Acknowledgment: Darryl H. Hwang, PhD.
Masses that are extradural in location may surround, compress, or displace the thecal sac (see Table 13.1). These lesions may show a “cutoff sign” of the CSF within the thecal sac due to the extrinsic compression. Lesions that arise within the extradural space may arise primarily within the epidural space or may be extension of masses from the spinal column or adjacent paravertebral tissues. The division of the spine into these regions is arbitrary as lesions may involve one, two, or all three of these spaces. The purpose of this division is to aid in generating a differential diagnosis for spine tumors.
Masses that arise from the bony spinal column may represent primary bone lesions, metastatic disease from a remote primary neoplasm, or direct invasion from an adjacent neoplasm. These lesions may extend peripherally away from the spinal canal and involve the adjacent paravertebral soft tissues and/or the neural foramina. The vertebrae are divided into the vertebral body, the pedicles, the lateral masses (including the superior and inferior facets and intervening pars interarticularis), and the posterior neural arch, which includes the lamina and spinous process. Tumors of the vertebra can be divided into lesions that commonly involve the vertebral body and/or the lateral masses versus lesions that have a predilection for the posterior neural arch.
Imaging considerations
Plain radiography of the spine is highly limited in the depiction of spinal tumors particularly when located within the spinal canal or when there is a significant soft tissue component. Boneremodeling, bone destruction, or pathologic fracture, however, may be depicted with x-ray. Large lytic or sclerotic lesions of the bone may be evident, though smaller or more subtle lesions can be difficult to detect. This limited sensitivity may be further confounded by underlying decreased bone density or from limitations in technique.
MR imaging is the modality of choice in both precisely localizing and characterizing spinal tumors within the central canal. T2-weighted images (T2WI) with the high signal intensity of CSF help in determining the relationship of tumors with respect to location (intramedullary, intradural extramedullary, or extradural/epidural). T2WI also help detect the presence of edema and cystic changes within the cord. The use of gadolinium contrast agents aids in determining tumor–soft tissue interfaces and characterizing lesions associated with the dura. For lesions associated with the spinal column, contrast is also useful in mapping out the extent and gross margins of the tumor. In adults, the hyperintense signal intensity of the marrow on T1-weighted images (T1WI) relative to intervertebral disk is due to marrow fat, which serves as helpful natural background contrast relative to the more isointense to hypointense masses that may involve the vertebra. Noncontrast T1WI may be the most sensitive sequence to depict marrow lesions. If contrast-enhanced images are used, it is helpful to perform fat-saturated T1WI so that the hyperintense signal intensity of fat does not hide underlying T1 shortening from enhancement of a mass. Fat-saturated T2WI or STIR images may be helpful in depicting marrow lesions when there is associated edema though the fat-saturated T2W and STIR signal intensity of marrow lesions may also be isointense to hypointense relative to background bone marrow on occasion and therefore may be less conspicuous.
The use of CT is complementary to MR imaging. Bone lesions can be well characterized on CT and may be seen as lytic or sclerotic. Zone of transition, presence of a sclerotic rim, other sclerotic changes, expansion of the vertebra, bone remodeling, or osseous erosion can be well characterized on CT and aid in differentiating benign from malignant lesions. The presence of a sharp transition zone, sclerotic margin, and bone remodeling may be indicative of a benign process. A wide or ill-defined transition zone, aggressive periosteal reaction, cortical erosion, permeative change, and frank osseous destruction are more indicative of a malignant process. The introduction of a nonionic contrast agent into the thecal sac in myelography and subsequent CT study may be used to help localize a lesion within the spinal canal if MR imaging is contraindicated or inconclusive, and the principles discussed earlier on MRI T2WI for tumor localization apply to myelography as well. Postmyelography CT is not performed as widely as in the past prior to the rise in MR imaging but has shown resurgence as a tool for radiation treatment planning to help distinguish the spinal cord and assess its relationship to the spinal tumor.
Bone scans may be utilized to define marrow lesions as these studies depict the physiologic activity of bony production and resorption. These studies are cost efficient in the characterization of metastatic disease and may have a role in analyzing primary bone tumors. However, in the setting of multiple myeloma, these studies are typically negative and other imaging modalities should be used. PET imaging using 18FDG–glucose is a useful adjunct in the depiction of spine tumors. However, due to high cost, these studies are usually not included in the initial workup of primary spine lesions and are used more frequently in the setting of disseminated disease.
Intramedullary Lesions (Table 13.2)
Table 13.2 Intramedullary Tumors
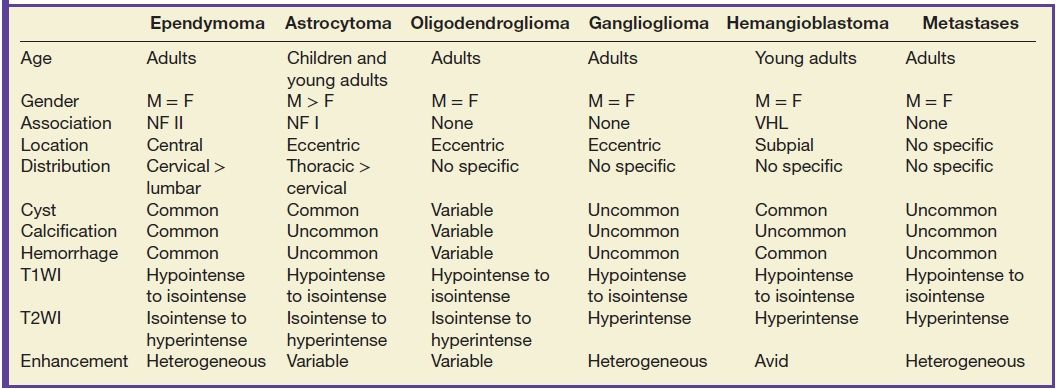
Tumors of the intramedullary space include ependymoma, astrocytoma, oligodendroglioma, ganglioglioma, hemangioblastoma, and metastatic disease. Location within the spinal cord may be helpful in distinguishing these lesions from each other. Ependymomas are midline lesions, while glial tumors such as astrocytoma, oligodendroglioma, and ganglioglioma are off center, though this may be difficult to determine based on the overall tumor size. Patient symptomatology and diffusion tensor imaging (DTI) with tractography may also give a sense as to whether a tumor is infiltrating versus displacing white matter tracts. Hemangioblastomas typically occur in a subpial location within the cord and tend to be along the cord periphery. Metastatic disease can always be considered in the differential diagnosis of a patient with a known primary neoplasm regardless of location.
Ependymoma
Ependymomas are the most common spinal cord tumor in adults and make up to 60% of all intramedullary tumors. These neuroepithelial tumors arise from the ependymal cells that line the central canal of the spinal cord. Therefore, they are typically midline. Histologically, the variants include myxopapillary type of the filum terminale (WHO grade I), myxopapillary type of the spinal cord and conus medullaris (WHO grade II), anaplastic type (WHO grade III), and subependymomas. These lesions are typically solitary, but multiplicity may be seen in patients with neurofibromatosis type II. The myxopapillary type constitutes 13% of all ependymomas with greater than 80% at the level of the conus medullaris or filum terminale (Fig. 13.1).
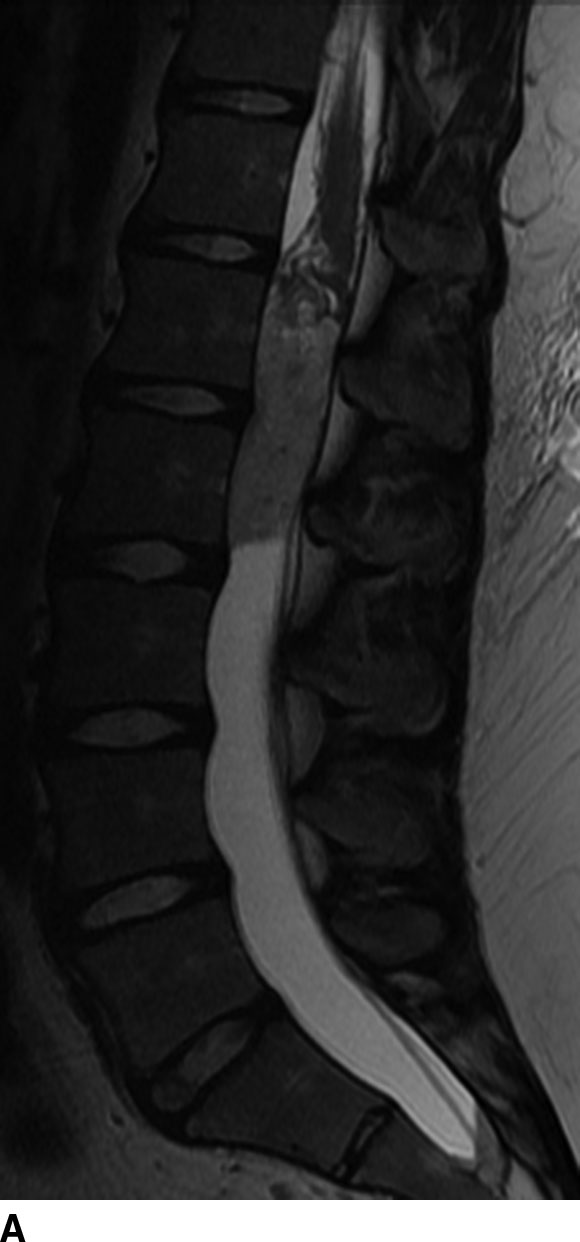
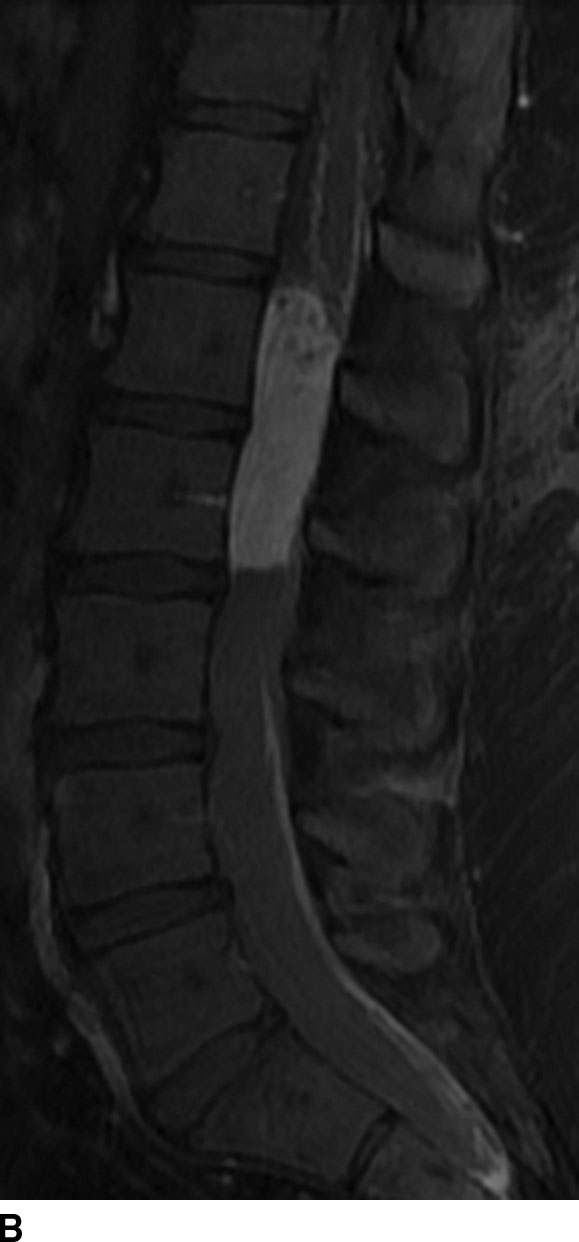
FIG 13.1 Myxopapillary ependymoma. A: Sagittal level of the T2WI demonstrates a hyperintense, intradural mass at the conus medullaris. B: There is avid enhancement of the mass and leptomeninges on contrast-enhanced sagittal T1WI.
On MR imaging, these lesions are typically well circumscribed and are isointense to hypointense on T1WI and hyperintense on T2WI and demonstrate variable patterns of enhancement. Due to repeated episodes of bleeding, these tumors may demonstrate a hypointense cap along their margins on T2WI and gradient echo imaging. Eighty percent of these lesions have an associated nonenhancing cyst, which represents an associated syrinx or a true tumoral cyst (Fig. 13.2). Surrounding spinal cord edema may also be present especially with larger lesions. Calcifications are rare in spinal cord ependymomas in contrast to intracranial ependymomas. Due to the high rate of drop metastasis associated with ependymoma, whole spine MR imaging is typically required when the diagnosis of ependymoma is confirmed. These tumors may also occur in patients with neurofibromatosis type II (1,2).
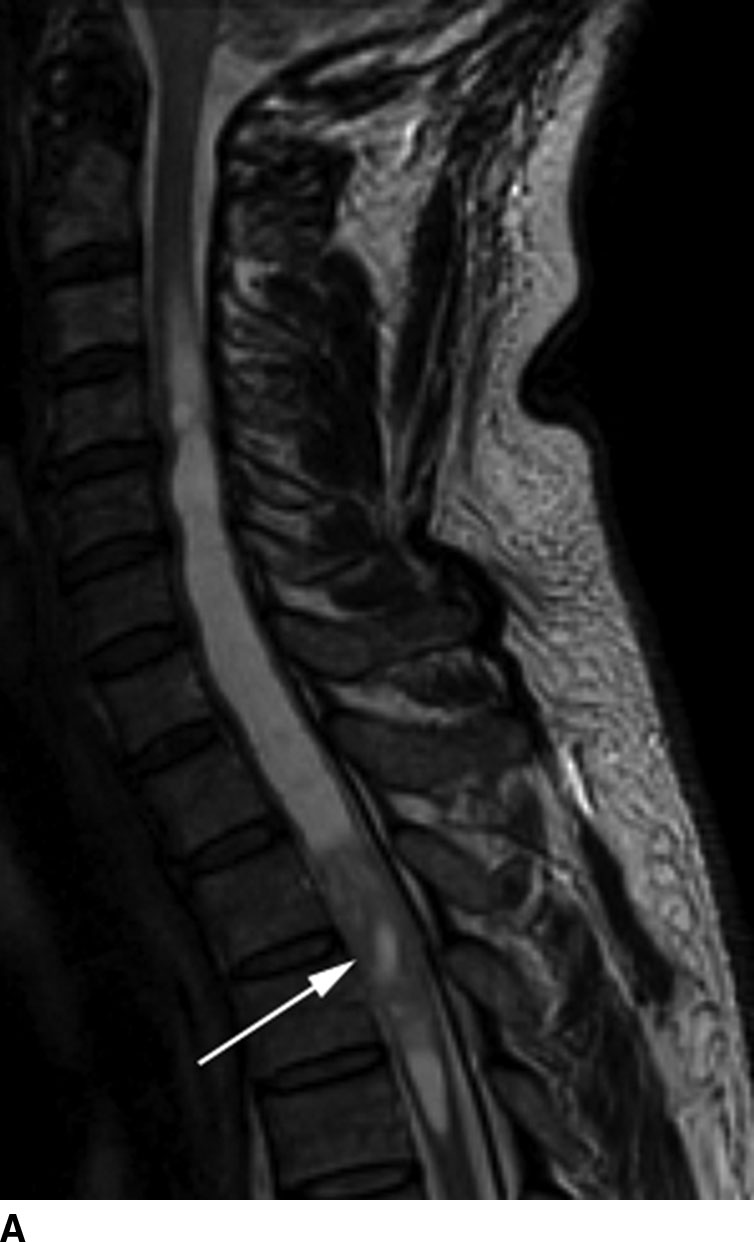
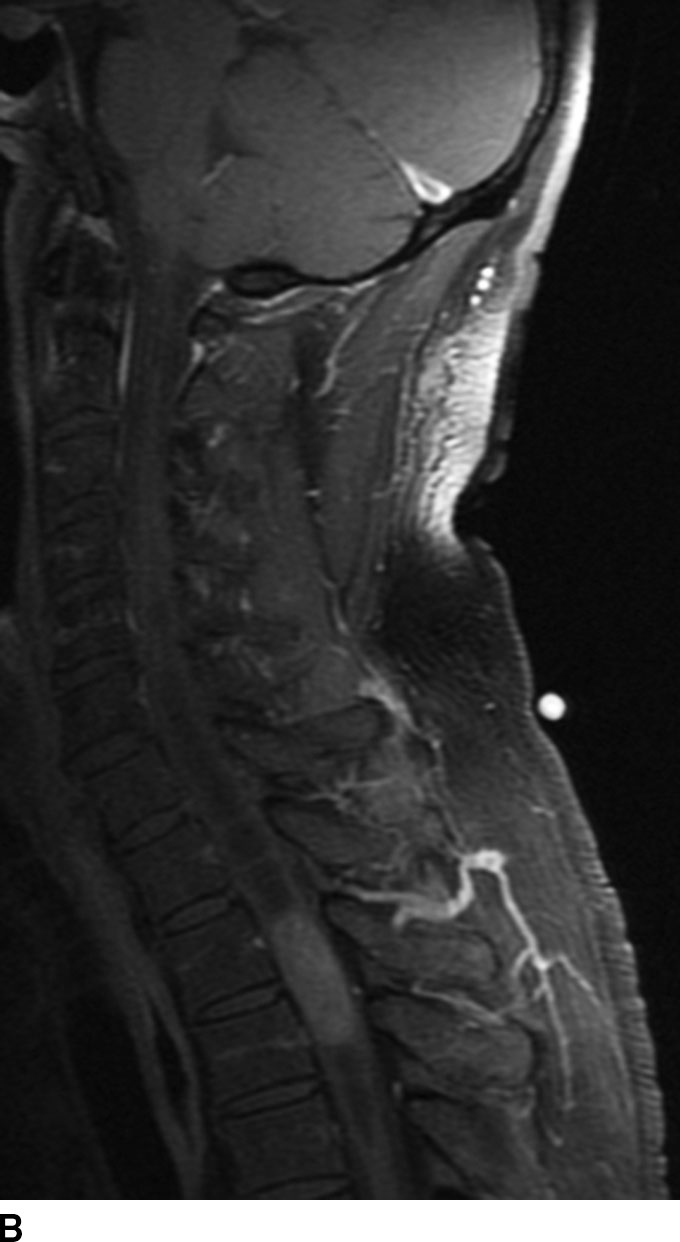
FIG 13.2 Intramedullary ependymoma. A: Sagittal T2WI shows a mass with the upper thoracic spinal cord. Notice the associated tumoral cyst distinct from the solid portion (arrow) of the mass. There is also a large syrinx on both ends of the mass. B: The tumoral cyst does not enhance following contrast administration on sagittal fat-saturated T1WI. The solid portion of the mass shows marked enhancement on sagittal fat-saturated T1WI.
Astrocytomas
Astrocytoma is the second most common spinal cord tumor and constitutes up to 30% of these lesions. Arising from astrocytic glial cells, these tumors are typically off midline. Tumor types include pilocytic type (WHO grade I); diffuse type (WHO grade II) including fibrillary, gemistocytic, and protoplasmic subtypes; anaplastic astrocytoma (WHO grade III); and glioblastoma multiforme or GBM (WHO grade IV). Both anaplastic and GBM are high-grade tumors and fortunately are rare in the spinal cord. Spinal cord astrocytomas are more common in children, in whom the pilocytic type is most common. Adult astrocytomas tend to occur in the third decade of life, earlier than ependymoma, and are more commonly the diffuse type. Unlike ependymomas, they rarely hemorrhage. These tumors are typically isointense to hypointense on T1WI and heterogeneously hyperintense on T2WI and have variable patterns of enhancement. Edema is usually seen in the higher grades. These lesions tend to be ill-defined, and in the diffuse type, the tumor–spinal cord interface is not as well seen compared to ependymoma. Tumoral cysts are more commonly seen with astrocytomas (especially with the pilocytic type), though associated syrinx may also form due to obstruction of CSF flow (Fig. 13.3). Multiple lesions are uncommon with astrocytoma, and if present, a high-grade malignancy should be suspected. Leptomeningeal or drop metastasis could also be indicative of a higher grade. Astrocytomas may also occur in patients with neurofibromatosis type I (3).
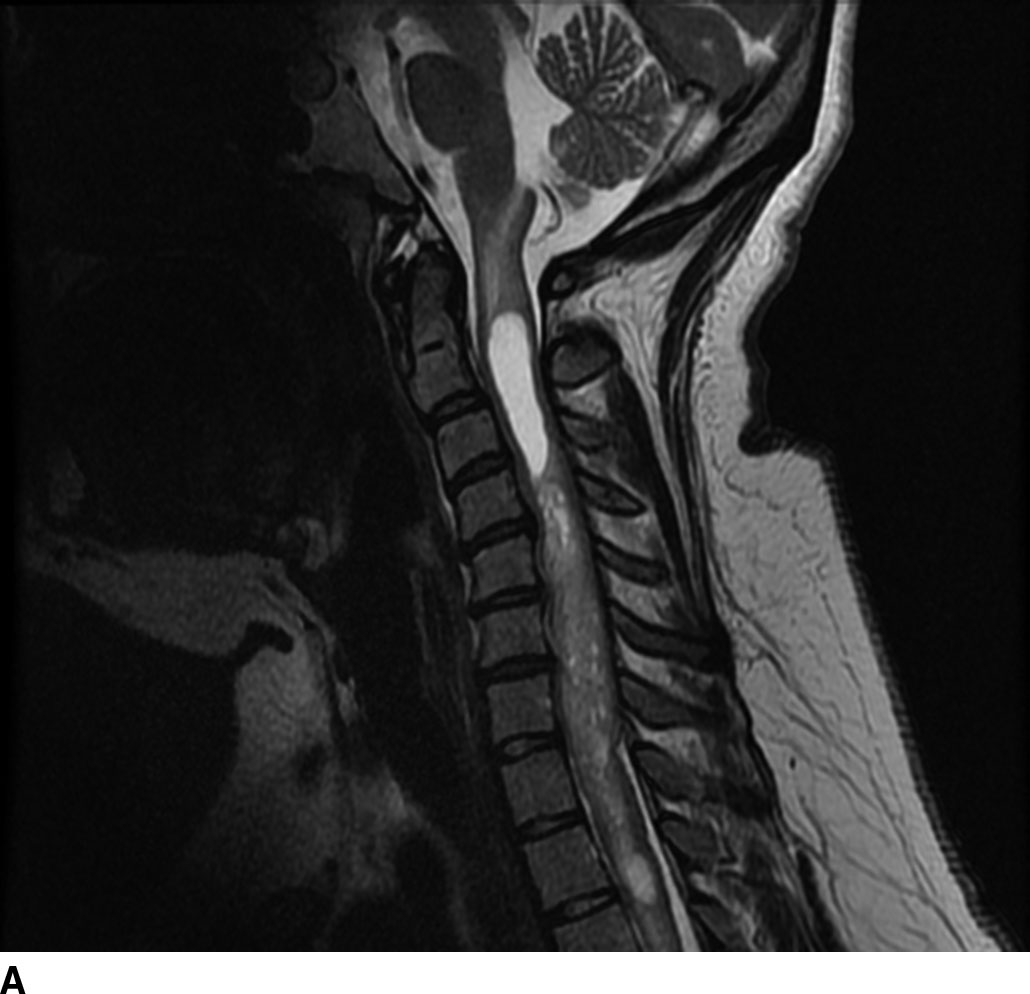
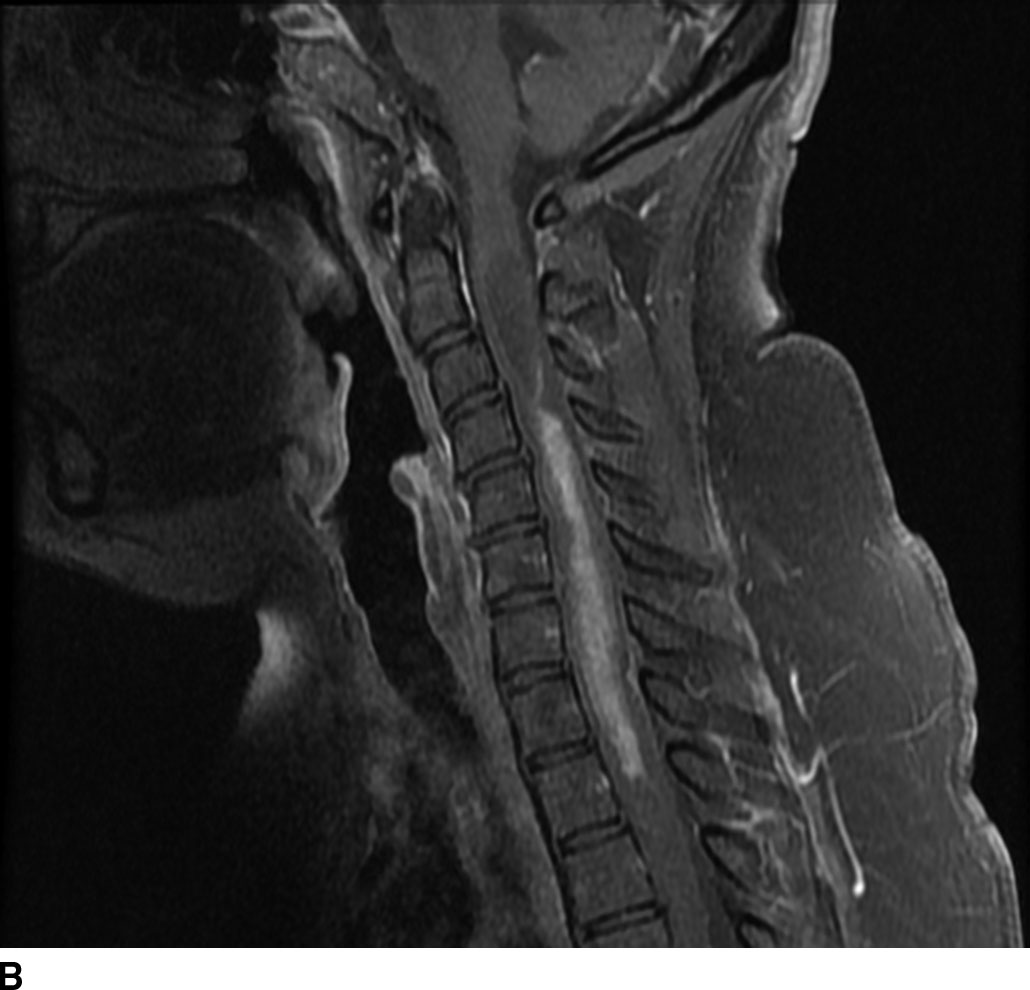
FIG 13.3 Astrocytoma. A: Sagittal T2WI reveals a mildly hyperintense expansile mass within the spinal cord consistent with an intramedullary tumor. There are small intratumoral cysts as well as a syrinx above the mass. B: The central portion of the mass shows enhancement, but there may be nonenhancing areas of tumor as well.
Oligodendrogliomas
Oligodendrogliomas represent the third most common glial tumor of the spinal cord yet constitute less than 2% of spinal cord tumors and 1.5% of all oligodendrogliomas. Classic oligodendrogliomas are WHO grade II tumors, though anaplastic variants are WHO grade III. Arising from oligodendroglial cells, these tumors are indistinguishable from astrocytoma on imaging and the histology is determined after biopsy or resection. The presence of calcification or hemorrhage may aid in making the diagnosis prior to surgery. These tumors have demonstrated variable patterns of enhancement and the presence of tumoral and reactive cysts. Surrounding edema may also be present (4).
Gangliogliomas
Gangliogliomas represent mixed neuronal glial tumors and, like their counterpart intracranially, are uncommon. These tumors constitute less than 1% of all spinal tumors and are extremely rare tumors. Like oligodendroglioma, the imaging of these tumors is similar to astrocytoma and thus cannot be distinguished based on imaging alone. These lesions are isointense to hypointense on T1WI but may demonstrate variable signal intensity due to the mixed neuronal and glial elements. On T2WI, these lesions are heterogeneously hyperintense with variable patterns of enhancement. These rarely involve the conus medullaris. Ganglioglioma is the fourth most common glial tumor of the spinal cord (5).
Hemangioblastomas
Hemangioblastomas are capillary-rich low-grade (WHO grade I) tumors that may occur sporadically (70%) or in the setting of von Hippel-Lindau syndrome (30%). These lesions are the third most common spinal cord tumors and constitute up to 6% of all spinal cord tumors. These tumors are usually seen between the second and fourth decades of life with a mean age of 30 years. The majority occur in a subpial location along the posterior aspect of the cord and thus appear along the surface of the cord. Cysts are common with these lesions and may be seen in up to 55% of cases. These may represent a tumoral or reactive cyst (Fig. 13.4). The size of the cyst is not dependent on the size of the tumor itself. Because of the hypervascular nature of these lesions, avid enhancement is usually seen. The solid component of the tumor may also exhibit the presence of blood products due to hemorrhage, and prominent signal flow voids may be seen on MR imaging secondary to tumor vascularity. Angiographic evaluation may be useful in distinguishing these lesions from other spinal cord tumors or vascular malformations. The solid component of these lesions is fairly well circumscribed. These lesions are typically hypointense to isointense on T1WI and heterogeneously hyperintense on T2WI. These lesions frequently do not demonstrate surrounding edema (6).
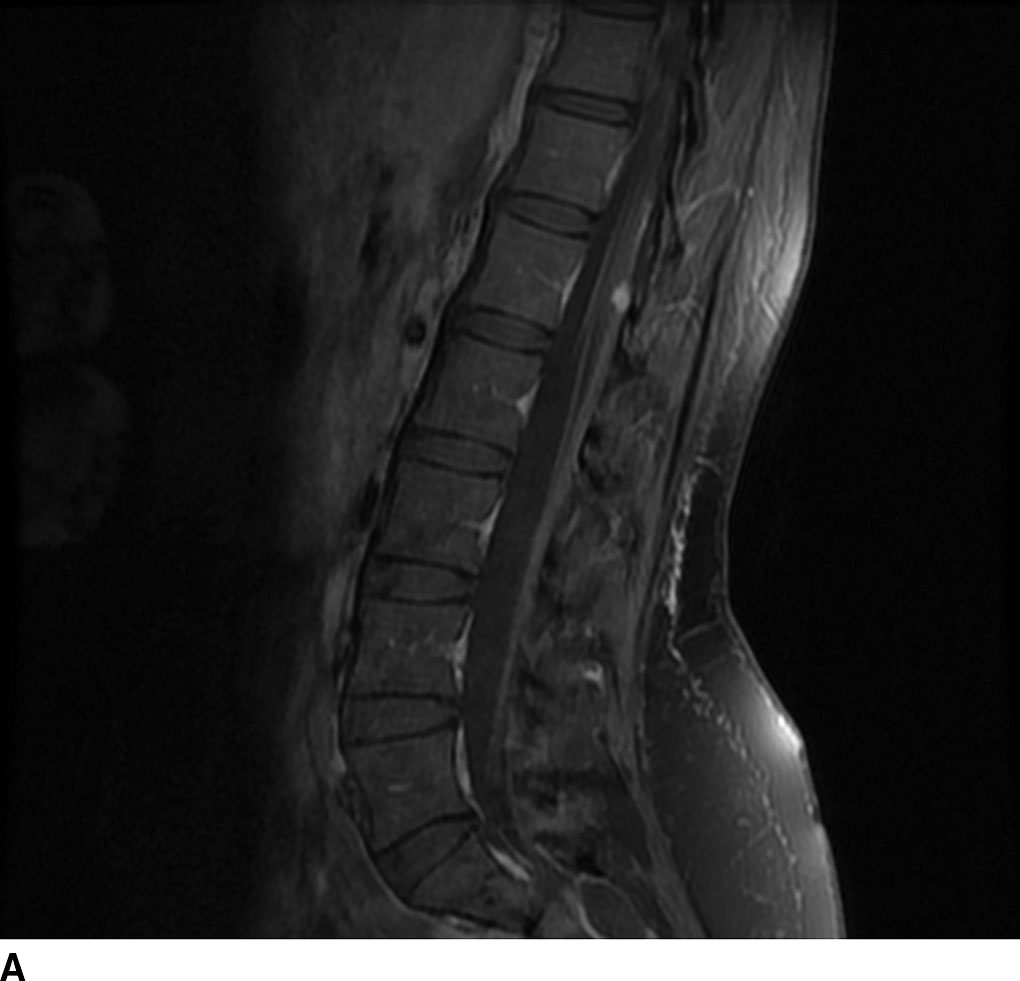
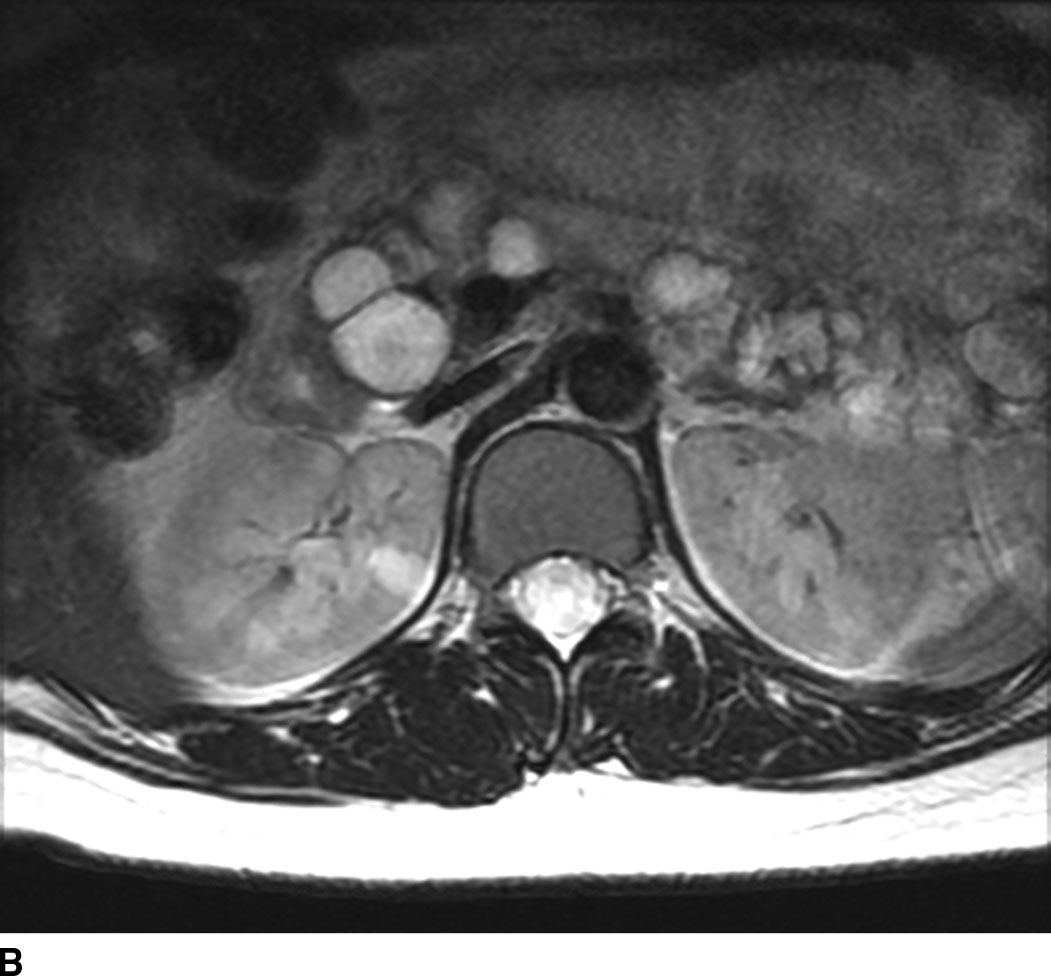
FIG 13.4 Hemangioblastoma. A: Postcontrast fat-saturated T1WI shows an enhancing mural nodule that extends to the cord surface suggesting a subpial location. There is also enhancement along the cauda equina. B: Axial T2WI also shows pancreatic and renal cysts suggesting underlying VHL in this patient.
Metastatic disease
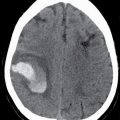
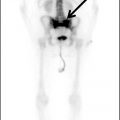
Metastatic disease to the spinal cord used to be a rare occurrence. Given the increased survival of patients with cancer due to new treatment regimens and early detection, there has been an increased incidence of metastasis to the spinal cord (Fig. 13.5). Metastatic disease should always be in the differential diagnosis for an enhancing mass within the cord in any patient older than 40 years. The most common spinal cord metastases are lung and breast cancer as these are the two most common malignancies that metastasize to the central nervous system. Hemorrhagic lesions within the cord should include hemorrhagic metastases such as melanoma, renal cell carcinoma, thyroid cancer, and choriocarcinoma. Cystic metastasis to the cord can be seen with the aforementioned primary neoplasms as well as mucinous adenocarcinomas (7).
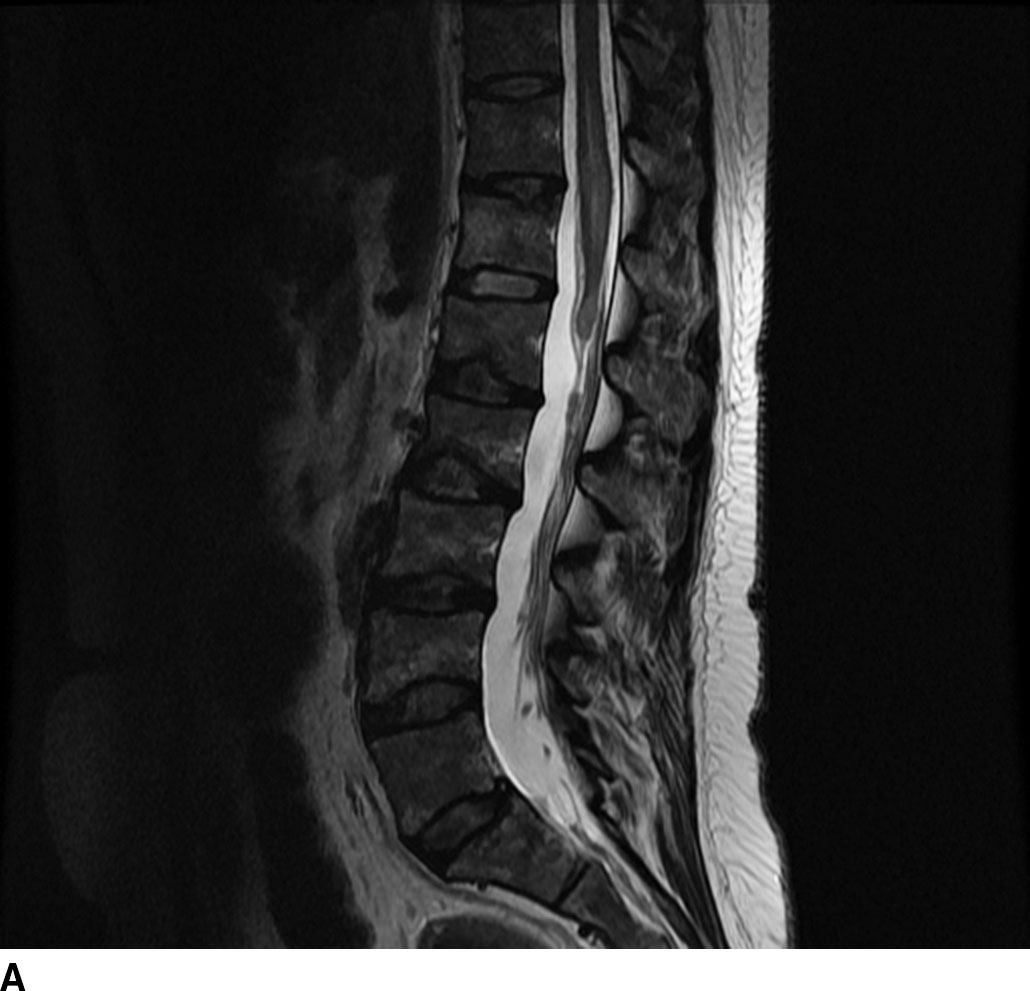
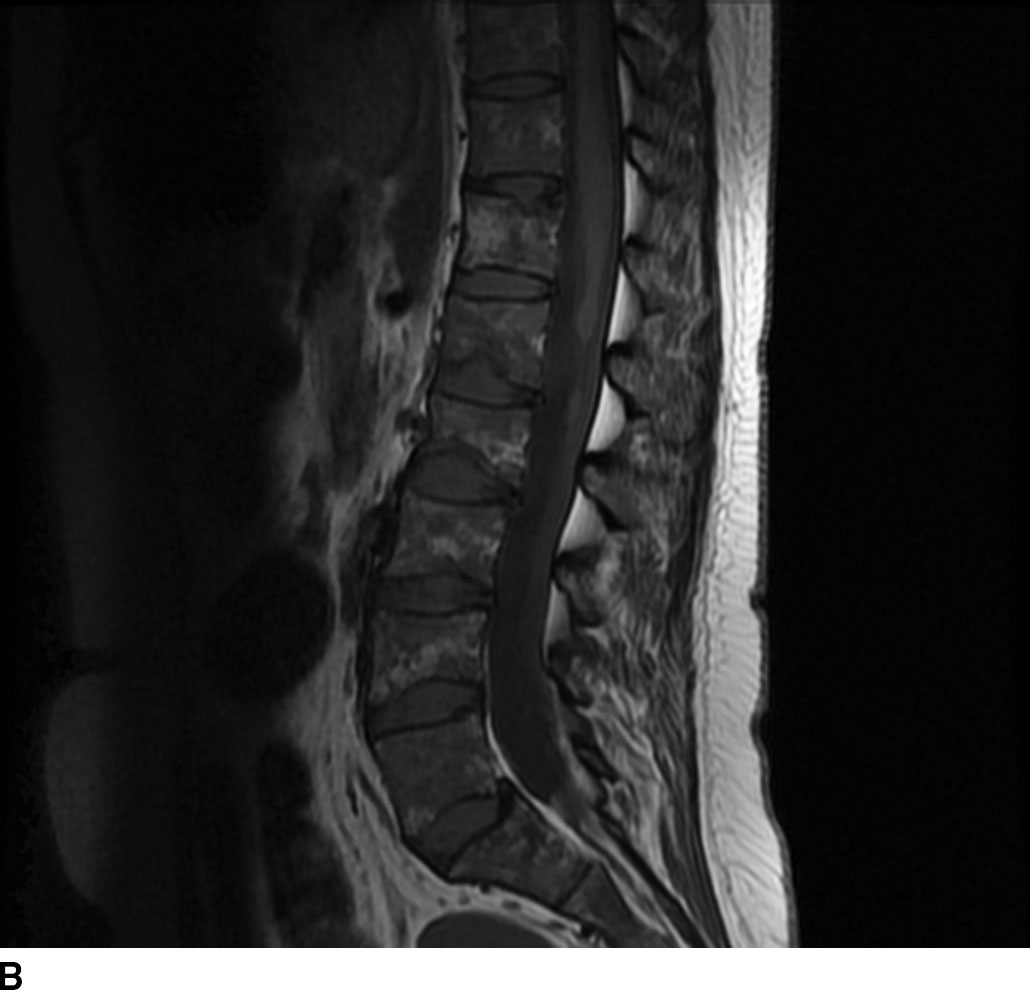
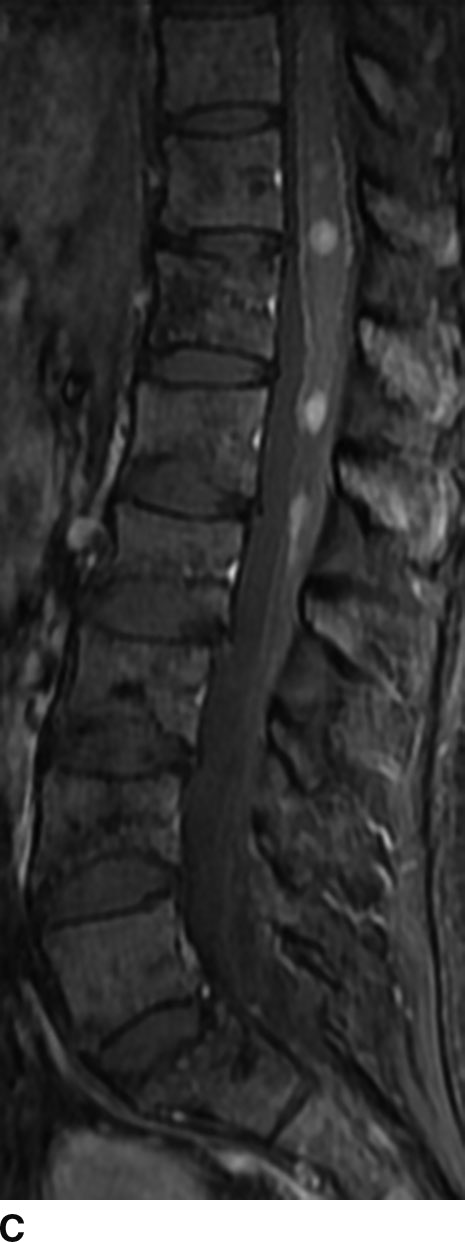
FIG 13.5 Intramedullary breast cancer metastases in a patient being treated with trastuzumab (Herceptin). A: Sagittal T2WI shows mass effect and increased signal in the distal spinal cord. Nodularity is also present among the cauda equina nerve roots. B: On sagittal T1WI, heterogeneous areas of low signal are seen in the bone marrow of the spine compatible with metastatic disease. C: Postcontrast fat-saturated sagittal T1WI shows multiple enhancing metastases within the distal spinal cord and along the cauda equina. There is also leptomeningeal enhancement along the conus. In addition, the bone metastases enhance.
Intradural/Extramedullary Lesions (Table 13.3)
Table 13.3 Intradural Extramedullary Tumors
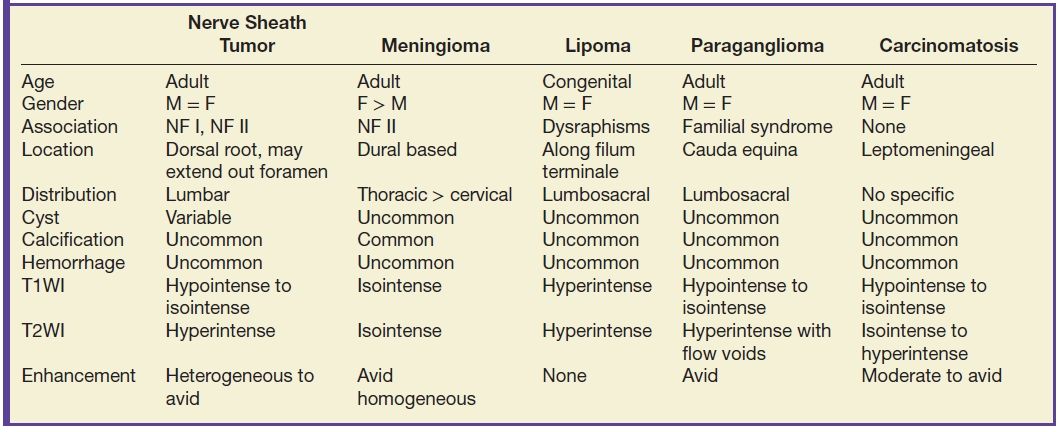
The most common primary intradural extramedullary space lesions are nerve sheath tumors and meningiomas. Both nerve sheath tumors and meningiomas may be seen in patients with neurofibromatosis type II.
Nerve sheath tumors
Nerve sheath tumors arise from the nerve sheath, which encapsulates the axons of peripheral nerves. Composed of Schwann cells and fibroblasts, these tumors are the most common benign intradural extramedullary tumors of the spinal column. The neural sheath begins at the root entry zone of the dorsal nerve root as it emerges from the spinal cord and extends along the course of the nerve root within the thecal sac, within the neural foramen, and beyond the foramen as the nerve leaves the spinal column.
Nerve sheath tumors are subdivided based on their histologic composition into schwannomas and neurofibromas. Schwannomas are composed primarily of Schwann cells, while neurofibromas contain a mixed population of Schwann cells and other cells. The imaging distinction between a schwannoma and a neurofibroma is often difficult. In the setting of a schwannoma, the nerve travels along the surface of the tumor and can be dissected from the tumor at surgery. The nerve in a neurofibroma is invested within the tumor and cannot be separated from the mass and is sacrificed at surgery. While neurofibromas can have central hypointensity with peripheral hyperintensity on T2WI or a “target sign,” this appearance can also occur with schwannomas though it is thought to be more common in neurofibromas.
The major cell type within nerve sheath tumors are Schwann cells, which are only found in the peripheral nervous system along the axons. The two histologic layers seen in schwannomas are the Antoni A and Antoni B layers. Antoni A regions are composed of spindle-shaped cells with a high nucleus-to-cytoplasm ratio. Antoni B regions are myxoid in appearance with a higher water content, lower nucleus-to-cytoplasm ratio, and an overall paucicellular distribution. The imaging appearance of nerve sheath tumors is dependent on the percentage of Antoni A and Antoni B areas. Those nerve sheath tumors with a higher percentage of Antoni A composition will be lower in signal intensity on T2WI due to the decrease in water content within the cells. Thosetumors with higher Antoni B composition will have a higher water content and thus will be more hyperintense in signal intensity on T2WI images.
In general, nerve sheath tumors tend to be heterogeneously hyperintense in signal intensity on T2WI. On T1WI, nerve sheath tumors are isointense to hypointense relative to other nerve or cord tissue. After the administration of contrast, these tumors demonstrate heterogeneous moderate to avid enhancement. On CT imaging, nerve sheath tumors are well-circumscribed masses that are isoattenuating to muscle on noncontrast images and demonstrate moderate to avid enhancement on postcontrast images. Nerve sheath tumors may cause bone remodeling and expansion of the neural foramen. Tumors in the neural foramen tend to have a dumbbell shape with a portion of the tumor extending into the central canal and a portion distal to the neural foramen. Within the thecal sac, these masses are situated separate from the spinal cord and may displace it. At the level of the cauda equina, these masses are associated with a nerve root. These tumors frequently have cystic component(s) (Fig. 13.6), but hemorrhage and calcification are rare with calcification occurring in less than 5%. Calcification may be used as a differentiating point between a nerve sheath tumor and the second most common tumor in the intradural extramedullary space, meningioma.
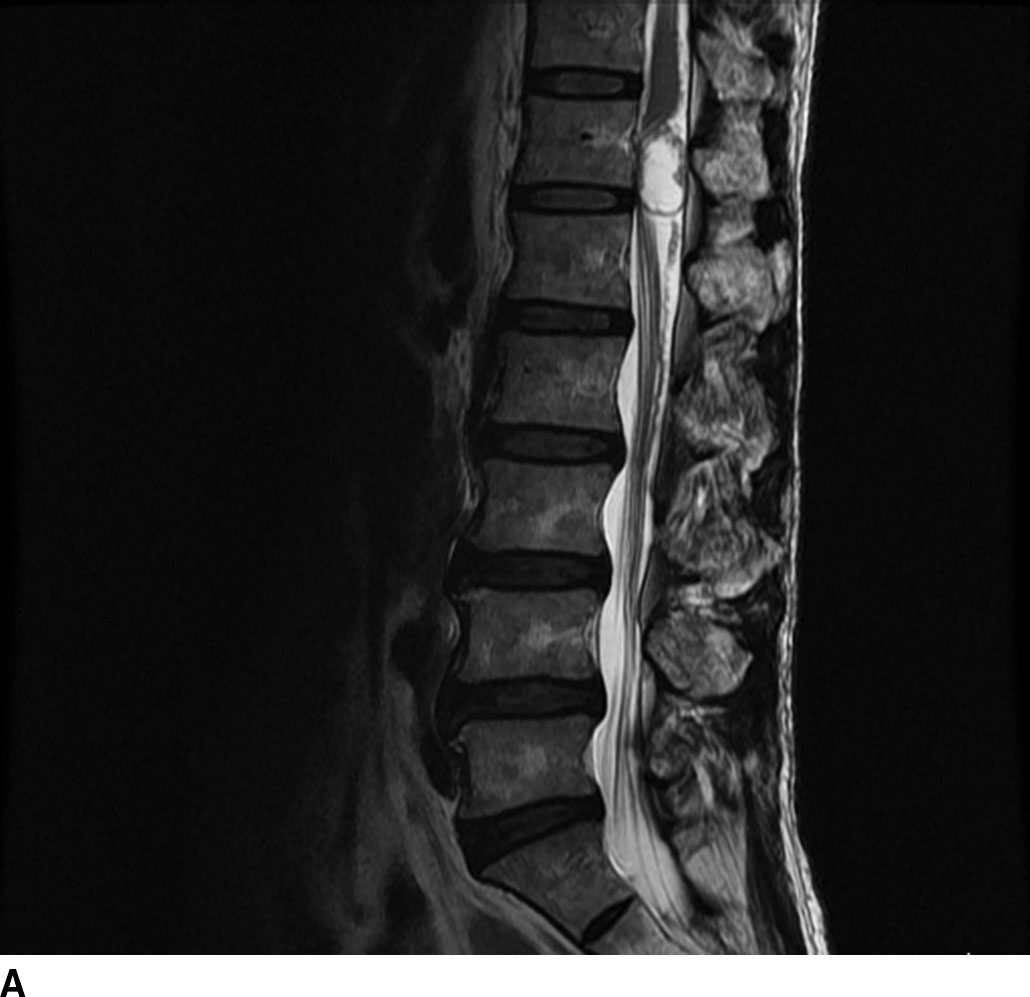
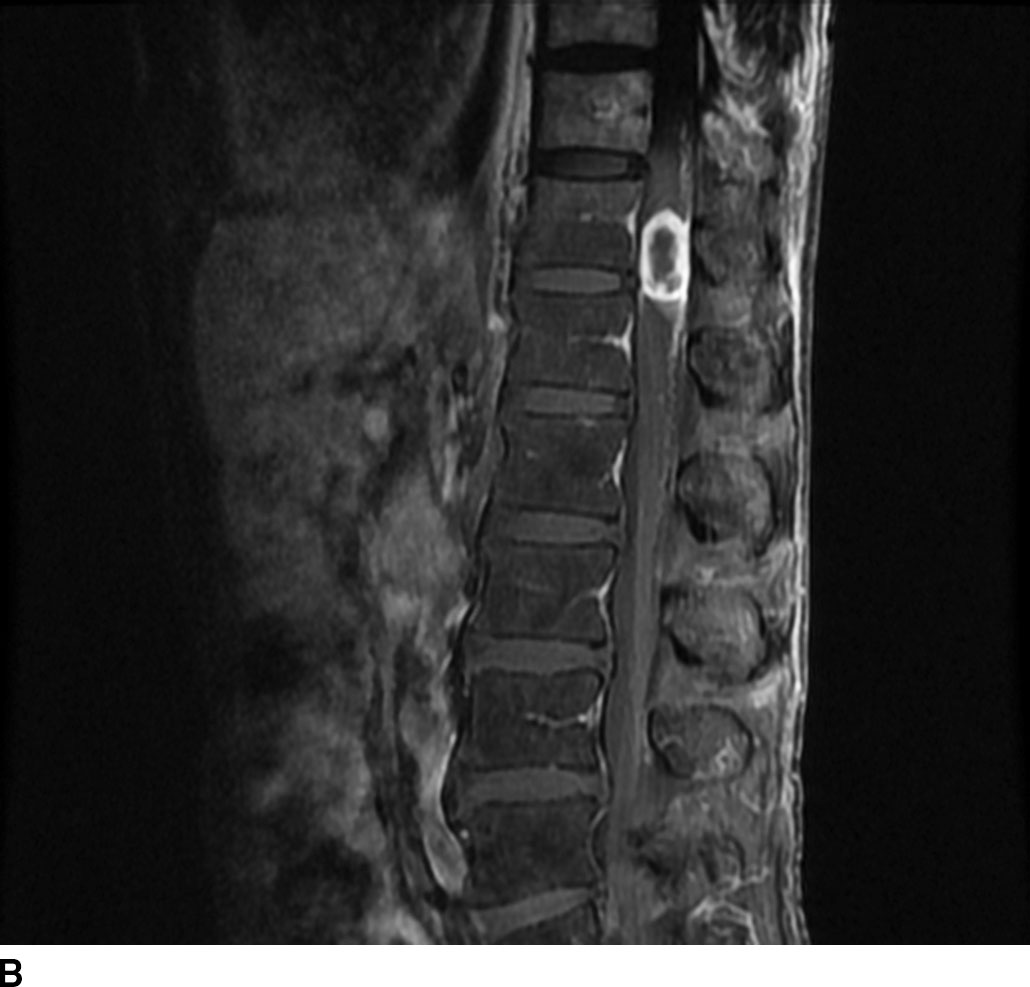
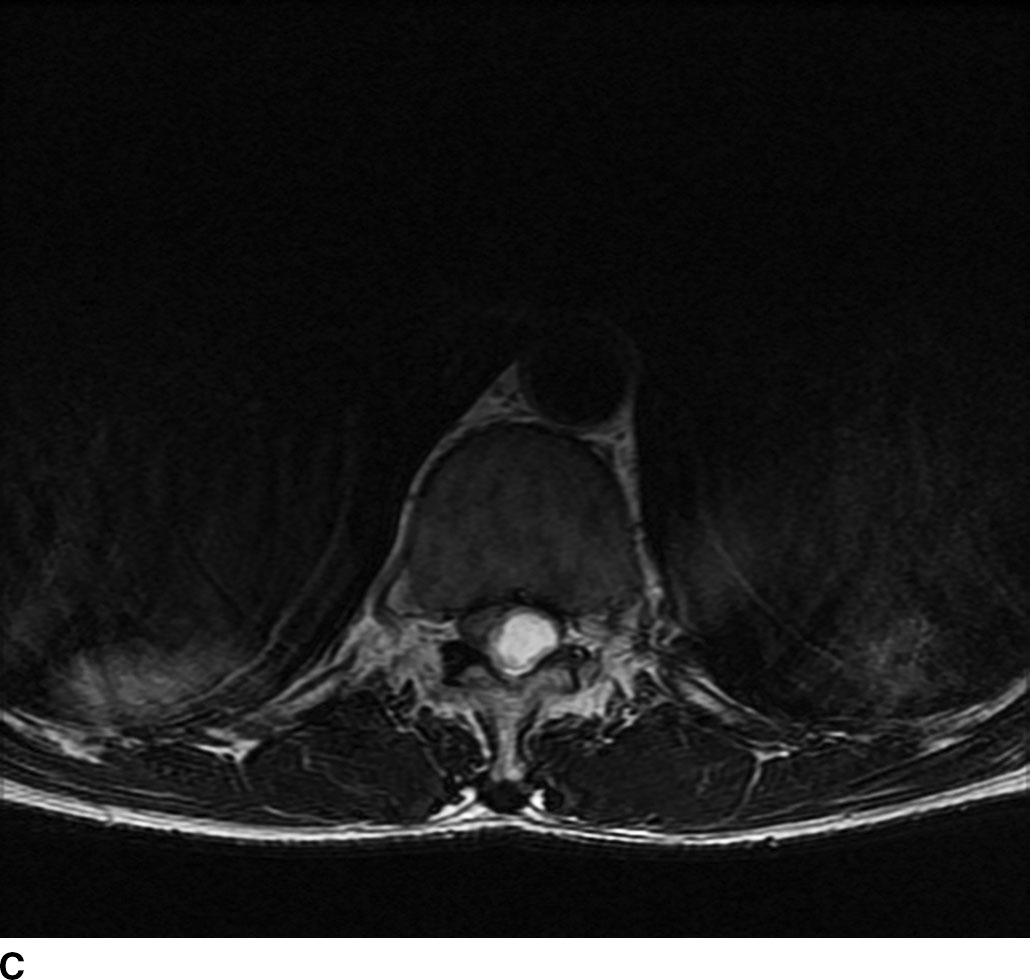
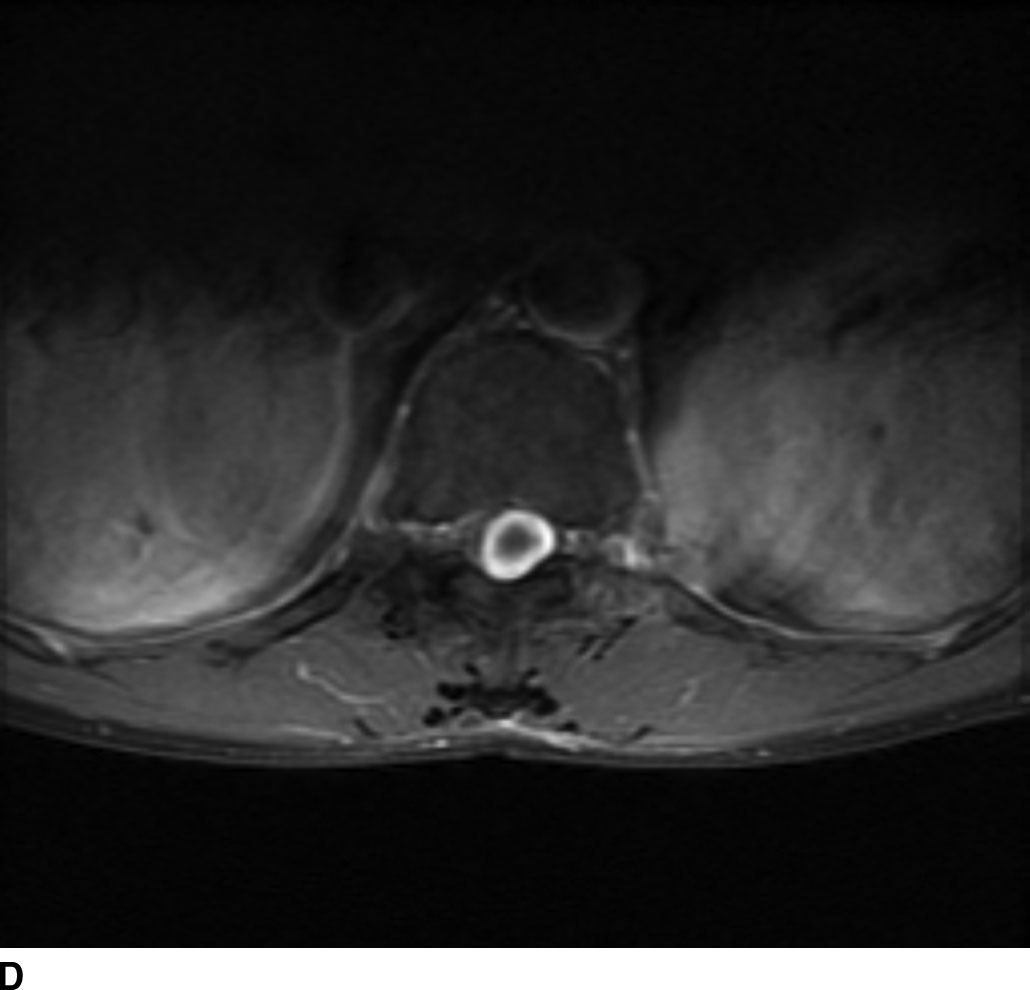
FIG 13.6 Schwannoma. A: An intradural mass can be seen near the level of the conus medullaris with internal hyperintensity on sagittal T2WI. B: Heterogeneous peripheral enhancement can be seen on a postcontrast fat-saturated sagittal T1WI. There is also dural enhancement. C: Axial T2WI shows peripheral displacement of the other cauda equina nerve roots. D: Axial postcontrast fat-saturated T1WI again confirms absence of central enhancement compatible with the “cystic” nature of this schwannoma.
The malignant equivalent of a nerve sheath tumor is a malignant peripheral nerve sheath tumor (MPNST). Other names include malignant schwannoma or neurofibrosarcoma. MPNST should be considered when there is bone invasion and destruction since benign tumors typically remodel bone.
Meningiomas
Meningiomas arise from meningothelial rests associated with the dura. Similar to meningiomas that occur intracranially, spinal meningiomas have a broad base along the dura with an associated dural tail on postcontrast images. Though these tumors may occur anywhere within the thecal sac, the most common location is in the thoracic region in a posterior or posterolateral location. Meningiomas are classically WHO grade I tumors. Distinguishing features for a meningioma include a broad dural attachment, relatively isointense appearance on T1WI and T2WI relative to the cord, and homogeneous avid enhancement (Fig.13.7). On CT imaging, meningiomas may be hyperdense relative to the cord on noncontrast CT and cause bony remodeling without bone destruction (8–10).
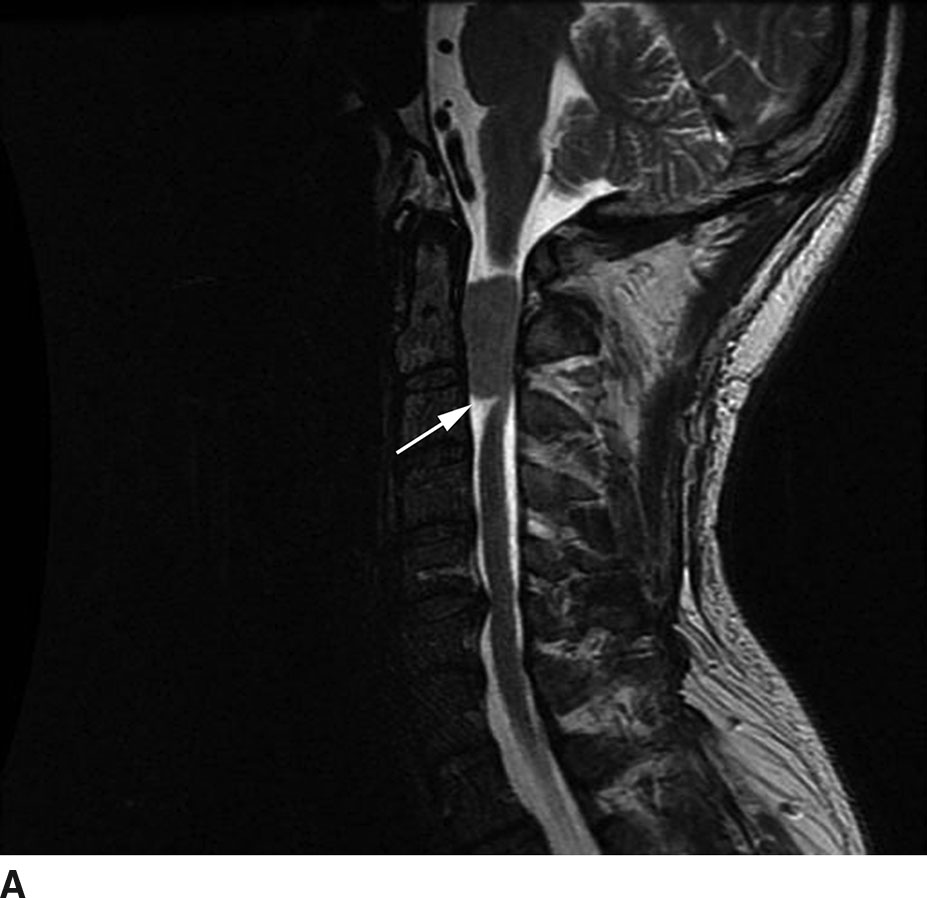
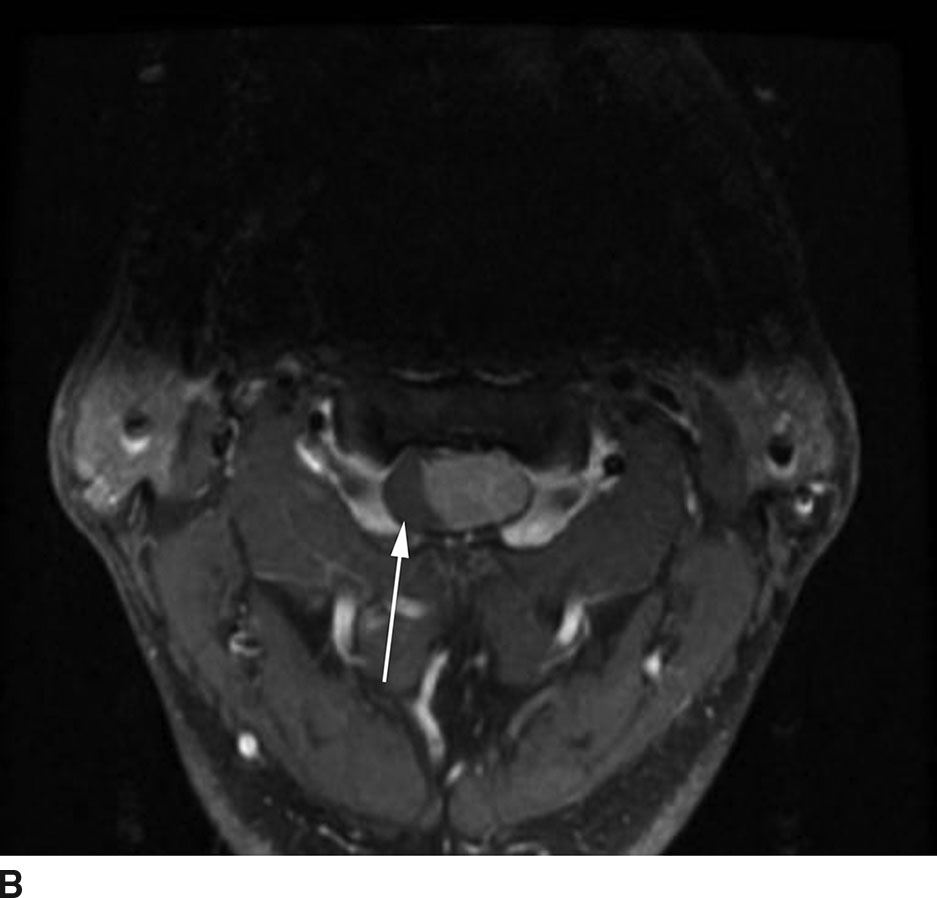
FIG 13.7 Meningioma. A: A meniscus sign (arrow) can be seen on this sagittal T2WI associated with this intradural extramedullary, isointense mass. B: On postcontrast axial T1WI, there is avid enhancement of the meningioma with the overall appearance relatively similar to an intracranial meningioma. There is no extension of the mass beyond the thecal sac or through the neural foramen as might be seen with a peripheral nerve sheath tumor. The spinal cord is compressed and displaced to the right (arrow).
Lipomas
Lipomas may occur within the thecal sac, most commonly along the filum terminale. Four to six percent of the population have a filum terminale lipoma. These lipomas may be tubular in appearance and follow the course of the filum terminale or may be mass-like (Fig. 13.8). These masses may also be a cause for tethering of the conus medullaris below its normal position near the L1-L2 level. Lipomas may also be associated with posterior spinal dysraphisms such as lipomyelomeningoceles or dermoids. As these lesions contain fat, imaging is relatively straightforward. These masses demonstrate fat attenuation on CT and fat signal intensity on MRI, which is hyperintense on both T1WI and FSE T2WI. On a fat-saturated sequence, the signal intensity is nulled with loss of signal. Simple lipomas do not enhance.
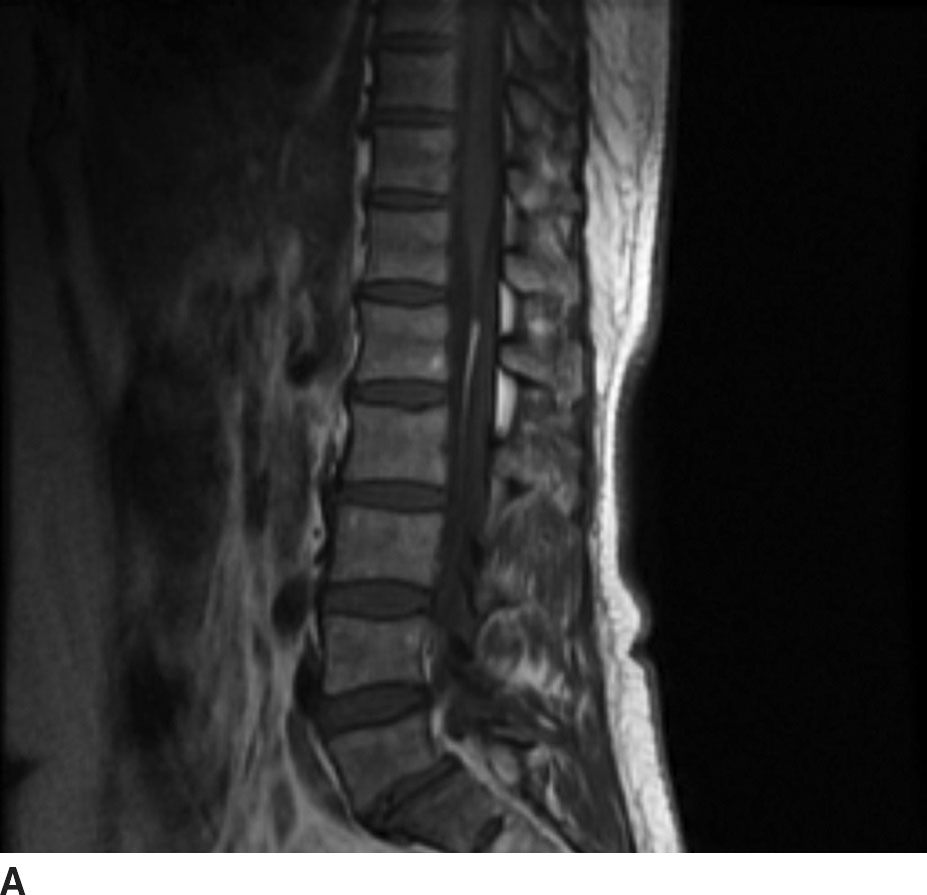
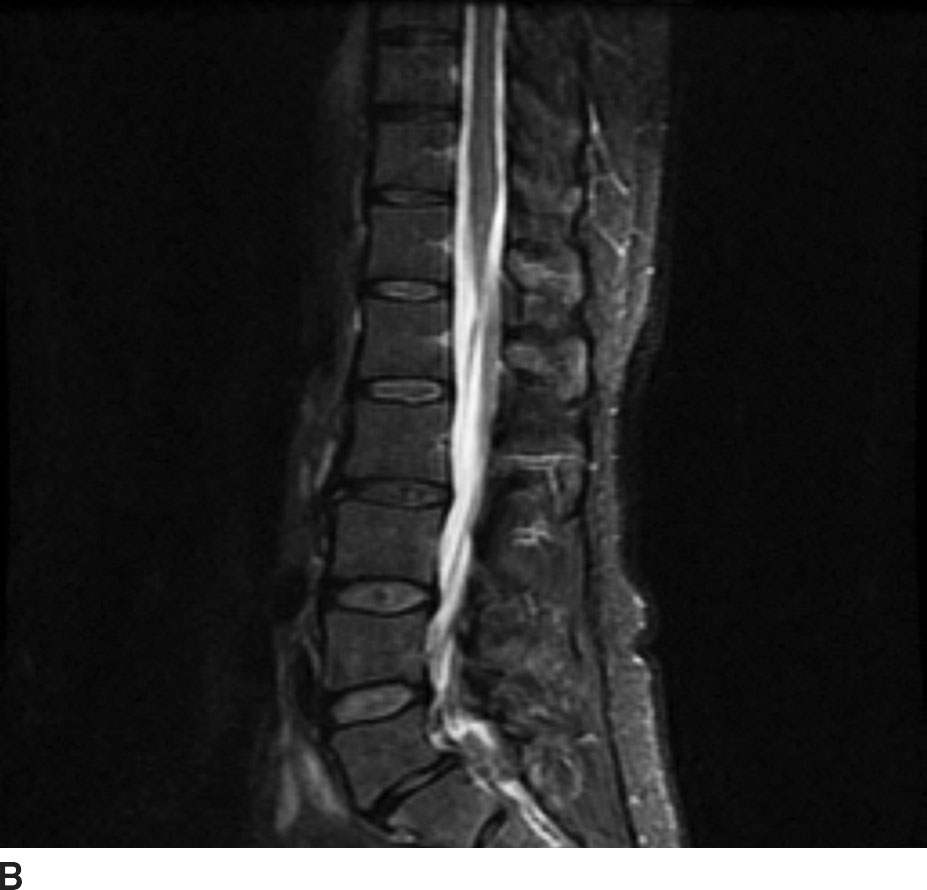
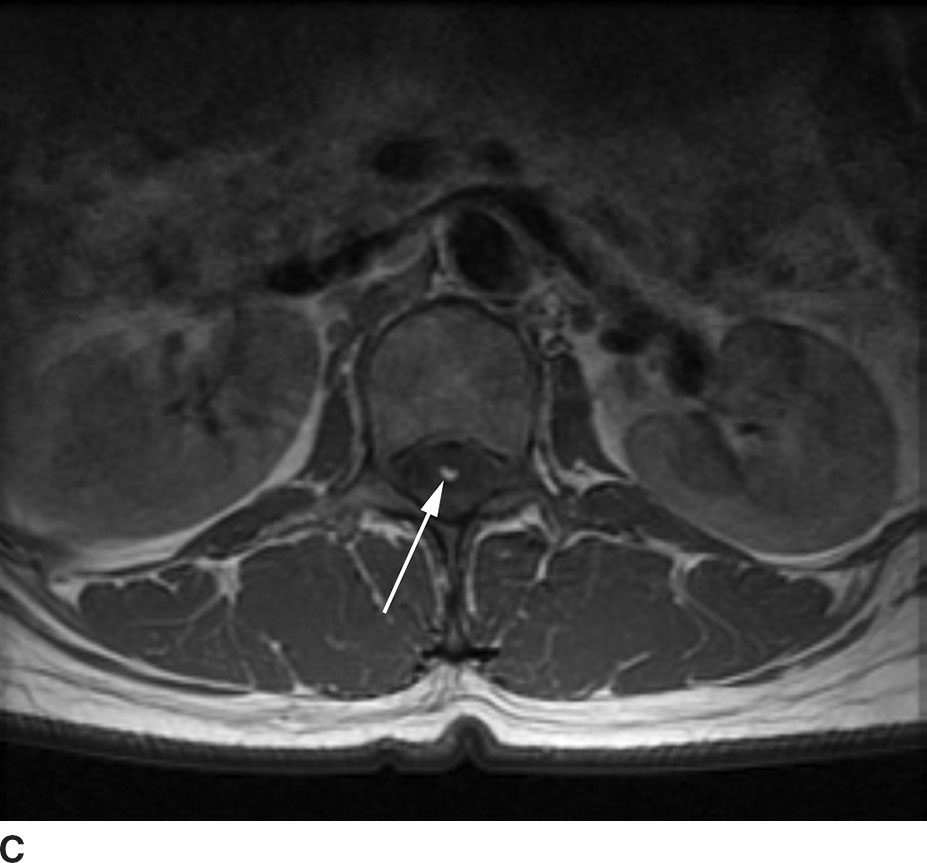
FIG 13.8 Lipoma. A: Sagittal T1WI demonstrates a linear hyperintense structure along the course of the filum terminale. B: This structure loses signal on sagittal STIR imaging confirming the presence of fat. C: Axial T1WI shows the lipoma (arrow) distinct from the cauda equina nerve roots.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree