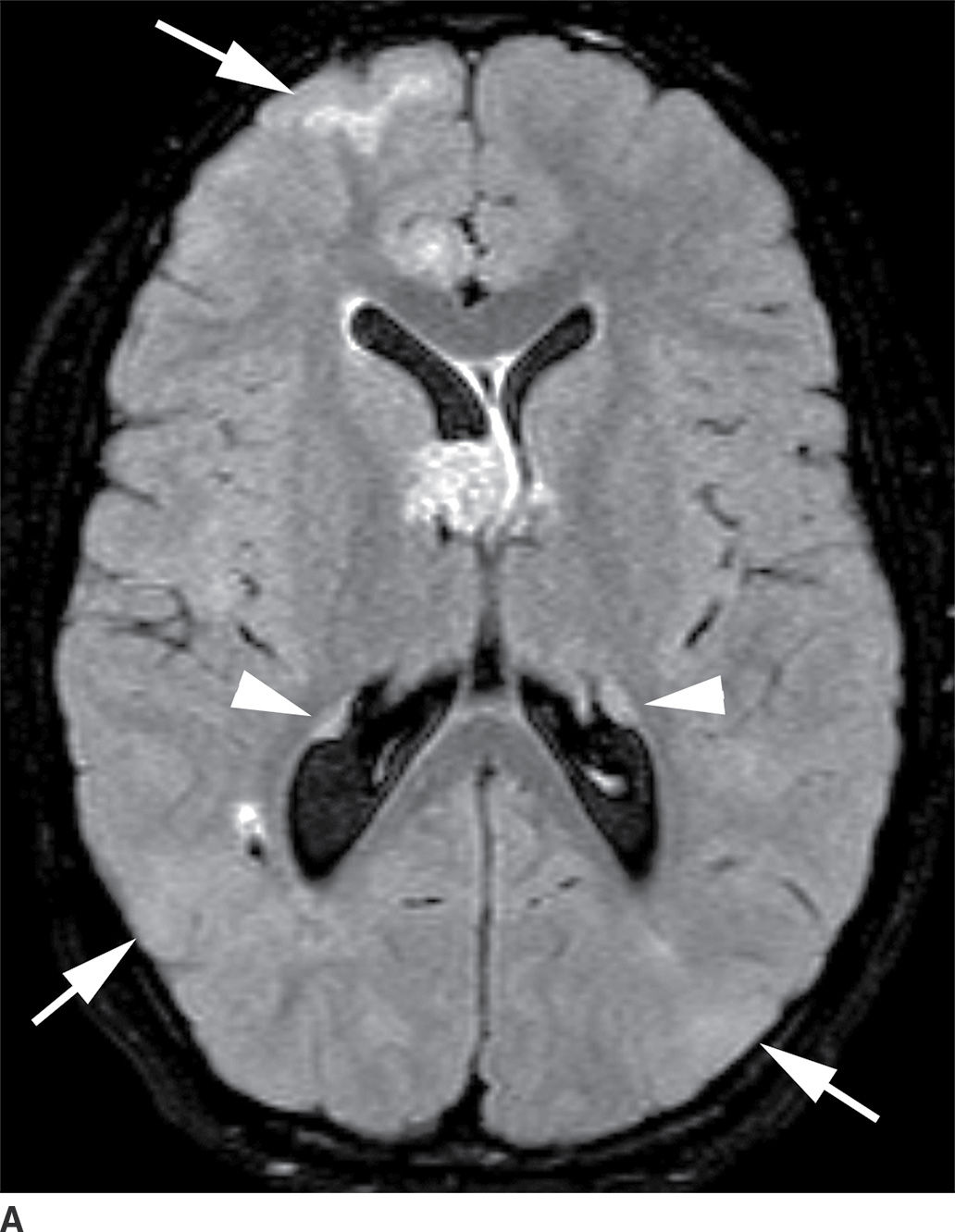
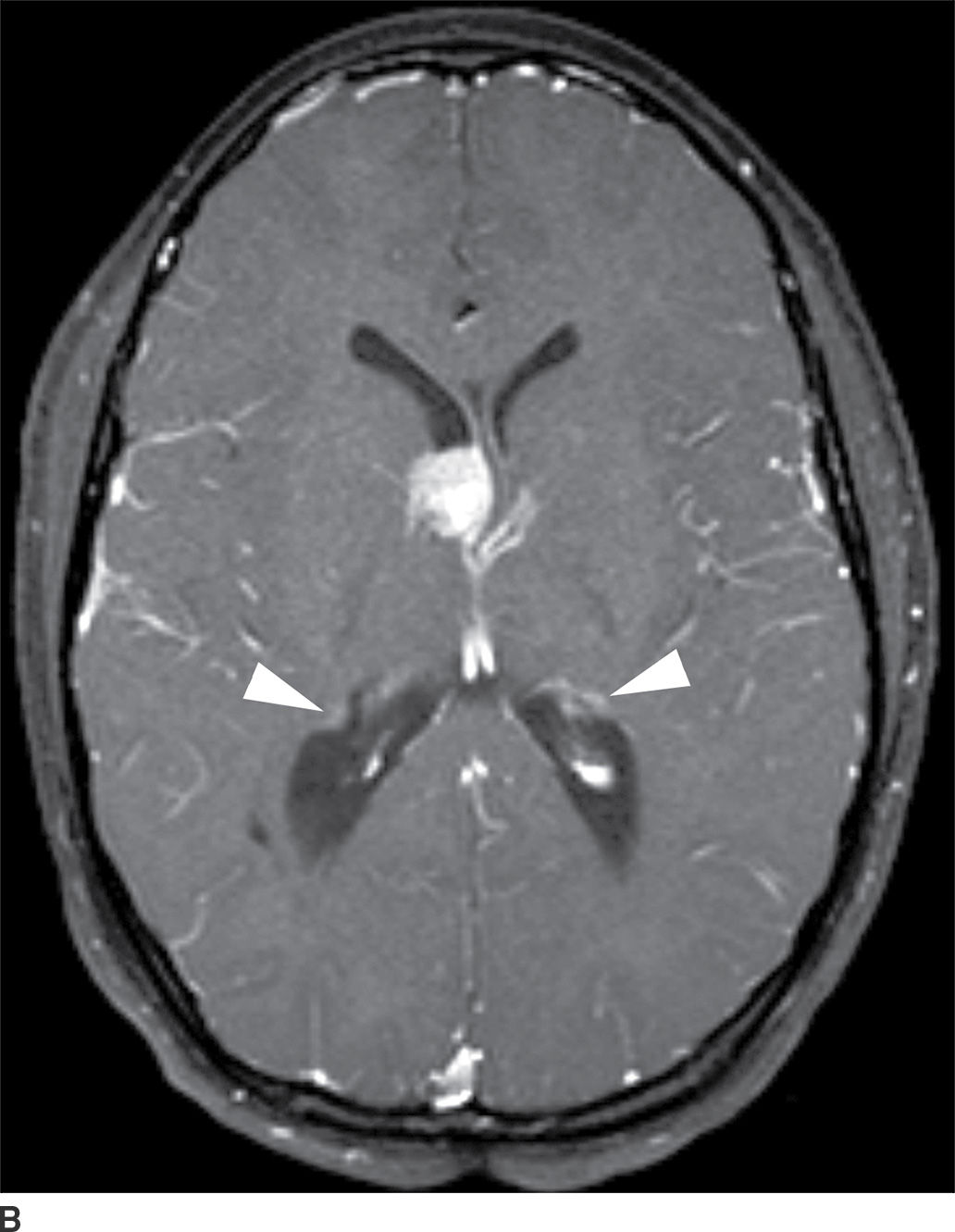
FIG. 4.2 Subependymal giant cell astrocytoma (SEGA) in a patient with tuberous sclerosis. FLAIR image (A) shows a subependymal giant cell astrocytoma in the region of the foramen of Monro. The cortical/subcortical tubers (arrows) and subependymal nodules (arrowheads) characteristic of tuberous sclerosis are also seen. Postcontrast T1WI (B) shows enhancement of SEGA and subependymal nodules.
Low-grade diffuse astrocytoma
These tumors are slowly growing grade II astrocytomas. Most of these tumors are neoplastic fibrillary-type astrocytomas. These tumors are more commonly supratentorial, and the clinical presentation is highly variable and dependent on the location. They can appear either localized or infiltrative on imaging. CT usually demonstrates a nonenhancing isodense to hypodense lesion, and calcification is occasionally seen. They are usually hypointense on T1WI and hyperintense on T2WI and FLAIR sequences and do not enhance with gadolinium (Fig. 4.3). Occasionally, low-grade gliomas can appear very round and well circumscribed, with a very high T2 and low T1 signal mimicking a cyst. However, closer inspection of the T2WI and FLAIR sequences with manipulation of the display window and level settings demonstrates that they are not cysts. The major differential diagnosis of grade II astrocytomas includes nonenhancing anaplastic astrocytomas and oligodendrogliomas. Small low-grade astrocytomas may also be hard to differentiate from focal cortical dysplasias. Many diffuse low-grade astrocytomas have an intrinsic tendency to transform and dedifferentiate into high-grade tumors (anaplastic astrocytomas or glioblastomas).
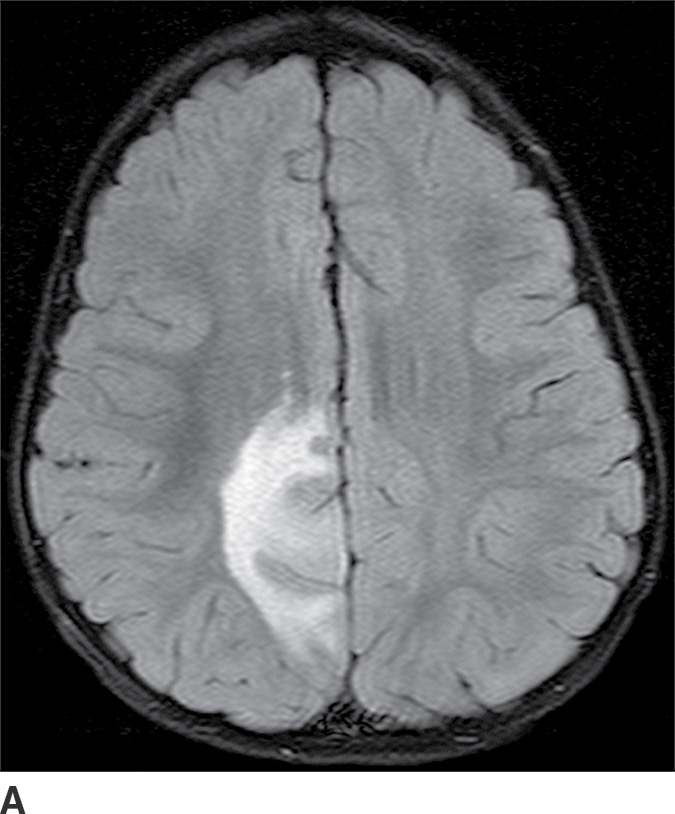
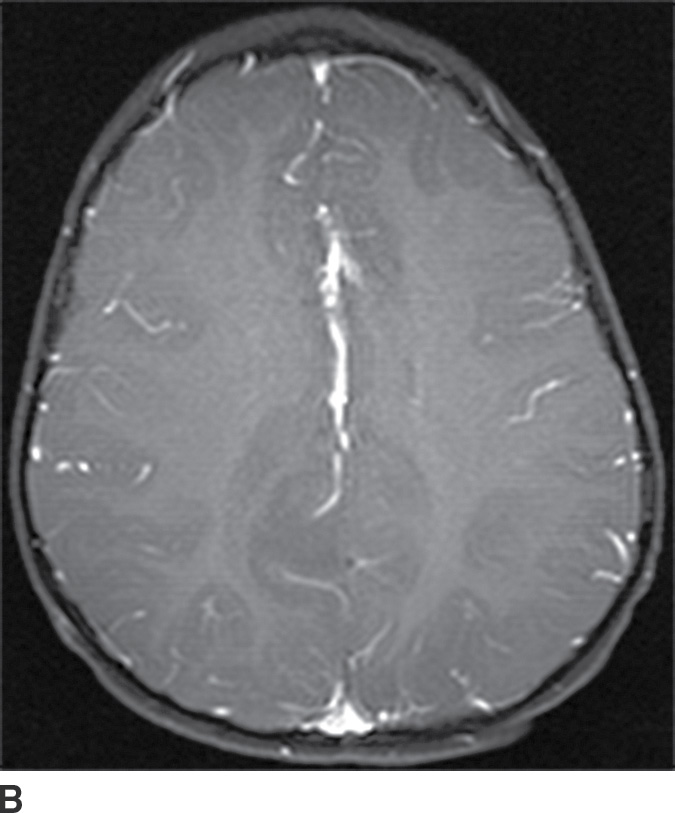
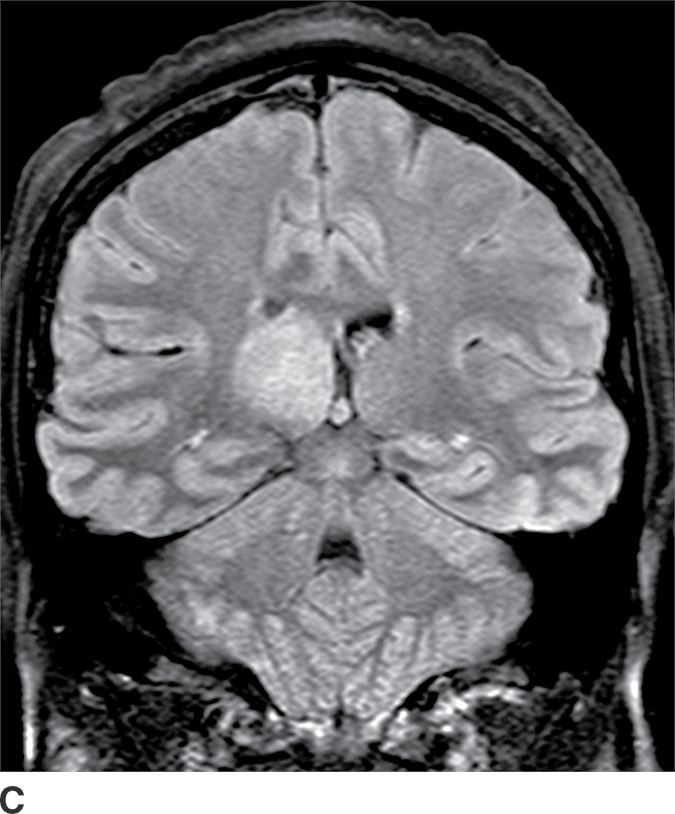
FIG. 4.3 Low-grade diffuse astrocytoma (glioma). FLAIR image (A) shows an infiltrative lesion with some expansion of the gyri. Postcontrast T1WI (B) shows no contrast enhancement, which is typical of hemispheric grade II astrocytomas. C: Coronal FLAIR image in another patient shows a hyperintense mass expanding the right thalamus.
Pleomorphic xanthoastrocytoma
These tumors are typically superficial, cortically based lesions in the cerebral hemispheres, most commonly seen in the temporal lobe (15). Seizure is the most common presenting symptom. They are WHO grade II neoplasms, although a small subset displays anaplastic features. PXAs primarily occur in older children and young adults. They are commonly cystic lesions with an enhancing solid nodule abutting the pial surface. MRI may demonstrate enhancement in adjacent meninges with a “dural tail” appearance. The main differential diagnosis of PXA is other low-grade cortically based tumors such as gangliogliomas, which can have the same imaging appearance of a peripheral cystic lesion with nodular enhancement (15).
Anaplastic astrocytoma
These tumors are WHO grade III (high-grade) malignant neoplasms. They can arise de novo or evolve from transformation of low-grade diffuse astrocytomas. They can also further progress to glioblastomas. They most commonly involve the cerebral hemispheres, but deep structures such as the thalami and basal ganglia are also frequently involved. These tumors can involve all age groups; however, they are more common in middle aged to older adults. CT usually demonstrates poorly defined expansile hypodense lesions that usually do not demonstrate enhancement. These tumors are usually hypointense on T1WI and hyperintense on T2WI. Contrast enhancement is variable on MRI; however, perfusion-weighted images usually demonstrate foci of increased CBV and can be helpful for differentiation of these tumors from low-grade diffuse astrocytomas (3,16). The major differential diagnosis includes other tumors such as low-grade astrocytomas and oligodendrogliomas and, for those that have contrast enhancement and elevated CBV, glioblastomas.
Glioblastoma
These tumors are WHO grade IV malignant astrocytomas and have a very poor prognosis, with less than a 3% 5-year survival (2). Unfortunately, they are the most common type of adult primary brain tumors. They are more commonly seen in older adults. In the newest WHO classification, they are no longer called glioblastoma multiforme (GBM), although the term remains prevalent. These tumors can occur de novo or transform from a lower-grade astrocytoma. The supratentorial cerebral hemispheres are the preferred site of involvement in adults, with common involvement of subcortical and periventricular white matter and crossing white matter tracts such as the corpus callosum. Involvement of both sides of the corpus callosum has been described as a “butterfly” appearance. Deep brain structures such as the basal ganglia and thalami are also frequently involved.
CT usually demonstrates an avidly enhancing heterogeneous mass, with frequent presence of hemorrhage and necrosis. These tumors often demonstrate significant mass effect and are surrounded by marked vasogenic edema. MRI usually demonstrates a poorly defined mass with mixed heterogeneous signal intensity indicative of hemorrhage, necrosis, cyst formation, and contrast enhancement (Fig. 4.1). Thick irregular enhancement surrounding a central necrotic focus is the most common imaging pattern. The solid portions of glioblastoma may be densely cellular and show restricted diffusion. Glioblastoma infiltrates to other brain structures via the white matter tracts and can also disseminate along the ependyma and via the CSF. The highly infiltrative nature of this neoplasm results in tumor cells being present beyond the margin of the visible enhancement and signal abnormality on MRI. Occasionally, glioblastomas are multicentric.
The differential diagnosis includes other high-grade neoplasms, CNS lymphoma, single large metastases, and brain abscesses. Advanced imaging techniques such as MR spectroscopy and perfusion can be helpful in differentiating between a glioblastoma and a single large metastasis. There is increased choline/creatine ratio and increased CBV in the region surrounding the enhancing portion of a glioblastoma because it contains infiltrating neoplastic cells. This is not true for the region surrounding a metastasis because it typically does not contain neoplastic cells (4). Other etiologies such as enhancing subacute infarction and tumefactive demyelination can also mimic high-grade gliomas.
Gliosarcoma is a variant of glioblastoma with both gliomatous and sarcomatous components. The imaging appearance is usually a heterogeneous broad-based peripheral intra-axial hemispheric mass with frequent involvement of the dura. When it invades the dura, it can mimic extra-axial invasive tumors such as anaplastic meningioma, dural metastases, and extra-axial lymphoma.
Oligodendroglioma
These tumors arise from oligodendrocytes, although some have histologic features of both oligodendroglioma and astrocytoma and are called oligoastrocytoma. These tumors can be WHO grade II or III based on the degree of differentiation and presence of anaplastic features (17). These tumors primarily occur in adults with a peak incidence within the fourth and fifth decade. They usually present as relatively well-delineated masses with involvement of the cortex and subcortical white matter, with the frontal lobe being the most common site of involvement. On CT, these tumors are usually hypodense but very frequently have calcifications (up to 90%) (Fig. 4.4). Cystic degeneration is also sometimes seen. Enhancement is variable and can be seen in both low- and higher-grade oligodendrogliomas. On MRI, these tumors are frequently heterogeneously hyperintense on T2WI sequences with frequent blooming on susceptibility images secondary to the presence of calcifications. The main differential diagnosis of oligodendroglioma is a low-grade diffuse astrocytoma.
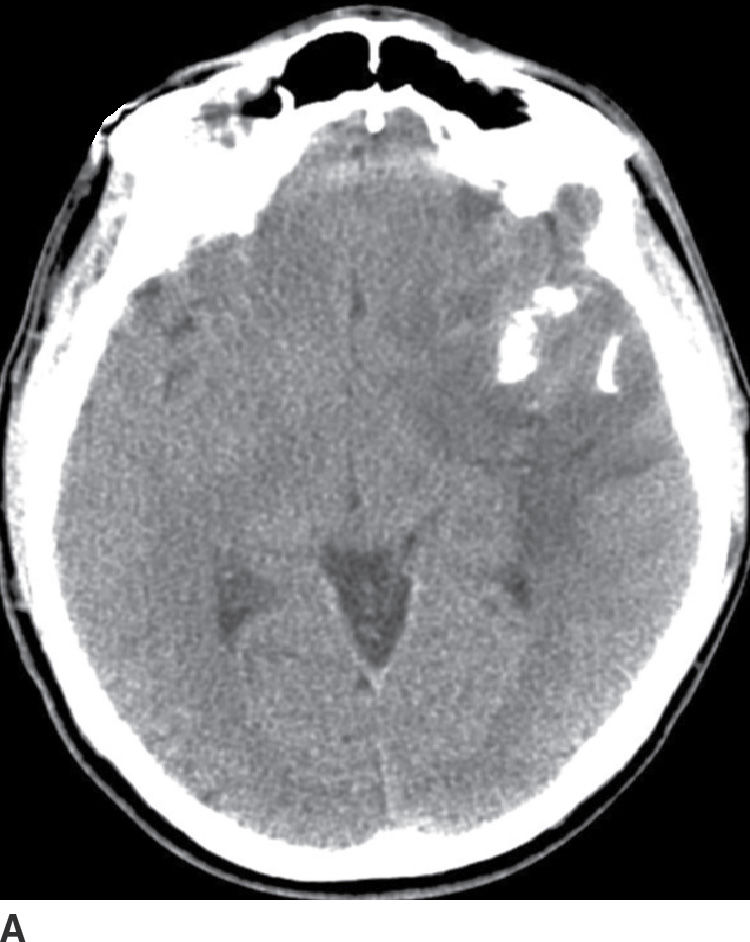
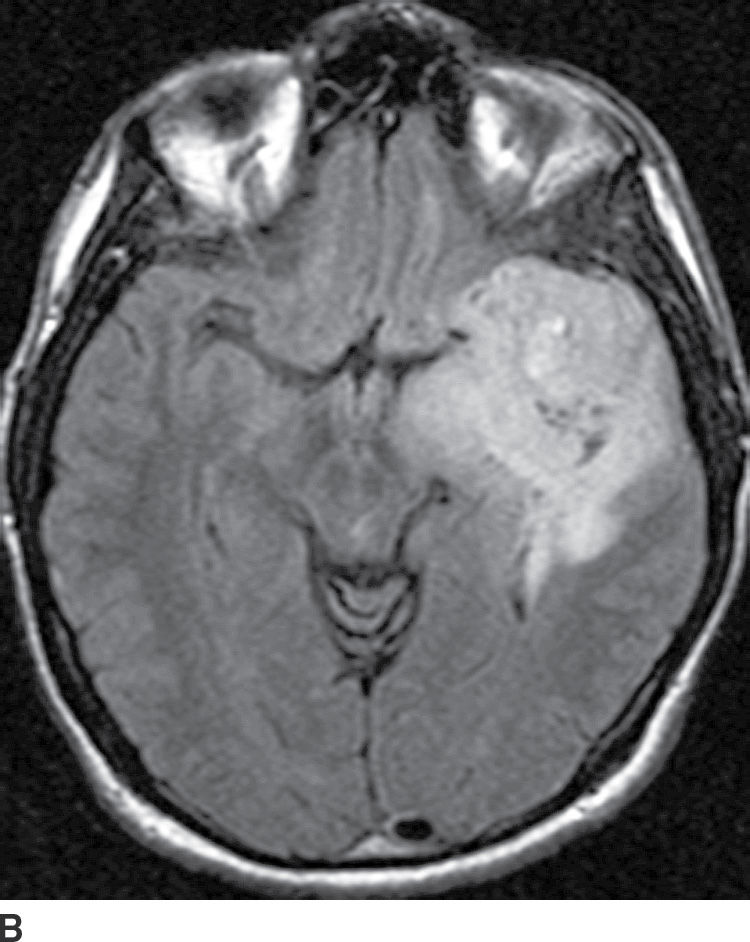
FIG. 4.4 Oligodendroglioma. Axial CT (A) shows a relatively dense, partially calcified intra-axial mass in the left temporal–frontal region. On axial FLAIR (B), the mass appears heterogeneous due to the calcifications and infiltrates and expands the temporal lobe.
Gliomatosis cerebri
Gliomatosis cerebri is an infiltrative tumor defined by involvement of at least three lobes of the brain. The basal ganglia, thalami, and deep white matter are also frequently involved. The grade varies from II to IV, but gliomatosis cerebri is usually classified as grade III. It can occur at any age but predominantly affects middle-aged adults (18). Typically, the infiltrative brain imaging appearance is worse than expected from the patient’s clinical symptomatology. These tumors can arise de novo or be a progression of a diffusely infiltrating glioma. CT usually demonstrates hypodensity in the involved areas with mild expansion and decreased gray–white differentiation. Enhancement is usually absent on CT. On MRI, the involved areas usually demonstrate T2–FLAIR hyperintensity with partial effacement of adjacent sulci and the ventricular system (Fig. 4.5). Enhancement is also usually absent on MRI, though if present can indicate foci of dedifferentiation with the lesion into higher grades. Perfusion MRI can also be helpful in determination of higher-grade foci for biopsy guidance.
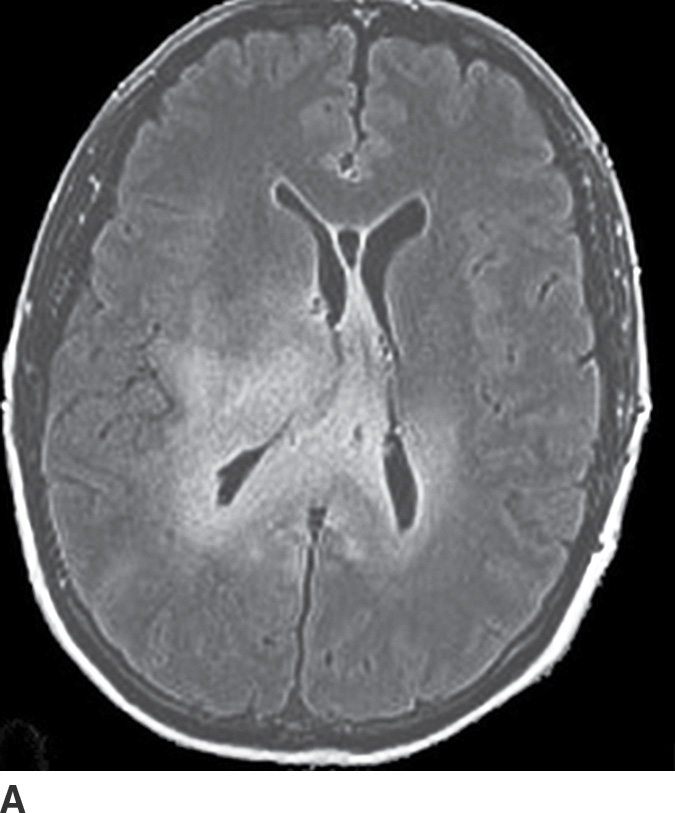
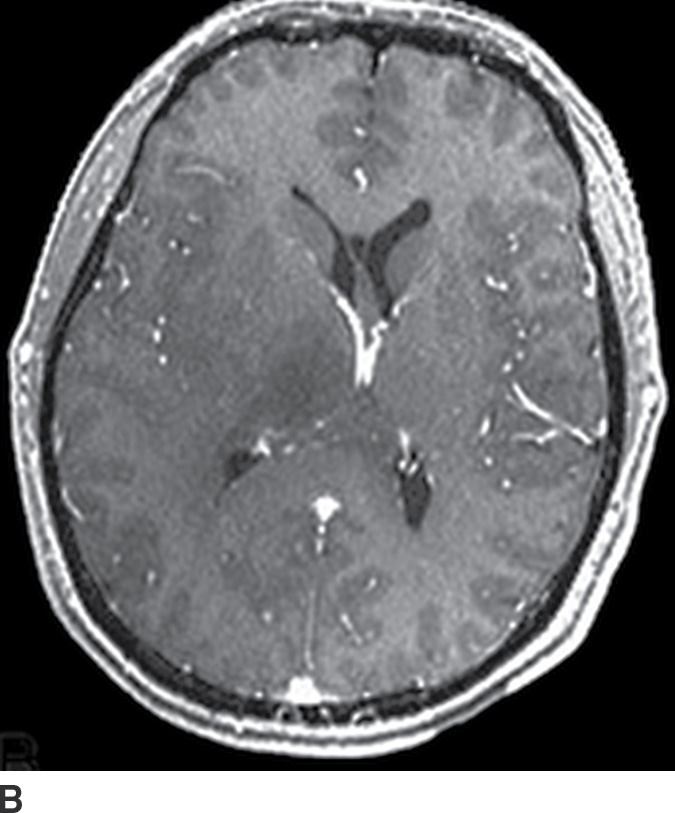
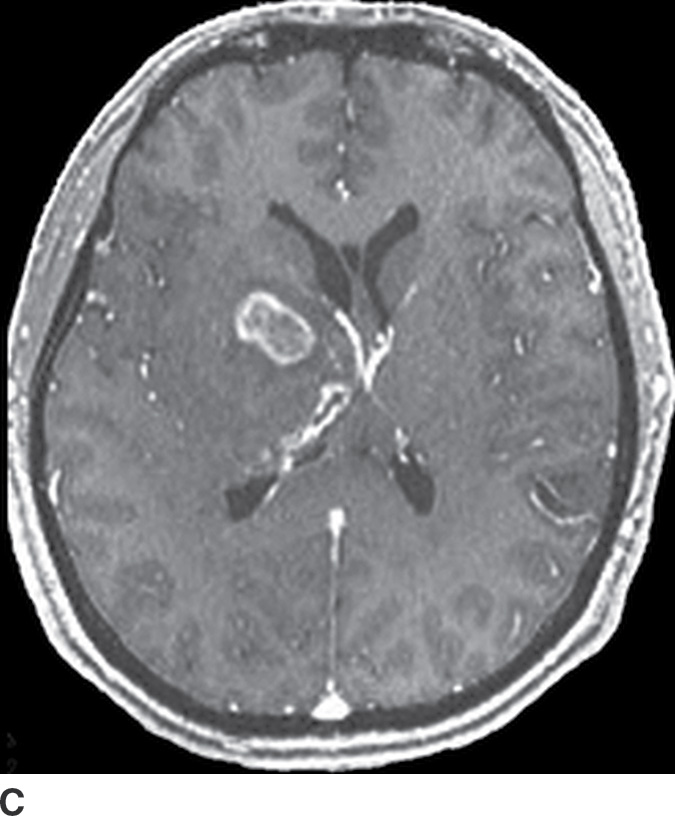
FIG. 4.5 Gliomatosis cerebri. Axial FLAIR (A) shows an infiltrating, hyperintense lesion involving multiple regions of the brain, including the corpus callosum, thalami, right internal capsule, cingulate gyrus, and periatrial regions. There is no associated enhancement on postcontrast T1WI (B). Six months later, on follow-up postcontrast T1WI (C), there is a focus of enhancement within the tumor, suggesting a focus of dedifferentiation.
Due to extensive brain involvement, gliomatosis cerebri can potentially mimic confluent white matter changes in small vessel ischemic disease; however, presence of mass effect rather than volume loss is a useful differentiating feature. Progressive multifocal leukoencephalopathy (PML) may also demonstrate extensive confluent white matter involvement and mimic gliomatosis cerebri, though it is typically found in immunocompromised patients and typically has less mass effect.
Mixed Neuronal–Glial and Nonglial Tumors
Mixed neuronal–glial tumors are a heterogeneous group of neoplasms, which contain both neuronal and glial elements. These tumors are frequently associated with seizures, are usually less aggressive compared to other gliomas, and typically occur in children and young adults.
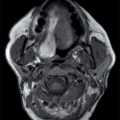
Ganglioglioma
These tumors are WHO grade I or II neoplasms and are composed of neoplastic ganglionic and glial cells. These tumors can occur throughout the brain; however, they are most frequently found in the temporal lobe (greater than 75%). They can be partially cystic with a mural nodule or completely solid (15). Anaplastic gangliogliomas are also occasionally seen. CT imaging may show a hypodense cystic lesion with an isodense mural nodule. Calcification is frequent. On MRI, the cystic component is hyperintense on T2WI. The mural nodule, if present, usually demonstrates enhancement on both CT and MRI (Fig. 4.6). MRI can sometimes demonstrate an area of cortical dysplasia adjacent to the tumor.
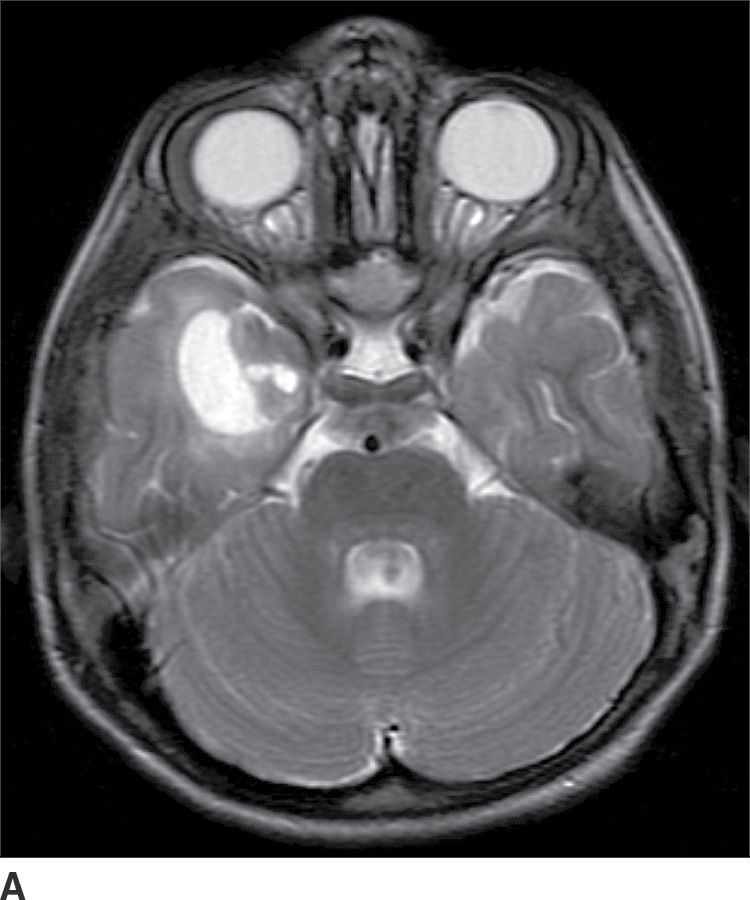
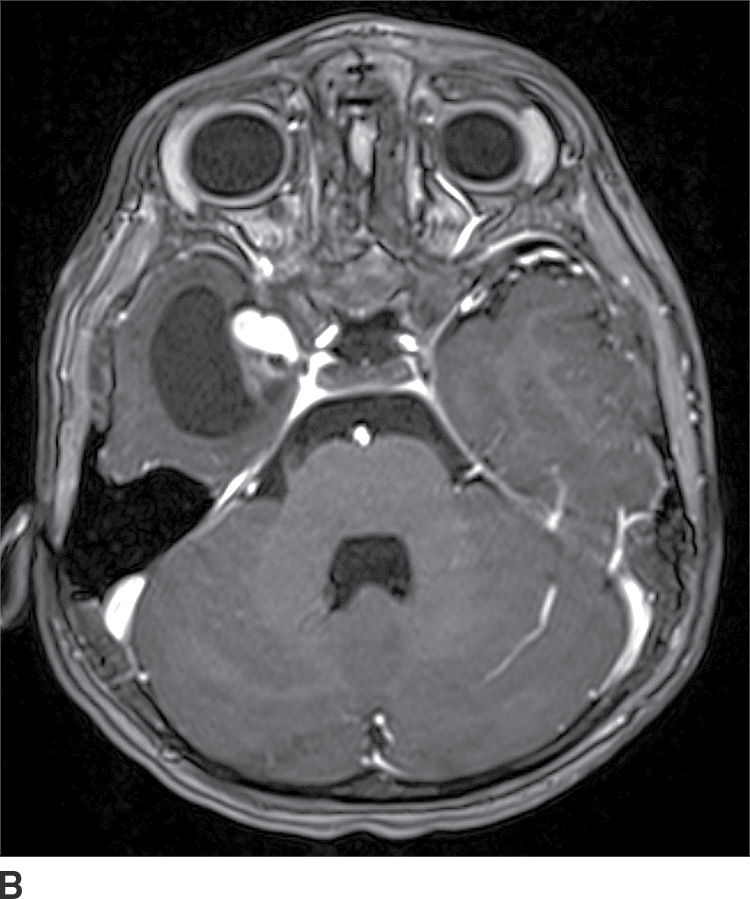
FIG. 4.6 Ganglioglioma. This 16-year-old patient presented with seizures. T2WI (A) demonstrates a cystic and solid mass in the anterior right temporal lobe. Postcontrast T1WI (B) shows a nodular focus of enhancement along the margin of the tumor.
The major differential diagnosis includes pilocytic astrocytoma and PXA, which can both demonstrate a similar imaging appearance of a cyst and enhancing mural nodule. Other differential diagnoses for these often superficial tumors include dysembryoplastic neuroepithelial tumor (DNET) and oligodendroglioma.
Dysembryoplastic neuroepithelial tumor
This tumor is a grade I neoplasm that usually occurs in the first three decades of life, typically presenting with complex partial seizures. DNETs are cortically based lesions that tend to extend toward the margin of the ventricles (15). On CT, a DNET tends to have a well-circumscribed wedge-shaped hypodensity. Remodeling and scalloping of the inner table of the adjacent skull are common. On MRI T2WIs, they have a well-demarcated hyperintense, “bubbly” appearance. On FLAIR, the tumor shows hyperintense rims around the hypointense “bubbly” areas (Fig. 4.7). Enhancement is usually absent or mild.
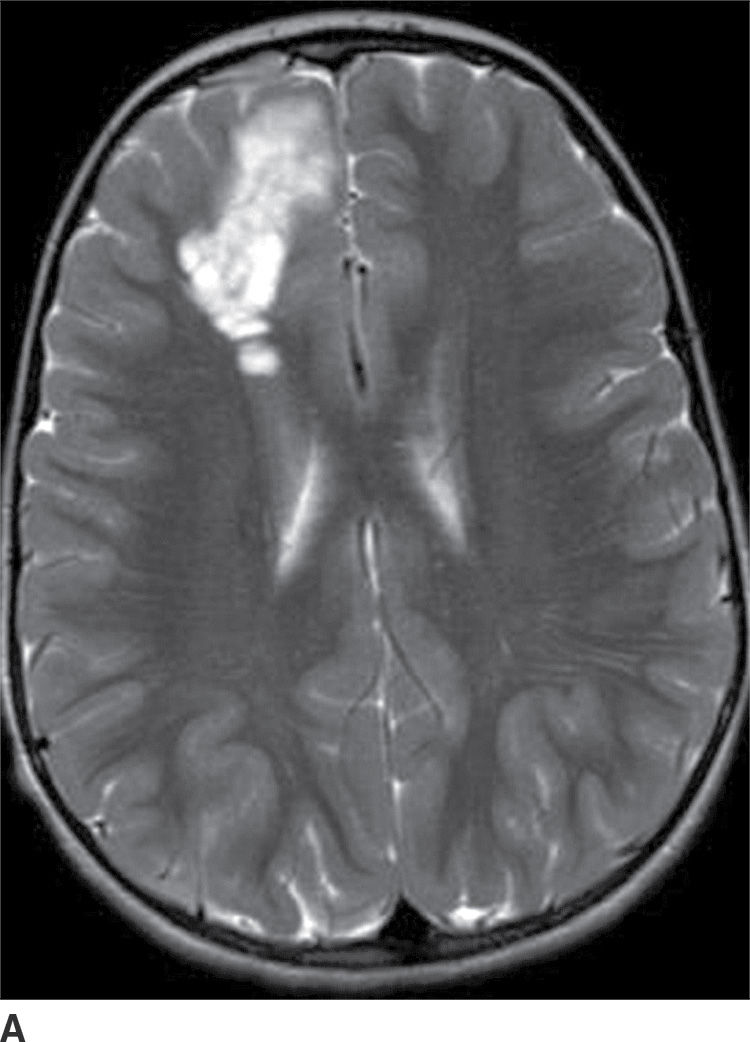
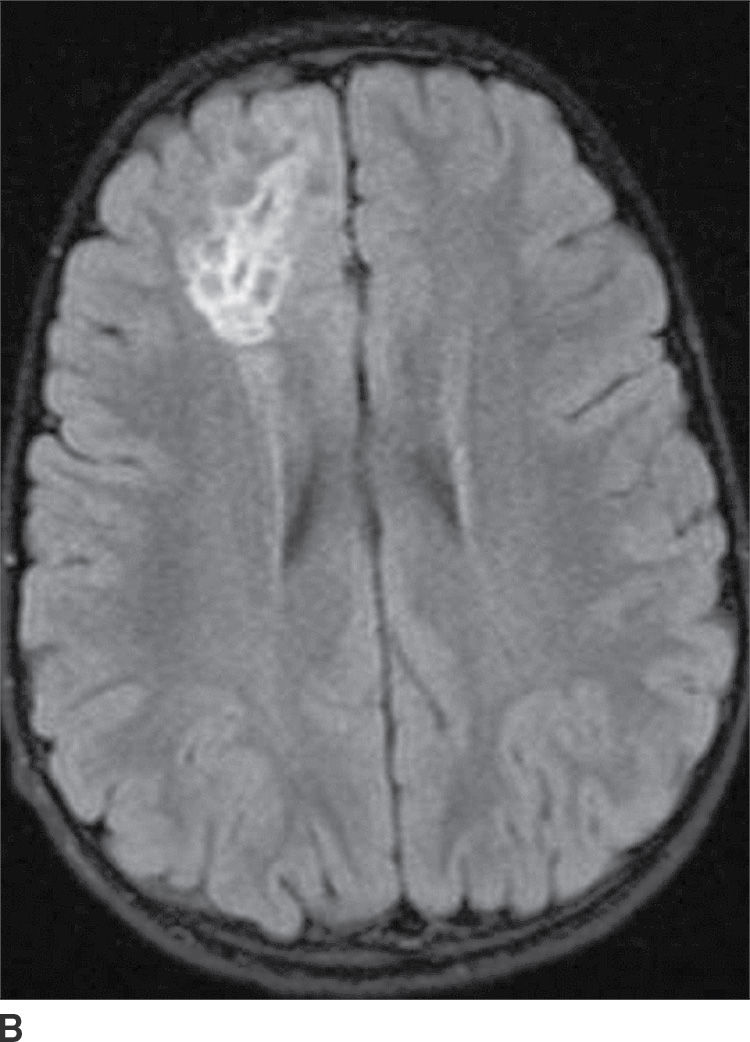
FIG. 4.7 Dysembryoplastic neuroepithelial tumor (DNET). Axial T2WI (A) in a 12-year-old patient with seizures shows a “bubbly” T2 hyperintense multicystic-appearing mass in the right frontal lobe, which extends from the cortex toward the ventricle. FLAIR (B) shows that the rims of these cystic-appearing foci are hyperintense, with hypointense centers.
Primary CNS lymphoma
PCNSL is a rare variant of extranodal non-Hodgkin lymphoma restricted to the central nervous system. Most PCNSLs are large B-cell lymphomas. They can occur both in immunocompetent (typically older patients) and in immunocompromised (younger) patients. They can affect any part of the brain, though the cerebral hemispheres are the preferred site of involvement, with a predilection for the periventricular white matter and corpus callosum. The basal ganglia and thalami are also frequently involved. PCNSL is a densely cellular tumor and therefore is usually hyperdense on CT and isointense to brain parenchyma on T1- and T2-weighted images and shows restricted diffusion. Peritumoral vasogenic edema is common (4) (Fig. 4.8). Presence of enhancement depends on the immune status of the patient; it is usually solid and homogenous in immunocompetent patients but can be ringlike in immunocompromised patients, including those with HIV. The main differential diagnoses include high-grade gliomas (glioblastoma) and metastases. Hemorrhage and necrosis is much more common in glioblastoma, and it tends to have higher CBV on perfusion imaging compared to PCNSL. In immunocompromised patients, an additional differential diagnosis includes toxoplasmosis, which can mimic rim-enhancing PCNSL, although restricted diffusion is usually absent.
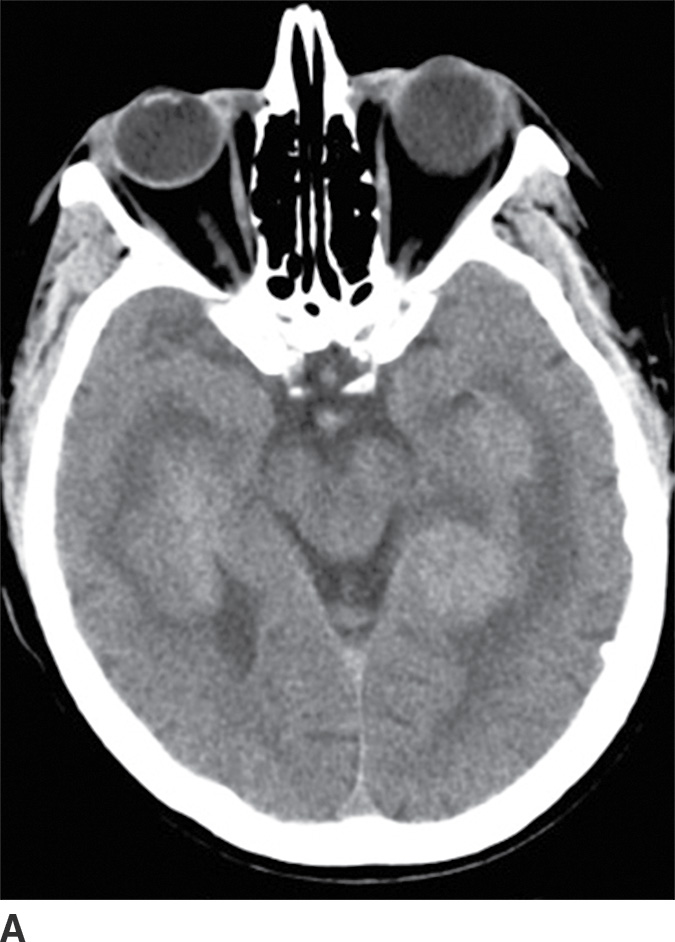
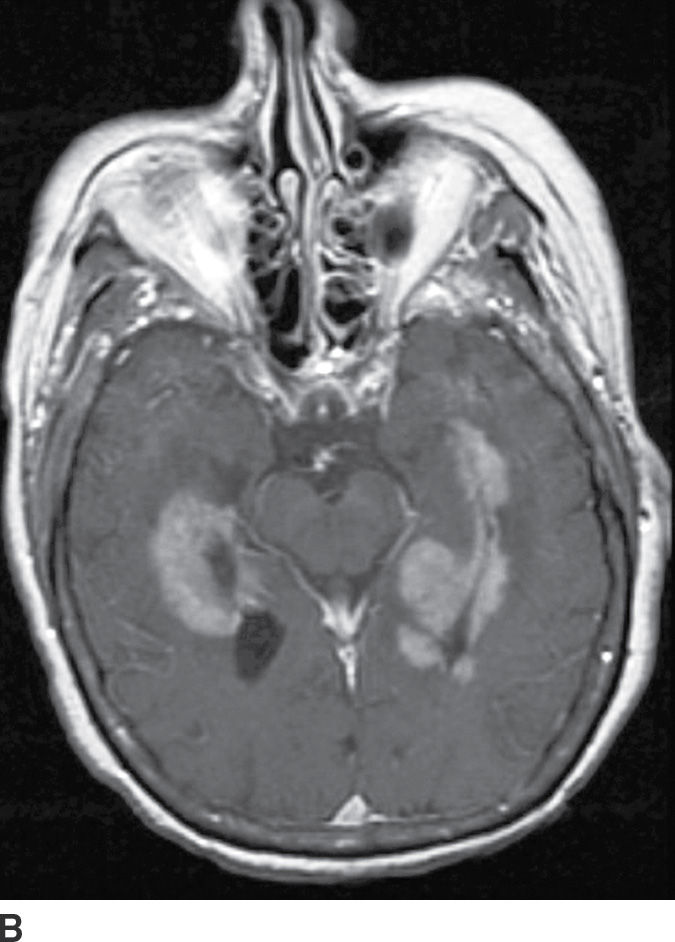
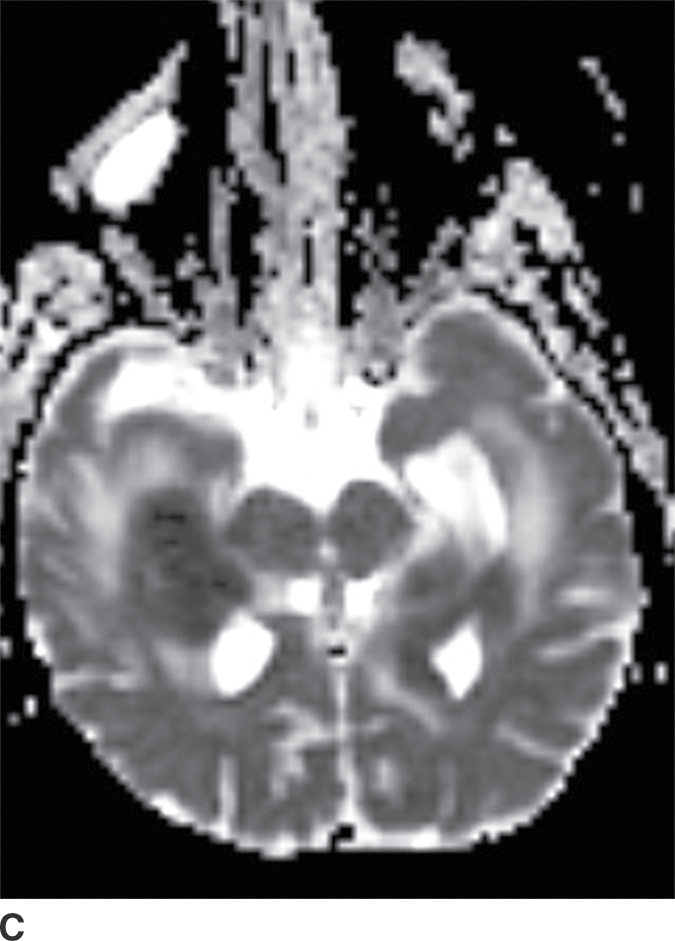
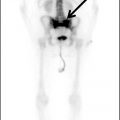
FIG. 4.8 Primary CNS lymphoma. Axial CT (A) shows multiple dense masses along the margins of the lateral ventricles. On a postcontrast T1WI (B), these lesions show avid enhancement, and they show restricted diffusion on ADC maps (C). Primary CNS lymphoma has a relative predilection of the periventricular regions. The density on CT and the restricted diffusion is a reflection of the high cellular density.
Intraventricular Tumors
Choroid plexus neoplasms
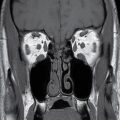
Choroid plexus papilloma (WHO grade I) is an uncommon tumor. Patient age has a strong effect on the location of these tumors. In children (usually infants), they are found in the trigone of the lateral ventricle, whereas in adults, they are more common in the fourth ventricle. They rarely occur in the third ventricle. Hydrocephalus is common, which can be due to a combination of obstruction from tumor cells and overproduction of CSF. CT usually demonstrates iso- to hyperdense lesions, with calcification in up to 25% of patient. MRI usually demonstrates lobulated masses with frond-like papillary projections that are iso- to hyperintense on T2WI. These lesions are highly vascular with frequent demonstration of vascular flow voids and avid contrast enhancement (Fig. 4.9).
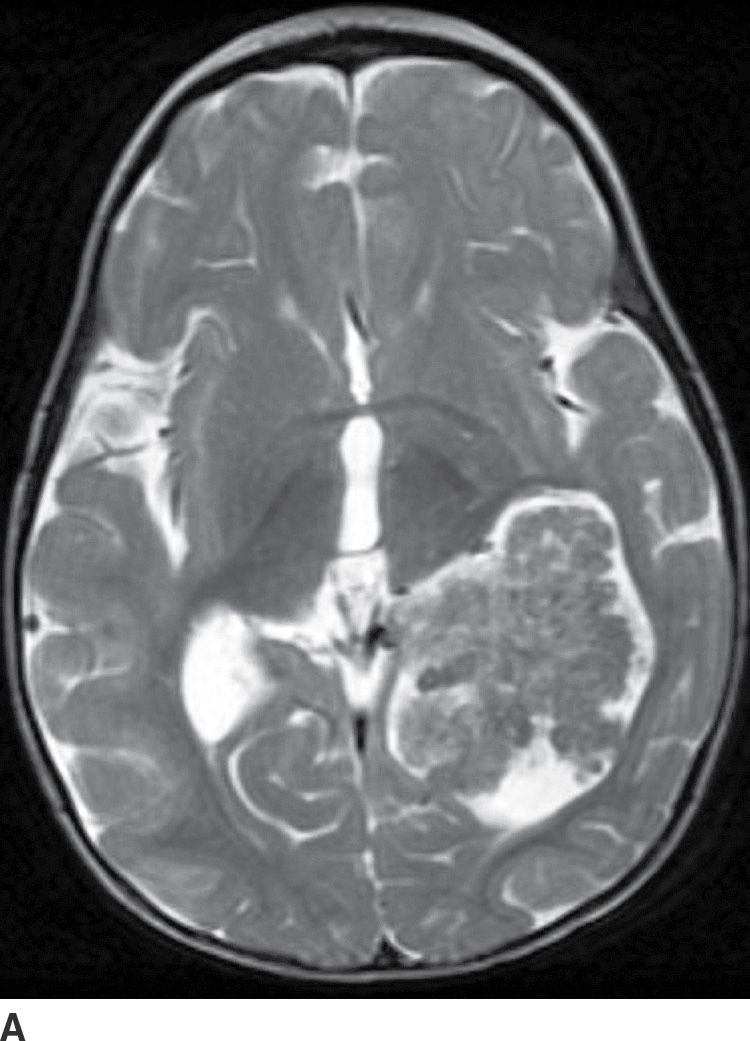
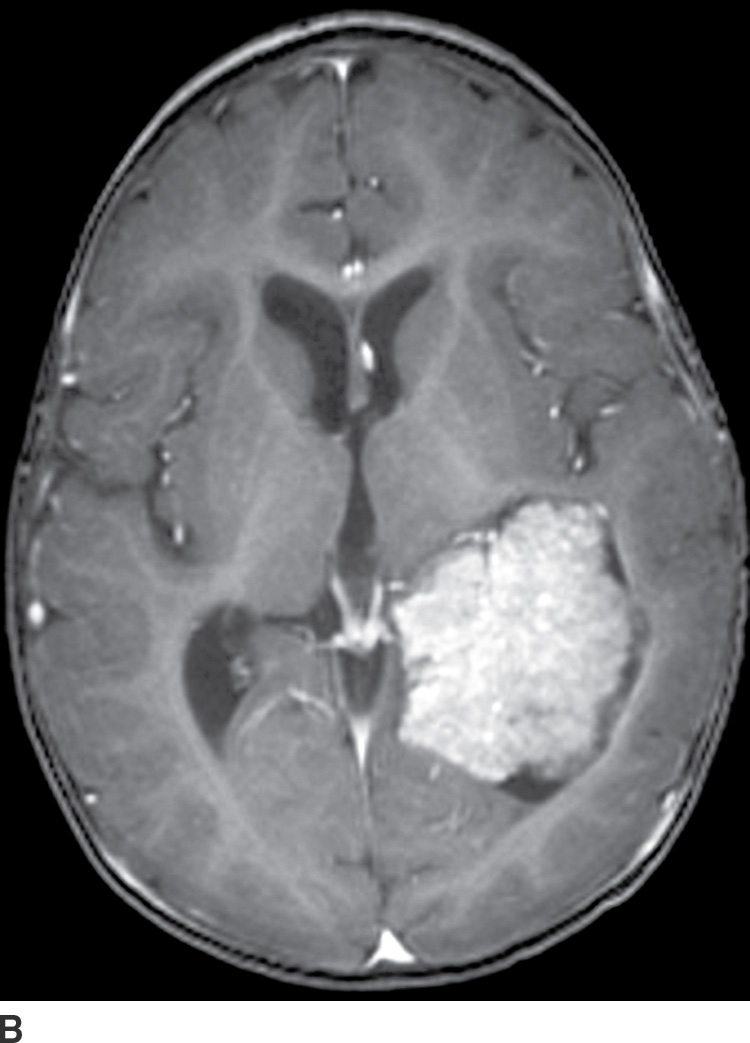
FIG. 4.9 Choroid plexus papilloma. An 11-month-old patient with increasing head circumference. Axial T2WI (A) shows a large intraventricular mass in the atrium of the left lateral ventricle with frondlike lobulations. Postcontrast T1WI (B) shows intense enhancement.
Choroid plexus carcinoma (WHO grade III) is a rare malignant tumor that almost invariably occurs in young children. The imaging appearance can be similar to choroid plexus papilloma, but these tumors often invade the adjacent brain, with frequent presence of edema, necrosis, and hemorrhage.
Subependymoma
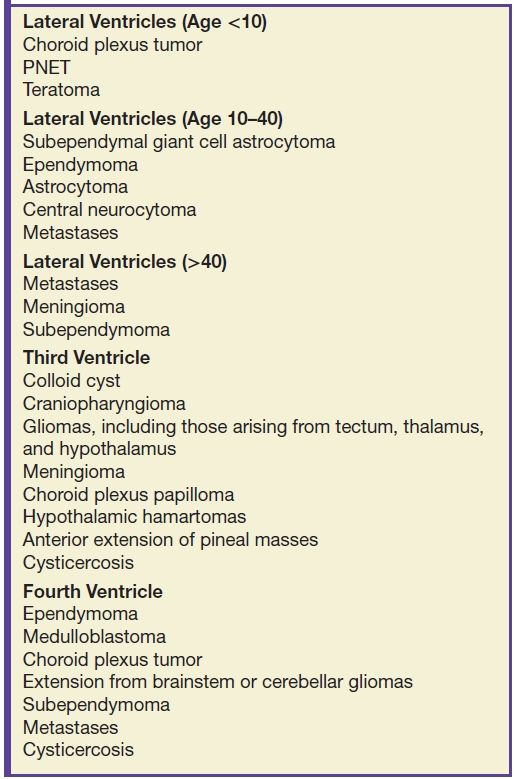
A subependymoma is a rare, slowly growing WHO grade I tumor, likely arising from astrocytes in the subependymal plate. The most commonly reported location is in the inferior fourth ventricle, followed by the frontal horn of the lateral ventricles with frequent attachment to the septum pellucidum. CT typically demonstrates hypodense lesions. The MR signal characteristics are not specific. Enhancement is variable but is frequently absent or mild. The differential diagnosis of subependymoma depends on the age of the patient and tumor location. In older patients, the main differential diagnosis, especially in the fourth ventricle, is an intraventricular metastasis. In younger patients, tumors such as ependymoma and central neurocytoma should be considered, though central neurocytoma usually demonstrates more enhancement. Choroid plexus papilloma is also in the differential diagnosis, but it enhances avidly. Other differential diagnoses are included in Table 4.2 (19).
Table 4.2 INTRAVENTRICULAR MASSES
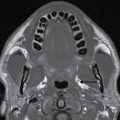
Central neurocytoma
These tumors are benign neoplasms and most commonly occur in young adults. They usually occur in the body of the lateral ventricles and are attached to the septum pellucidum. Given the location, patients usually become symptomatic secondary to obstructive hydrocephalus. CT usually demonstrates a mixed solid and cystic intraventricular neoplasm with attachment to the septum pellucidum (Fig. 4.10). Intratumoral calcification can be seen. MRI demonstrates heterogeneous signal intensity with frequent presence of flow voids. Heterogeneous enhancement is usually seen following contrast administration on both CT and MRI (15). The main differential diagnoses include subependymoma, ependymoma, and intraventricular metastases.
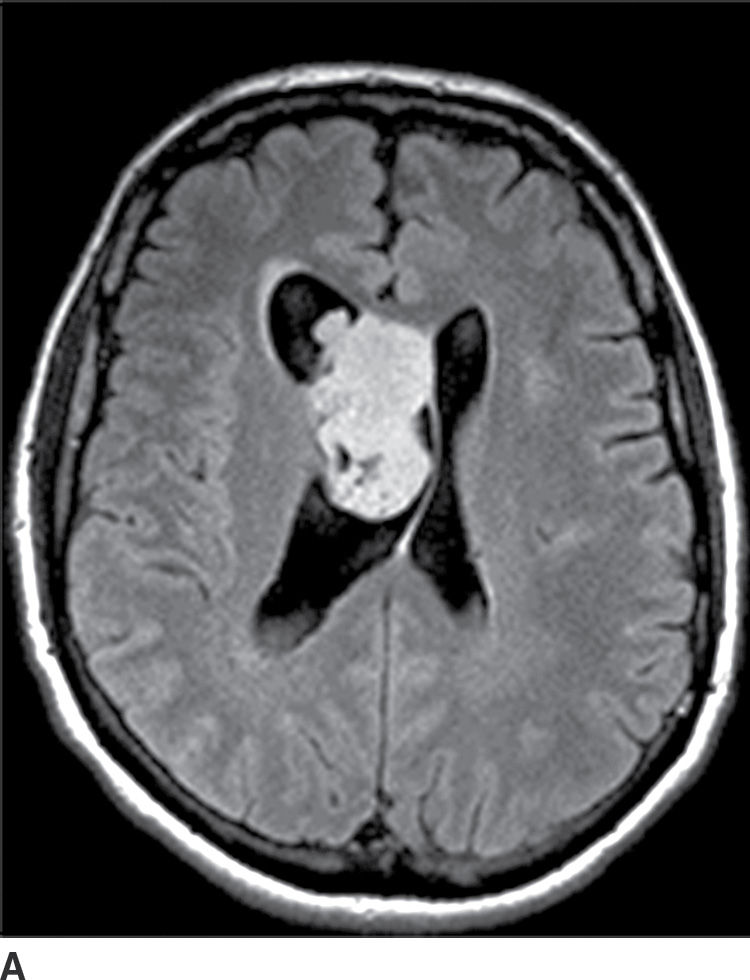
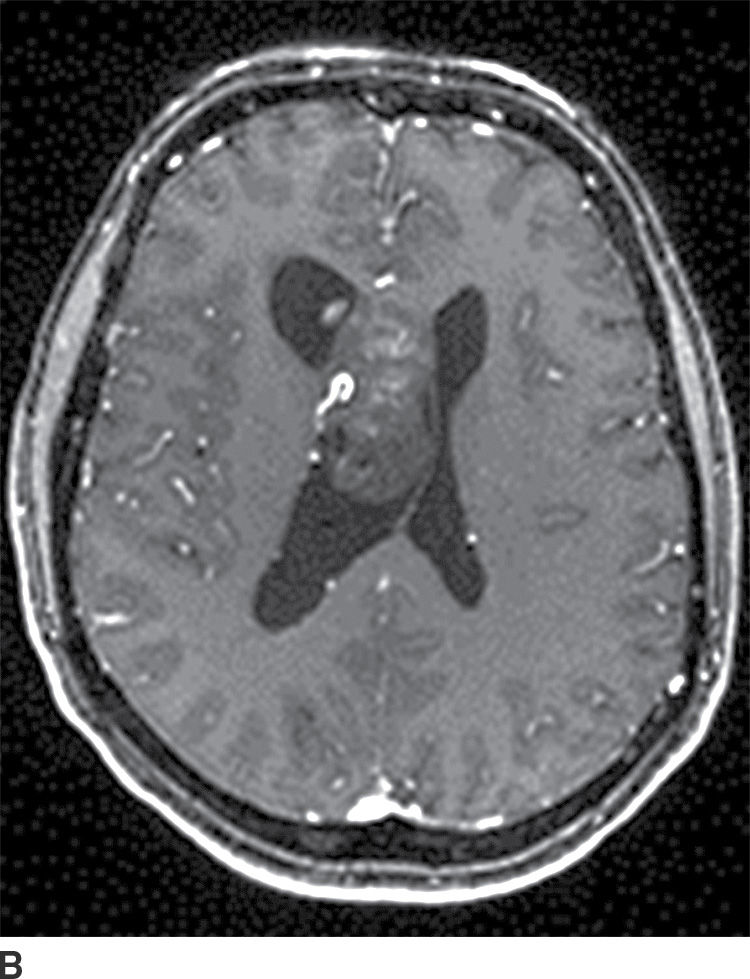
FIG. 4.10 Central neurocytoma. Axial FLAIR (A) and postcontrast T1WI (B) images demonstrate a mildly enhancing mass in the right lateral ventricle, in contact with and displacing the septum pellucidum.
Other intraventricular masses
Subependymal giant cell astrocytomas are intraventricular grade I gliomas that occur near the foramen of Monro in patients with TS and were discussed under gliomas. Colloid cysts of the third ventricle are technically not tumors, but can mimic tumors and cause hydrocephalus of the lateral ventricles. They are typically small round cysts along the anterosuperior aspect of the third ventricle near the foramina of Monro. The CT density and MRI signal intensity depend on the proteinaceous contents of the cyst. They do not have malignant potential. Meningiomas can occasionally occur in the ventricles, with the atrium of the lateral ventricle being the most common location.
Pineal Region Masses
Pineal region tumors typically present with hydrocephalus or difficulties with upward gaze (Parinaud syndrome) due to compression of the nearby midbrain tectum. Germ cell tumors constitute more than half of all pineal region tumors with germinoma being the most common subtype (Table 4.3). Pineal germinomas have a very high male predilection. They occur most commonly in older children and young adults. Other subtypes are less common, with the second most common type being a teratoma. Germinomas tend to engulf a potentially calcified pineal gland, may have tiny cystic areas within them, and typically avidly enhance with contrast (Fig. 4.11). They can disseminate in the CSF.
Table 4.3 PINEAL REGION MASSES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree