Fig. 1
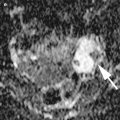
The typical features of endometriomas: diffuse low-level internal echoes (“ground glass”) and hypoechoic focal lesion in the wall in the absence of particular neoplastic features
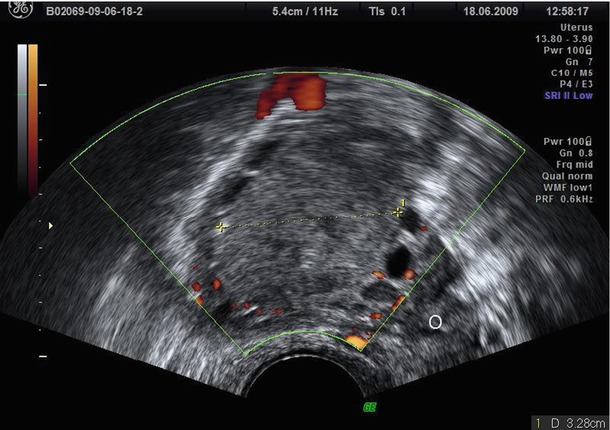
Fig. 2
Diffuse low-level internal echoes (“ground glass”) cyst with a clear demarcation from ovarian parenchyma (O)
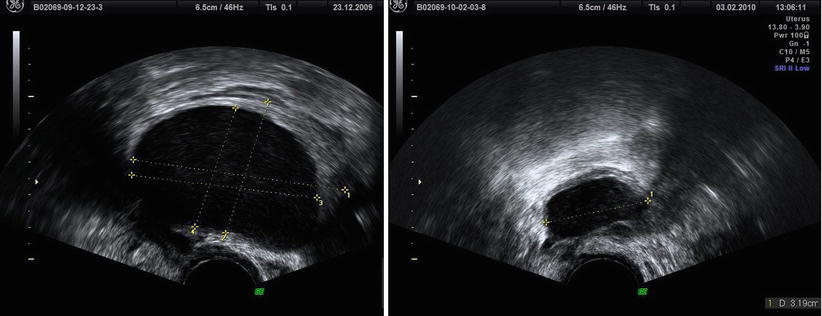
Fig. 3
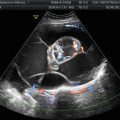
Less frequent content in two endometriomas: less intense hypoechoic and homogeneous diffuse low-level internal echoes
Ovarian endometriosis may be unilocular or multilocular (Fig. 4) (appearing as multiple cysts separated by septations) and is often multiple or bilateral. Van Holsbeke et al. [3] found that only 51 % of the endometriomas were unilocular cysts with ground glass echogenicity of the cyst fluid. These characteristics were found less often among other benign tumors or malignancies or among the small set of endometriomas (4 %) that were found in postmenopausal patients. In fact the endometriomas in the postmenopausal patients were less often unilocular cysts (40 % vs. 66 %), and they present ground glass echogenicity in only 40 % of cases (vs. 74 %) [3].
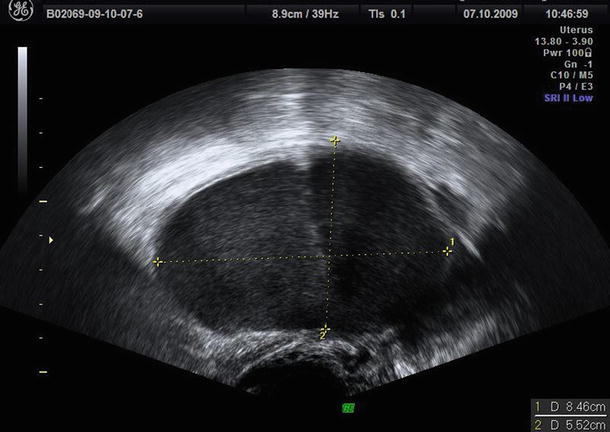

Fig. 4
Bilocular endometrioma
Patel et al. [23] demonstrate that hyperechoic wall foci (Fig. 5) in a mass with low-level echoes with the absence of neoplastic features are strongly predictive of an endometrioma although the pathologic basis of these hyperechoic wall foci has not been established. Patel et al. [23] suggest that these foci may contain cholesterol, perhaps from the breakdown of cell membranes. In fact they are similar in appearance to hyperechoic wall foci seen in the gallbladder wall in patients with hyperplastic cholecystosis, due to the presence of cholesterol within polyps.
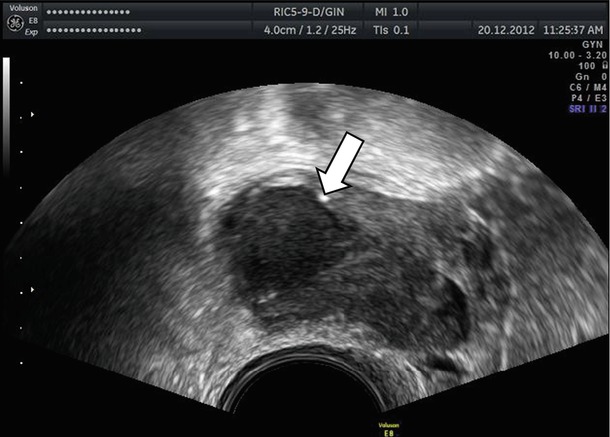

Fig. 5
Hyperechoic wall foci (see arrow) in a mass with low-level echoes
Ash and Levine [27] report that in the 10 % of cases, the endometriomas were described as hemorrhagic cysts. A small percentage of endometriomas have less typical US features such as a fluid-fluid level. These authors [27] suggest that in an endometrioma, the supernatant fluid layer should be hypoechoic, with a hyperechoic dependent layer representing blood. On the contrary in a dermoid, the supernatant layer will be echogenic, representing fat.
Atypical endometriomas included cases with retracted clots that appeared solid but without blood flow (Figs. 6 and 7). As correctly discussed by Brown et al. [28], endometriomas may contain a small solid area in 4–20 % of cases, and these can simulate the mural nodule of malignant neoplasm [23, 26]. Based on IOTA study [3], an irregular wall can present in the 26 % of endometriomas in premenopausal women, and in the 10 % of these, a papillary projection was present. The Van Holsbeke study [3] reports a proportion of “atypical” endometriomas higher than in any other in the literature [2, 22–27]. In particular Guerriero et al. [29] found that the 83 % of endometriomas in premenopausal patients demonstrated the typical appearance of a unilocular cyst with ground glass echogenicity of cyst fluid [29] vs. only 53 % in IOTA study [3]. The causes of this difference are unknown.
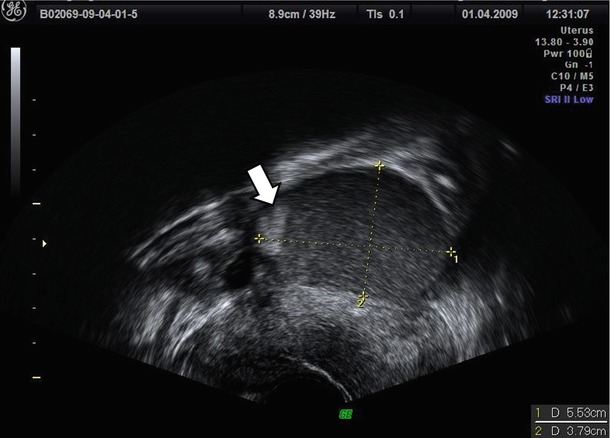
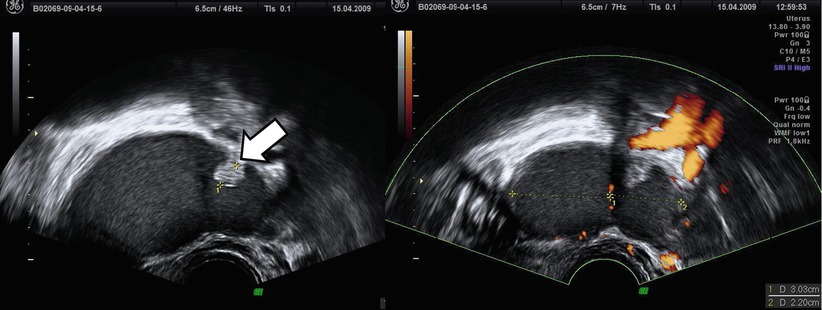

Fig. 6
Atypical endometriomas with irregular wall due to retracted clots (see arrow)

Fig. 7
Another atypical endometriomas with a small solid area that can simulate the mural nodule of malignant neoplasm but without blood flow (see arrow)
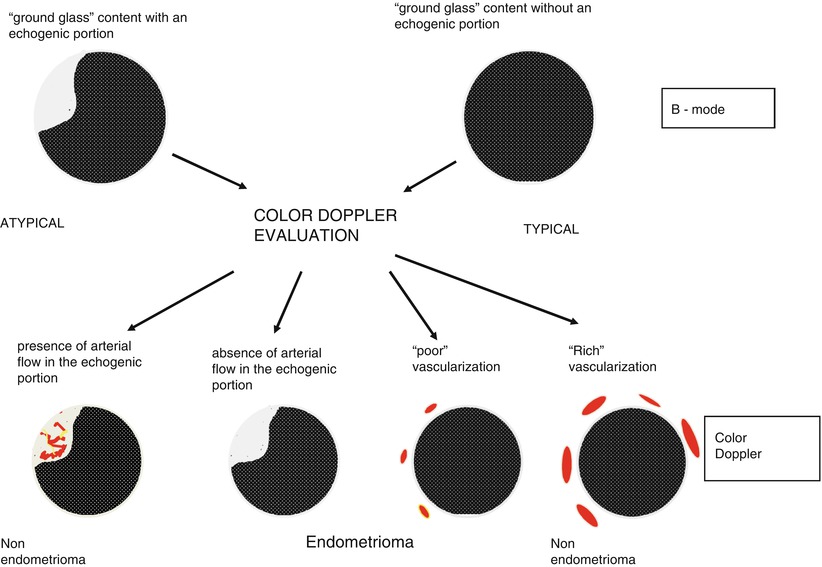
To evaluate these “atypical” endometriomas, Guerriero et al. [29] propose to use color Doppler. Usually endometrioma showed an absent or scanty peripheral vascularization at color Doppler (Fig. 8). In the IOTA study [3], the proportion of masses with color score 1 or 2 (absent to minimal) was 78 %. The use of color Doppler as a secondary test permits to diagnose the presence of endometrioma also in case of “atypical” endometriomas in which no flow is detected in the echogenic portion due to the presence of a clot and enables them to be differentiated from an intracystic vegetation. On the contrary, this simple color Doppler imaging flow chart (Fig. 9), based on the localization of vessels and intensity of arterial flow, permits exclusion of ground glass appearance but “rich vascularization” frequently associated with the presence of corpus luteum cysts or mucinous cystadenoma [29].
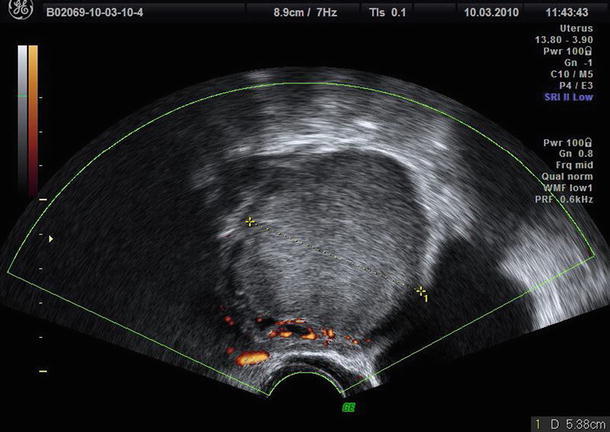

Fig. 8
The typical scanty peripheral vascularization at color Doppler of endometrioma
Alcázar reports that vascularization of ovarian endometriomas in patients presenting with pelvic pain is higher than in asymptomatic patients. This could be an indicator of endometriosis activity [30]. From anecdotal point of view (because without any clinical value), Aleem et al. [31] evaluating pulsed Doppler analysis found that mean of the resistance index (RI) and pulsatility index (PI) for the endometriomas were 0. and 0.95, respectively. All endometriomas showed an RI of >0.5 with a range of 0.5–0.74, while the PI was 0.59–1.59.
The reproducibility of ultrasonographic B-mode findings is high [32]. Guerriero et al. [32] performed a study to evaluate the reproducibility and the accuracy of B-mode ultrasonographic features of ovarian endometrioma (“ground glass appearance”). They used digitally stored B-mode sonographic images of 98 women submitted to surgery for the presence of an adnexal mass performing an evaluation by five different examiners with different degrees of experience. The intraobserver agreement was good or very good for all examiners. Also the interobserver agreement was good for all experts (kappa = 0.66–0.78). Interobserver agreement between experts and highly experienced operators was good or very good (kappa = 0.70–0.83) [32].
The diagnostic value of transvaginal B-mode ultrasonography is well established. In Table 1 the diagnostic performance of B-mode ultrasonography among the most important studies published in the literature [2, 3, 24, 26, 29, 33–36] is reported. Several studies report very high values of specificity with values of sensitivity usually ranging from 87 to 77 % (Table 1). Only the IOTA study [3], for unknown reason, reports a value of sensitivity of 68 %. Alcázar et al. [2], analyzing pre- and postmenopausal women separately, found that diagnostic performance is different. As a matter of fact, transvaginal ultrasound is more sensitive for diagnosing endometrioma in premenopausal women in comparison with postmenopausal population (89 % vs. 67 %). A possible explanation for this, in the case of endometrioma, is that ultrasound appearance of this kind of cyst may change from premenopause to postmenopause because bleeding within the lesion would stop in menopausal women and cyst mucosa will become atrophic as also suggested by Van Holsbeke et al. [3] that observe the increase of anechoic content in this latter population. The different prevalence of endometrioma in premenopausal and postmenopausal women may be also an explanation because examiner’s knowledge of different prevalence may bias his/her impression [2].
Table 1
The diagnostic accuracy of ultrasonography in the diagnosis of endometrioma
Reference | Sensitivity (%) | Specificity (%) |
|---|---|---|
Mais et al. [24] | 84 | 90 |
Kurjak and Kupesic [33] | 84 | 97 |
Volpi et al. [34] | 82 | 98 |
Guerriero et al. [35] | 84 | 95 |
Alcázar et al. [26] | 89 | 91 |
Guerriero et al. [29] | 81 | 96 |
Sokalska et al. [36] | 77 | 98 |
Van Holsbeke et al. [3] | 68 | 98 |
Alcázar et al. [2] | 89 | 96 |
The differential diagnosis should be performed by mainly considering hemorrhagic cysts (Fig. 10), teratomas, and malignant neoplasms. An appropriate anamnesis focused on symptoms and gynecologic history of the patient may be helpful, in fact usually hemorrhagic cysts have often a more acute symptomatology which generally resolves in 4–6 weeks. Alcázar et al. [2] confirm that the main source of false-positive cases in premenopausal population (29/42, 69 %) was the hemorrhagic cysts. In this large series of more than 2,000 adnexal masses, no cases of malignancy were erroneously suspected to be endometriomas in both populations. On the contrary Van Holsbeke et al. [3] observe that a large proportion of masses with ground glass echogenicity in postmenopausal patients are malignant (34/77, 44.2 %). These authors [3], in contrast with Alcázar et al. [2], conclude that masses in postmenopausal women whose cystic contents have a ground glass appearance have a high risk of malignancy and should be observed with caution. We agree with this latter authors due to the very low incidence of endometrioma in postmenopausal population previously reported [2, 3].
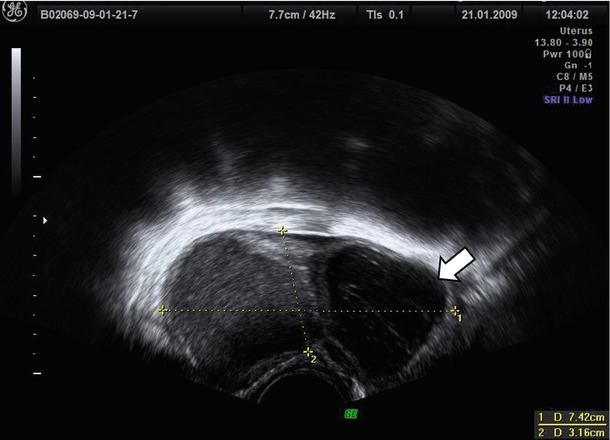

Fig. 10
The hemorrhagic cysts (see arrow) are often associated with endometrioma
Regarding false-negative cases in the 40 % (25/61), a malignant lesion was suspected due to the high rate of irregular walls and presence of papillations previously described [2].
Some additional features have been proposed to reduce the number of false positive and negative. Although promising after first studies [37], the absence of acoustic streaming (defined as movement of particles inside the cyst fluid during gray-scale and/or color Doppler examination provided that the probe had been held still for two seconds to ensure that the movement of the particles was not caused by movement of the probe or the patient) in endometrioma seems unable to discriminate reliably between these masses and other adnexal lesions, in an IOTA study performed on more than 400 adnexal masses [38].
To further reduce the number of false positive and negative, the use of color Doppler has been proposed. Guerriero et al. [29] using the flow chart previously described (Fig. 9) obtain a specificity of 97 % associated with a very high sensitivity of 90 %.
Very few data are present in the literature about three-dimensional (3D) ultrasonographic characteristics of endometrioma. Few studies explore the potential of this new technology in the field of endometriosis [39–42]. Raine-Fenning et al. [39] suggest that some features such as the texture and homogeneity of the endometriotic material within the body of the cyst and the thickened fibrotic capsule and echodense nodules within the wall of the cyst can also be seen on a conventional two-dimensional image but are more apparent when some 3D modalities such as speckle reduction imaging are used to enhance the contrast between different interfaces (Figs. 11 and 12). Rendering can also be used to display Doppler information within a three-dimensional dataset in different formats. The vascular tree can also be seen as a separate entity through subtraction of the gray-scale information, allowing an immediate impression of the vessel pattern in terms of its distribution and progressive branching. At 3D the endometrioma wall is generally well perfused, and the vessels often appear to have a short course, with minimal variation in their diameter, that surrounds the cyst in a uniform manner; Raine-Fenning et al. suggest to call this pattern “bird’s nest” appearance [39] (Fig. 13). This technology can be also used for teaching purpose using stored three-dimensional volume for “virtual navigation” [43].
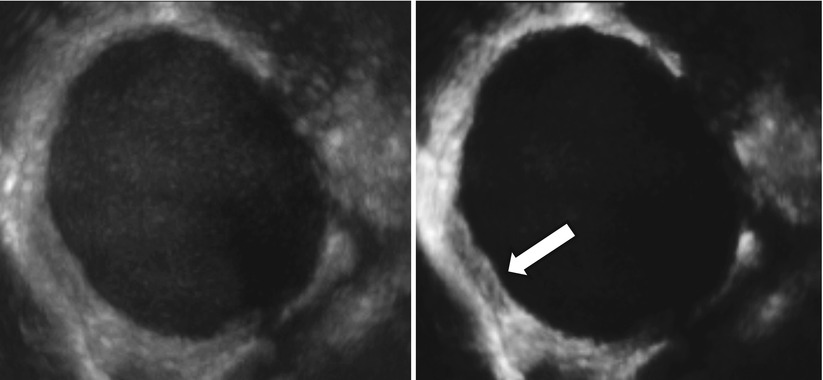
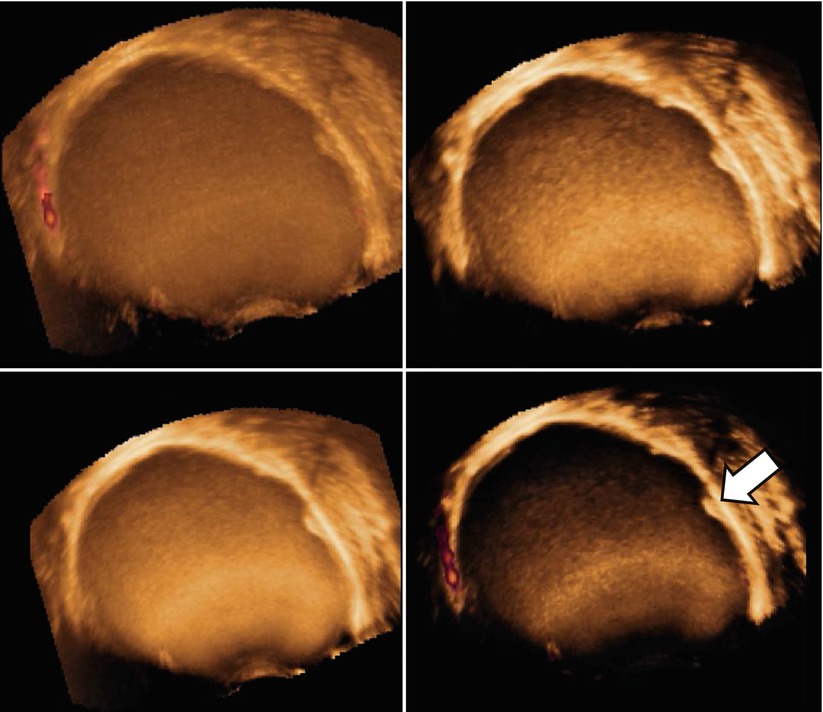

Fig. 11
Using three-dimensional ultrasonography and speckle reduction, the thickened fibrotic capsule is well defined (see arrow)

Fig. 12
Another cases of the use of three-dimensional ultrasonography and speckle reduction used to improve the visualization of echodense nodules within the wall of the cyst (see arrow)

Fig. 13
At 3D a well-perfused wall with vessels with “bird’s nest” appearance
Recently Alcázar et al. [44] found that three-dimensional ultrasonography mean gray value (MGV) that represents the mean intensity of gray-scale voxels (the smallest unit of volume) within a region of interest can discriminate ovarian endometriomas from other unilocular ovarian cysts in premenopausal women [40]. From a conceptual point of view, this is quite similar to the Hounsfield Unit (HU) values that are used in computed tomography (CT) to analyze the tissue properties and composition. MGV of cysts content is significantly higher in ovarian endometrioma (Fig. 14) when compared with all other kinds of cyst. The receiver-operating characteristics curve shows that using an MGV cutoff ≥ 15.560 had a sensitivity of 85 % and a specificity of 76.5 % for diagnosing ovarian endometrioma (area under the curve, 0.831; 95 % CI, 0.718–0.944). These figures are similar to those for B-mode diagnosis (sensitivity, 90 %; specificity, 82 %). Combining B-mode and MGV gives a sensitivity of 80 % and a specificity of 91 % [44].
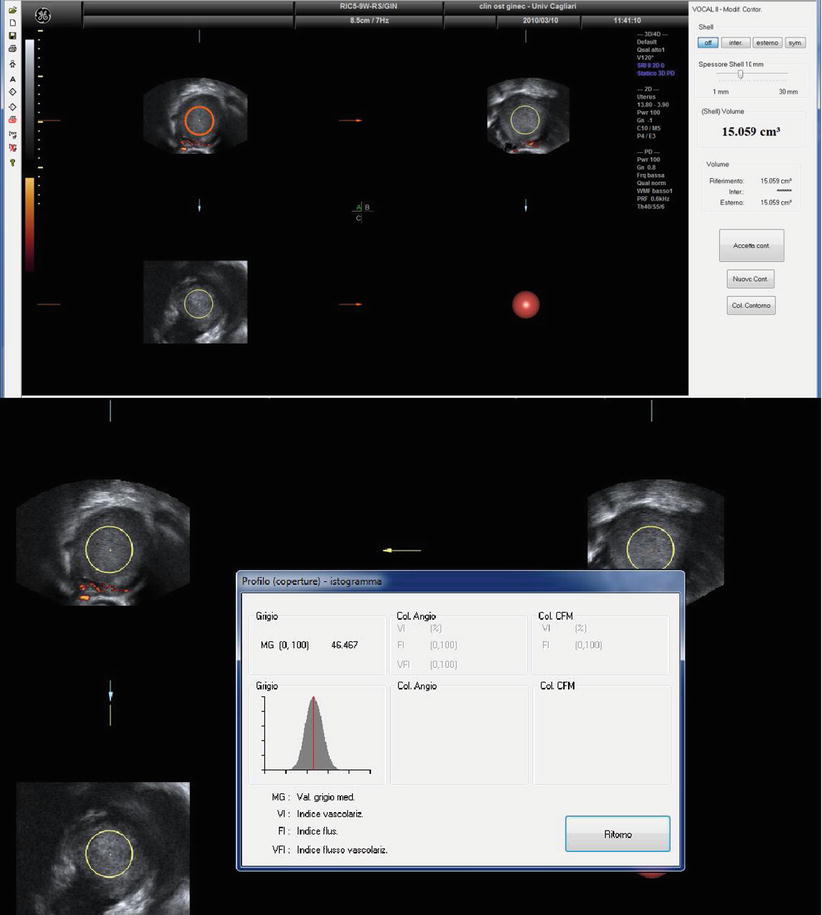

Fig. 14
Three-dimensional ultrasonography mean gray value (MGV) of an endometrioma
Use of Tumor Markers
In the diagnosis of endometrioma, the use of cancer antigen 125, or carbohydrate antigen 125 (CA-125), has been proposed. CA-125, a 220 kD cell surface glycoprotein, is present in more than 80 % of non-mucinous epithelial ovarian carcinomas [45] but is also increased in several benign conditions, such as superficial and deep endometriosis [45], ovarian cysts [46], pelvic inflammatory disease [45], resolution of ovarian torsion [47], and uterine fibroids [45], or in physiological conditions such as menstruation and early pregnancy [48].
In the literature controversial results [46, 49] are present using CA-125 alone in the diagnosis of endometrioma. Some authors [46] found 100 % of endometriomas with CA-125 higher than 20 U ml and 100 % of non-endometriomas with CA-125 lower than 20 U ml. On the contrary, using a different cutoff level, other authors [49] reported a sensitivity of 36 % and specificity of 87 % in the diagnosis of endometrioma. Further studies confirmed the presence of a significant difference between the values of CA-125 in endometrioic and non-endometrioic cysts [4, 35]; the use of CA-125 alone in the differential diagnosis of endometrioma is associated with very poor agreement also in combination with another tumor marker as carbohydrate antigen 19–9 (CA19-9) [35].
Recently Alcázar et al. [50] in a large series of cases found that the median CA-125 level was significantly higher in endometrioma (71.9 IU/mL; range: 5–2,620 IU/mL) compared to all other tumor types (P < .001). The CA-125 level was 35 IU/mL or higher in 74 % of endometriomas. In the diagnosis of endometrioma, the positive likelihood ratio for sonography plus CA-125 (55.0; 95 % confidence interval, 27.5–109.9) was significantly higher than for sonography alone (19.2; 95 % confidence interval, 13.6–27.1). For these reasons from the clinical point of view, an elevated CA-125 level (although not routinely suggested) associated with the presence of typical ultrasonographic findings significantly increases the probability of such lesion.
Ultrasonography and Malignant Transformation of Endometriomas
Although malignant transformation is a rare complication of endometriosis (<1 % of cases), it is very important to follow the disease. Several studies have found an increased overall cancer incidence in women with endometriosis, and many retrospective and epidemiologic studies have reported an increased rate of endometriosis in women with ovarian cancer, especially endometrioid and clear cell histologies showing that endometriosis is correlated to an increased risk of developing ovarian cancer [51, 52]. Luckily it represents an uncommon complication, and considering that the overall frequency of endometrioma in the general population may range from 1 to 10 % and combining this data with the incidence of ovarian cancer in the general population (10 cases per 100,000 person per year), we can assert that most of ovarian endometrioma does not develop ovarian cancer [51, 52].
Testa et al. [53], in 2011, demonstrated in their study, for the first time, the excellent diagnostic value of TVUS in the discrimination of benign vs. malignant ovarian masses arising in endometrioid cysts and demonstrated also that these masses might not represent a specifically difficult category of ovarian masses (compared with the general population of ovarian masses) if the assessment is conducted by an expert US examiner. Women with malignant findings (borderline ovarian tumors and cancers) were older (median age 52 (range, 28–79) years) than those with benign endometrioid cysts (median age 34 (range, 18–76) years) (P < 0.0001), and the prevalence of postmenopausal status was significantly higher in malignant cases. All (15/15) malignant tumors vs. 16 % (50/309) of benign tumors were characterized by the presence of solid tissue (P < 0.0001).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree