Fig. 1
Whole-body 111In-octreotide scintigraphy in a patient with lung and bone metastases from papillary thyroid cancer (from Stokkel et al. (2004)—Fig. 1)
Two studies concluded that octreotide scintigraphy has a lower diagnostic accuracy in patients with nonradioiodine-avid DTC compared with conventional imaging modalities, including chest X-ray, ultrasound of the neck, spiral computed tomography, magnetic resonance imaging, bone scintigraphy, and 18-Fluorodeoxyglucose (18F-FDG) PET (Valli et al. 1999; Giammarile et al. 2004). Nevertheless, the sensitivity of SRS was high for the detection of mediastinal lesions (93 %), whereas most false-negative results were observed in neck and lung metastases.
2.2.2 Treatment
111In-octreotide, [90Y-DOTA]-TOC, and [177Lu-DOTA]-TATE have all been evaluated for treatment of patients with iodine-refractory DTC with varying success (Table 1). Initial studies have shown that somatostatin-based radiopeptide therapy is feasible in iodine-refractory DTC (Chinol et al. 2002). The reported rates of disease control, defined as responses plus stable disease, vary between 20 and 56 % (Valkema et al. 2002; Virgolini et al. 2002; Stokkel et al. 2004a, b). Studies on the follow-up after treatment revealed a time to progression between 9 and 43 months (Gorges et al. 2001; Waldherr et al. 2001a, b; Teunissen et al. 2005). Comparison of these studies, however, is difficult because of differences in radionuclides, somatostatin analogs, and maximum activities administered. Nevertheless, radiolabeled somatostatin analogs appear to be less effective in treating nonradioiodine avid DTC than other NETs such as GEPs.
Table 1
Treatment results with radiolabeled somatostatin analogs in progressive dedifferentiated thyroid cancera
n | Histology | Progression prior therapy | Radiopharmaceutical | Cumulative dose (GBq) | Responsesb (TTP in months) | References | ||
|---|---|---|---|---|---|---|---|---|
Radionuclide | Chelator | Peptide | ||||||
3 | 3 HCTC | 3/3 | 90Y | DOTA | TOC | 1.7–9.6 | 1 SD (21), 2 PD | (Gorges et al. 2001) |
7 | 3 FTC, 4 PTC | 7/7 | 90Y | DOTA | TOC | 1.7–14.8 | 2 SD (9), 5 PD | |
2 | 2 PTC | NA | 90Y | DOTA | TOC | >7.4 | NA | (Chinol et al. 2002) |
5 | 1 FTC, 4 PTC | 4/5 | 111In | DTPA | Octreotide | 29.5–82.2 | 1 SDc, 4 PD (NA) | (Valkema et al. 2002) |
25 | 25 unknown | 25/25 | 90Y | DOTA | LAN | 0.9–7.0 | 3 MR (NA), 11 SD (NA) | (Virgolini et al. 2002) |
1 | 1 HCTC | NA | 90Y | DOTA | TOC | NA | NA | (Christian et al. 2003) |
5 | 3 FTC, 2 PTC | NA | 90Y | DOTA | TOC | 5.6–7.6 | 5 SD | (Gabriel et al. 2004) |
9 | 4 FTC, 5 PTC | 9/9 | 111In | DTPA | Octreotide | 14.3–33.1 | 4 SD (NA), 5 PD | |
5 | 1 FTC, 1 PTC, 3 HCTC | 3/5 | 177Lu | DOTA | TATE | 22.4–39.1 | 1 PR (22 +), 1 MR (43), 2 SDd (18, 24 +), 1 PD (4) | (Teunissen et al. 2005) |
24 | 17 FTC, 5 PTC, 2 TC | 24/24 | 90Y | DOTA | TOC | 1.7–30.3 | 7 responders | (Iten et al. 2009) |
The largest study to date investigated response, long-term safety profile, and survival after [90Y-DOTA]-TOC treatment in 24 patients with progressive metastasized iodine-refractory thyroid cancer (Iten et al. 2009) and a positive pretherapeutic 111In-Octreoscan. Response, defined as decreasing thyroglobulin levels after therapy, was found in 7 (29.2 %) patients. However, the visual grade of tumor uptake in the pretherapeutic 111In-Octreoscan and multiple regression analyses did not reveal any significant pretherapeutic predictor of treatment response. The median survival was 33.4 months (range, 3.6–126.8 months) from time of diagnosis and 16.8 months (range, 1.8–99.1 months) from time of the first [90Y-DOTA]-TOC treatment. Response to treatment was associated with longer survival from time of diagnosis (hazard ratio [HR], 0.17; 95 % confidence interval [CI], 0.03–0.92; P = .04) and from time of first [90Y-DOTA]-TOC therapy (HR, 0.20; 95 % CI, 0.04–0.94; P = 0.04) (Fig. 2). Eight (33.3 %) patients developed hematologic toxicity grade 1–3, and 4 (16.7 %) patients developed renal toxicity grade 1–4.
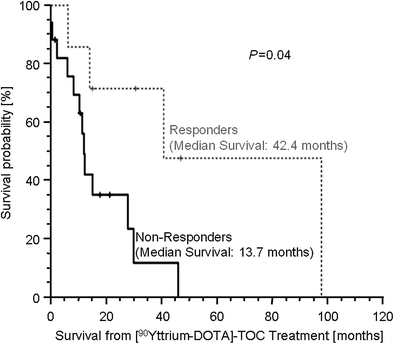

Fig. 2
Survival of responders versus nonresponders at time from diagnosis (a) and at time from first [90Y-DOTA]-TOC treatment (b) in patients with dedifferentiated thyroid cancer (from Iten et al. 2009—Fig. 3b)
2.3 Summary
Ten studies including a total of 86 patients with iodine-refractory thyroid cancer reported the results of treatment with 111In-octreotide, [90Y-DOTA]-TOC, and [177Lu-DOTA]-TATE (Table 1). While not overwhelmingly positive in terms of response rate, those patients that do respond have prolonged survival compared to nonresponders from time of diagnosis and first [90Y-DOTA]-TOC treatment (Fig. 2).
Although therapy with radiolabeled somatostatin analogs is generally well tolerated, there are currently no reliable predictors of response. SRS can aid in the detection of noniodine avid thyroid malignancies in patients where recurrence is suspected, however, no correlation exists so far between uptake on a pretherapeutic 111In-Octreoscan and response to therapy with [90Y-DOTA]-TOC. The sstr expression profile in DTC is more heterogeneous than in other types of neuroendocrine neoplasms, such as GEP tumors, and the observed discordance between tumor uptake during SRS and tumor response to radiotherapy with somatostatin analogs may in part be due to the relative expression of somatostatin receptor subtypes (sstr1-5) and their density on the tumor cell surface. For example, uptake on 111In-octreotide scintigraphy is considered to be mainly associated with sstr2 expression, and less with sstr1, sstr3, sstr4, and sstr5. (John et al. 1996). Thus, there is a need to understand the pathophysiology underlying response and to develop predictive indices that will identify those patients with DTC likely to respond to radiolabeled somatostatin analog therapy.
The higher prevalence of sstr2 in HCTC provides a more favorable profile of somatostatin receptor subtype expression compared with PTC and FTC, suggesting that this subgroup of DTC patients may be more responsive to therapy with radiolabeled somatostatin analogs. Preliminary evidence supports this (Table 1), although these results including palliation, prolonged stable disease, minor, or partial remission must be considered anecdotal given the small number of patients (Gorges et al. 2001; Christian et al. 2003; Teunissen et al. 2005). However, when conventional treatment is no longer an option and SRS shows sufficient uptake of 111In-octreotide, treatment with [90Y-DOTA]-TOC and [177Lu-DOTA]-TATE should be considered in patients with HCTC.
The ongoing development of somatostatin-based radioligands with a broader receptor subtype profile is of interest given the variability in DTC subtype expression (Reubi et al. 2005). The radiolabeled somatostatin analog DOTA-NOC has high affinity for sstr2, sstr3, and sstr5. Preclinical data and preliminary results with 111In and 177Lu-DOTA-NOC for imaging and therapy are especially encouraging (Wild et al. 2003).
3 Medullary Thyroid Cancer
3.1 Introduction
Medullary thyroid carcinoma (MTC) arises from calcitonin-producing parafollicular cells and accounts for roughly 5 % of all thyroid cancers (Ball et al. 2000). Localized disease can be successfully managed with surgery (Leboulleux et al. 2004) and, in select cases, external beam radiation (Vini and Harmer 2002). However, about 50 % of patients develop disease recurrence and survival after the discovery of distant metastases is approximately 20 % at 10 years. The main cause of MTC-related death is the development of distant metastases, which are often diffuse and numerous, and typically affect the liver, lungs, and bone (Schlumberger et al. 1991). Often, patients at this stage are not considered surgical candidates, given the poor localization of disease.
No systemic therapy has been established for metastasized medullary thyroid cancer (Vini and Harmer 2002). Treatment with Adriamycin, cyclophosphamide, vincristine, dacarbazine, 5-fluorouracil, or combination regimes have shown only minor response rates (~20 %) with considerable hematologic, nephrologic, cardiologic, or gastrointestinal toxicities and no improvement in survival rate (Vitale et al. 2001; Leboulleux et al. 2004). Preliminary studies with radiolabeled anti-carcinoembryonic antigen antibodies (Chatal et al. 2006) and metaiodobenzyl-guanidine (Kaltsas et al. 2004) have shown more encouraging results, indicating that targeted therapies as opposed to broadly cytotoxic therapies may be more successful in the treatment of MTC (see “Medullary Thyroid Carcinoma“).
3.2 Radiolabeled Somatostatin Analogs for Imaging and Treatment of Medullary Thyroid Cancer
Using immunohistochemistry, the distribution of the somatostatin receptors subtypes sstr1-5 in MTC specimens was shown to be heterogeneous with expression of the subtypes sstr2, sstr3, and sstr5 in 75 % of cases (Papotti et al. 2001). The low density of sstr2 expression is posited to account for the fact that only 40–75 % of MTCs can be visualized by scintigraphy with radiolabeled octreotide (Krenning et al. 1992a, b; Kwekkeboom et al. 1993; Baudin et al. 1996a, b; Kurtaran et al. 1996; Berna et al. 1998). Additionally, the expression of sstr is directly related to tumor differentiation and intra-tumoral calcitonin levels, which typically are higher in differentiated lesions (Reubi et al. 1991). This observation has been confirmed in vivo, as SRS with 111In-octreotide has a high sensitivity for cervical and upper mediastinum lymph nodes in patients with occult MTC, which are typically associated with less aggressive behavior. Conversely, SRS is less sensitive in MTC patients with distant metastases and progressive disease (Behr et al. 1999).
3.2.1 Imaging
Variable results of SRS imaging with 111In-octreotide of medullary thyroid carcinoma have been reported (Fig. 3). The initial report was promising, with 8 of 12 (75 %) medullary thyroid carcinomas imaged successfully (Krenning et al. 1992a, b). Two following studies demonstrated primary tumor localization in 11 of 17 patients (65 %) and 7 of 11 patients (64 %) with MTC, and both concluded the technique is insensitive in detecting metastases (Kwekkeboom et al. 1993; Kurtaran et al. 1996). Additionally, the ratio of serum calcitonin to CEA concentrations was significantly higher in patients whose lesions were visualized during octreotide scintigraphy than in those whose tumors were not. These findings concerning metastases and biochemical profiles were confirmed in later studies (Frank-Raue et al. 1995; Berna et al. 1998). Other reports are less encouraging, with sensitivities lower than conventional imaging (37 %), leading the authors to conclude that SRS has a limited role in the management of MTC (Baudin et al. 1996a, b).
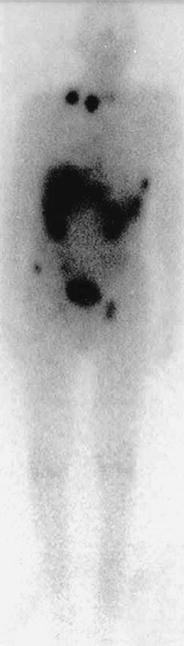

Fig. 3
Whole-body 111In-octreotide scintigraphy in a patient with medullary thyroid cancer metastasized into supraclavicular lymph nodes, the spine, and the pelvis (Source: Institute of Nuclear Medicine, University Hospital Basel, Switzerland)
The combination of anti-CEA monoclonal antibodies (MAbs) and octreotide scinitigraphy may yield diagnostic results superior to those achieved with other techniques for detection of occult MTC and the staging of known MTC (Behr et al. 1997). Higher serum CEA and better detectability with anti-CEA antibodies seem to be associated with more aggressive forms of MTC, whereas somatostatin receptor expression at normal CEA plasma levels and weaker MAb targeting may be associated with a more benign clinical course. This is in accordance with the studies showing higher CEA serum levels to be associated with a worse prognosis (Busnardo et al. 1984), as well as with the in vitro findings (Reubi et al. 1991) that lower somatostatin receptor expression was present in less differentiated MTC.
3.2.2 Treatment
In a pilot study with [90Y-DOTA]-TOC in 12 MTC patients with refractory metastatic disease, treatment was effective in 42 % (objective response and stable disease) (Waldherr et al. 2001a, b). A following study evaluated the response rate of patients with progressive MTC and positive SRS to [90Y-DOTA]-TOC therapy. When assessed by conventional imaging techniques, 10 % of patients had complete remission, 57 % had disease stabilization, and 33 % experienced progressive disease following treatment. Using biochemical parameters (calcitonin and CEA), a complete response was observed in one patient (5 %), while a partial response was seen in five patients (24 %) and stabilization in three patients (14 %). Twelve patients had progression (57 %). The duration of the response ranged between 3–40 months. Complete responses were observed in patients with lower tumor burden and calcitonin values at the time of enrolment, which suggests [90Y-DOTA]-TOC therapy should be performed earlier in the course of disease (Bodei et al. 2004a, b).
In a retrospective review of the efficacy of high-activity 111In-pentetreotide as a therapy for metastatic NETs, the response of two patients with MTC was evaluated (Buscombe et al. 2003). Over an average of 3.5 treatments the two patients received total of 10.95 GBq of activity, after which they experienced a complete response at 6 months with undetectable serum calcitonin levels and no anatomically or scintigraphically visible tumor seen, thought the patients were noted to have small tumor burdens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








