Pathologic features
Incidence (%)
Ductal adenocarcinoma
80–90
Intraductal papillary mucinous neoplasm
3–5
Pancreatic neuroendocrine tumor
3–4
Serous cystadenoma
1–2
Mucinous cystic neoplasm
1–2
Acinar cell carcinoma
1–2
Solid pseudopapillary neoplasm
1–2
Pancreatoblastoma
<1
A particular group is represented by neuroendocrine tumors (NETs) that could be classified into functioning (85%) and not functioning (15%). Although Chapter 18 of this book is dedicated to NETs in general, we deem it fitting to mention also in this Chapter the most relevant issues regarding NETS arising in the pancreas. Nonfunctioning NETs may not present with clinical symptoms until they produce tumor mass effects at a late stage of tumor growth, so they are often diagnosed because of nonspecific abdominal symptoms or weight loss that lead the physician to refer the patient for a CT scan of the abdomen.
Functioning NETs, on the contrary, produce dramatic symptoms because of excess release of various hormones by the tumor into the blood. Table 17.2 lists the functioning NETs of the pancreas, their clinical symptoms, and biochemistry used for diagnosis. In 2000, the World Health Organization (WHO) proposed a clinicopathologic classification (revised on 2004) with a prognostic intent, in consideration of tumor size, local invasion of surrounding tissue, angioinvasion, tissue atypia, mitotic activity, and evidence of regional and distant metastasis (Table 17.3) [8, 9].
Table 17.2
Classification of NETs of the pancreas
Tumor name and incidence | % Malignant | Clinical symptoms | Biochemical analysis |
|---|---|---|---|
Insulinoma (70%) | 10 | Hypoglycemia | Blood glucose levels, elevated plasma insulin |
Gastrinoma (20%) (Zollinger–Ellison) | 70 | Peptic ulcer, abdominal pain, diarrhea | Gastrin |
VIPoma (2–8%) (Verner–Morrison) | 60 | Watery diarrhea, hypokalemia, hypochlorhydria | Vasoactive intestinal polypeptide (VIP) |
Glucagonoma (5%) | 75 | Anemia, glucose intolerance or mild diabetes, weight loss, erythema necrolytica migrans | Glucagon |
Somatostatinoma | 70 | Diabetes, diarrhea, steatorrhea, gallstones | Somatostatin |
GHRFoma | 0 | Acromegaly | Growth hormone releasing factor (GHRF) |
Functioning NET causing carcinoid syndromes and hypocalcemia | 100 | Diarrhea, flushing, wheezing, hypotension, symptoms of hypercalcemia | Serotonin, prostaglandin, parathyroid hormone releasing peptide (PTHP) |
Table 17.3
Clinicopathologic classification of pancreatic endocrine tumors
Well-differentiated endocrine tumor |
Benign behavior: confined to pancreas, <2 cm in size, nonangioinvasive, ≤2 mitoses/high-power field (HPF), and ≤2% 67Ki-positive cells, no perineural invasion |
Benign or low-grade malignant (uncertain malignant potential): confined to pancreas, ≥2 cm in size or angioinvasion, or >2 mitoses/HPF, or >2% Ki-67-positive cells, or perineural invasion |
Well-differentiated endocrine carcinoma |
Low-grade malignant |
Gross local invasion of adjacent organs and/or metastases (could be functioning or not functioning) |
Poorly differentiated endocrine carcinoma |
High-grade malignant: >10 mitoses per HPF |
All pancreatic NETs < 20 mm in largest diameter without local invasion or angioinvasion and no evidence of regional lymphatic or distant metastasis were classified as “benign.” Tumors confined to the pancreas and without metastasis were said to be “uncertain” if the tumor size was > 20 mm or local invasion had occurred (or both). Tumors with extended invasion of peripancreatic tissue or regional or distant tumor spread were classified as “malignant.” These tumors can have heterogeneous microscopic findings, and immunohistochemical staining with markers, such as chromogranin A, synaptophysin, and neuron-specific enolase, can usually confirm their neuroendocrine origin. The European Neuroendocrine Tumor Society has recently published guidelines on the management of pancreatic NETs [10].
Approaches to Staging
The staging system for pancreatic exocrine cancer continues to evolve. The importance of distinguishing between resectable and unresectable tumor is uncertain since state-of-the-art treatment has demonstrated little impact on survival. To obtain a uniform definition of disease, however, it is necessary to know the real disease’s dimension. Furthermore, another classification regards the mass localization within the pancreas (head, body, tail, or uncinate process) in order to plan the surgical intervention. The AJCC has designated [11] staging by TNM classification (Tables 17.4 and 17.5).
Table 17.4
AJCC definitions of TNM for the pancreas
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situa |
T1 | Tumor limited to the pancreas, 2 cm or less in greatest dimension |
T2 | Tumor limited to the pancreas, more than 2 cm in greatest dimension |
T3 | Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery |
T4 | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Table 17.5
Anatomic stage and prognostic groups for pancreas
Group | T category | N category | M category |
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage IA | T1 | N0 | M0 |
Stage IB | T2 | N0 | M0 |
Stage IIA | T3 | N0 | M0 |
Stage IIB | T1 | N1 | M0 |
T2 | N1 | M0 | |
T3 | N1 | M0 | |
Stage III | T4 | Any N | M0 |
Stage IV | Any T | Any N | M1 |
Since the survival rate of patients with any stage of pancreatic exocrine cancer is poor, clinical trials are appropriate alternatives for treatment of patients and should be considered prior to selecting palliative approaches. Of course, surgical resection remains the primary modality when feasible because it can lead to long-term survival and provides effective palliation [12–14]. The role of postoperative chemotherapy or chemoradiotherapy remains contentious as randomized studies provide contradictory results [15–20].
Stages I and II pancreatic cancer could be treated by local surgical resection with an operative mortality rate of approximately 1–16% [21–24]. Complete resection can yield 5-year survival rates of 18–24%, but ultimate control remains poor because of the high incidence of both local and distant tumor recurrence [12, 14, 25]. Standard treatment options are surgery or surgery plus postoperative fluorouracil (5-FU) chemotherapy and radiation therapy. Radical pancreatic resection of the tumor could be performed by the Whipple procedure (pancreaticoduodenal resection of head and uncinate process masses) or by distal pancreatectomy for tumors of the body and tail of the pancreas. To obtain negative margins of the surgical specimen, total pancreatectomy could be necessary. Other therapeutic options (e.g., with other chemotherapeutic agents) are under clinical evaluation [26–28]. Patients with stage III pancreatic cancer have tumors that are technically unresectable. This condition is determined by the locoregional spread:
Invasion of the portal vein, superior mesenteric artery and vein, common hepatic artery, and proper hepatic artery
Involvement of the neural plexus around the celiac axis or the superior mesenteric artery
Invasion of contiguous structures such as the stomach, colon, spleen, left adrenal, kidney, or spine
Presence of metastasis to the liver and peritoneum
Chemotherapy with gemcitabine is the standard treatment option for unresectable patients. In addition, patients may benefit from palliation of biliary obstruction by endoscopic or percutaneous radiologic biliary stent placement. Gastric bypass could be performed if necessary [29–31]. Other treatment options under clinical evaluation are intraoperative radiation therapy and/or implantation of radioactive sources [32, 33].
Patients with stage IV or recurrent disease show low objective response rates, so every clinical trial must be considered as a suitable treatment. Some patients have palliation of symptoms when treated by chemotherapy with 5-FU or gemcitabine [34–36].
A recent study has showed that the use of capecitabine and erlotinib in patients with gemcitabine-refractory first-line treatment of advanced pancreatic cancer is associated with modest improvements in overall survival [37]. Pain-relieving procedures such as celiac or intrapleural block and palliative biliary bypass or stent placement must be considered.
Current Role of Radionuclide Imaging
Before considering the role of nuclear medicine, it should be noted that CT, magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), endoscopic ultrasound (EUS) (useful in evaluating small lesions and as a guide for biopsy sampling), and endoscopic retrograde cholangiopancreatography are the most widely applied methods for diagnosis and staging of pancreatic cancer. In fact, morphologic imaging allows visualization of any infiltration of the vessels, affecting the assessment of tumor resectability. The limit of radiology is that it only provides spatial information and is lacking in metabolic information, which is useful in staging and, above all, posttherapy restaging in many patients.
Scintigraphy
At present, conventional nuclear medicine is employed almost exclusively in the study of endocrine pancreatic neoplasms, taking advantage of biologic targets that are uniquely expressed or markedly overexpressed in these kinds of tumors. Tumor receptor imaging emphasizes important emerging themes in molecular imaging: characterizing tumor biology, identifying therapeutic targets, and delineating the pharmacodynamics of targeted cancer therapy.
123I-VIP Receptor Scintigraphy
Since human adenocarcinomas of the gastroenteropancreatic system seem to overexpress vasoactive intestinal peptide (VIP) receptors, two decades ago some centers began performing 123I-VIP receptor scintigraphy (VIP-RS), which appeared to detect tumor tissue, especially pancreatic metastatic tumors. However, very low sensitivity and specificity were observed for the detection of pancreatic cancers, and so VIP-RS is not at present commonly used [38].
Somatostatin Receptor Scintigraphy
Somatostatin is a peptide secreted by endocrine D cells sited in the gastrointestinal (GI) tract and in the pancreas, and it inhibits the release of endocrine and exocrine factors in the GI system itself [39]. Somatostatin receptors (SSTRs) are membrane receptors for which six subtypes have been identified by molecular analysis (named sst1, sst2a, sst2b, sst3, sst4, and sst5). Each receptor has a different binding affinity for natural somatostatin and somatostatin analogs. In addition to normal tissues, SSTRs are overexpressed in pancreatic NETs but also in pheochromocytomas, paragangliomas, medullary thyroid cancer, and pituitary adenomas.
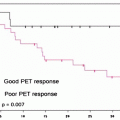
Since somatostatin has a short plasma half-life, analogs with longer half-lives are commonly used for SSTR imaging; most of the centers usually employ octreotide labeled with 111In (111In-DTPA-d-Phe1-octreotide, also called 111In-pentetreotide or with its commercial name, OctreoScan®) (Fig. 17.1). Octreotide binds with high-affinity sst2 and with moderate affinity sst3 and sst5. The presence of octreotide-binding sites on tumors allows their in vivo visualization after the injection of a radionuclide-labeled octreotide analog.
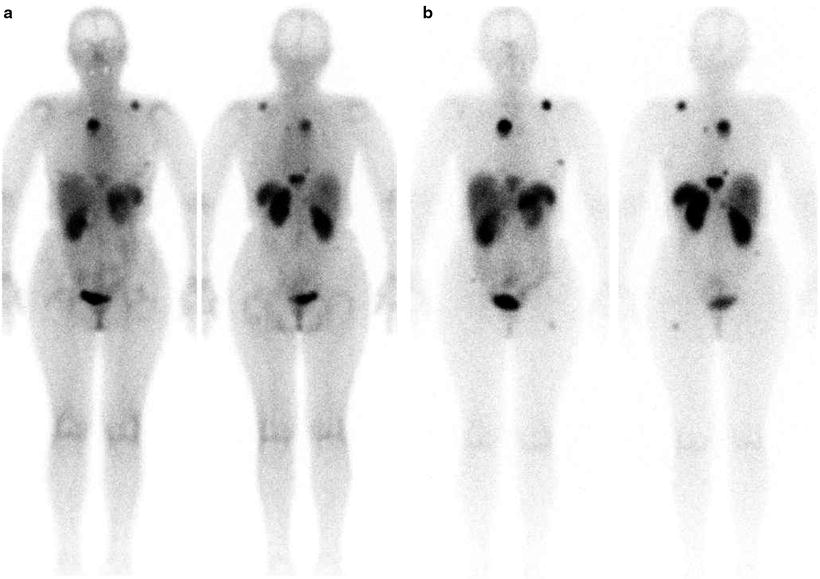

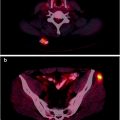
Fig. 17.1
OctreoScan® scintigraphy in a patient with relapsed disease after surgery for pancreatic carcinoma. Total body images were acquired at 4 h (a) and 24 h (b) after injection. Multiple areas of increased tracer uptake at right paramedian retrosternal region, left posterior mediastinum, left scapula, left rib, thoracic spine, VII hepatic segment, right lumbar abdominal quadrant, and left proximal femur shaft are noted and consistent with areas of metastasis with elevated expression of SSTR
Alternatively to OctreoScan®, 99mTc-labeled EDDA-hydrazinonicotinyl (HYNIC)-Tyr3-octreotide or EDDA/HYNIC-octreotate can be used.
Sst2 and sst5 are overexpressed in 70–90% of pancreatic NETs; therefore, radiolabeled somatostatin analogs, such as 111In-pentetreotide, can successfully visualize these tumors binding all tissue expressing a significant concentration of somatostatin subtype two receptors.
Somatostatin receptor scintigraphy (SRS) is accurate for detection and staging of NETs by demonstrating tumor sites (sensitivity of OctreoScan® varies between 75 and 100%), which are difficult to detect by conventional imaging above all when below 2 cm of diameter, supporting its ongoing use in clinical practice. Low sensitivity (50–60%) is instead demonstrated for detecting benign insulinoma since sst2 expression is absent [40].
In addition, centers that own a SPECT/CT hybrid imaging system can provide surgeons with more information about localization for tissue sampling and resectability [41–43]. Moreover, SRS may prevent surgery with curative intent in those patients whose tumors have already metastasized, and it may be used to select potentially responsive patients for treatment with the currently available octapeptide somatostatin analogs or with tumor-targeted radioactive treatment with radiolabeled (with 90Y or 177Lu) somatostatin analogs.
The role of SRS as a response predictor to therapy is commonly accepted: In particular, changes in functional volume, assessed by OctreoScan®, seem to be more useful than CT and response evaluation criteria in solid tumors (RECIST) in monitoring tumor response after treatment and correlate well with the later clinical response [44].
Nevertheless, some debated issues could reduce the widespread use of OctreoScan® in response prediction, such as the lack of quantitative data and the effect on SSTR expression of the SSTR-directed therapy, which is not well known [45, 46].
Moreover, in the last years, better accuracy has been seen with the introduction of new peptides (somatostatin analogs) labeled with the positron emitter 68Ga (produced by a generator, therefore without the need of a cyclotron), with higher spatial resolution and easier image quantification offered by positron emission tomography (PET) than by SPECT. This issue is discussed in greater detail in the following section on “68Ga-labeled peptides” [47, 48].
Positron Emission Tomography
PET allows, among other properties, the study of in vivo tissue metabolism, and thus demonstrates malignant tumors as hypermetabolic lesions, showing an increase of tracer uptake. Nowadays, [18F]fluorodeoxyglucose ([18F]FDG) is the most commonly employed compound in the clinical practice.
[18F]FDG is initially carried into the cell by glucose transporters (GLUT-1) as the normal glucose then is rapidly phosphorylated and trapped in the cells. Cancer cells are known to have increased anaerobic glycolytic activity and higher expression of glucose transporters.
In the past years, the main limitation of PET imaging was the lack of anatomical data: The introduction of PET/CT hybrid systems has enabled both morphological and metabolic imaging to be performed in a single session, reducing false-positive findings and inconclusive studies, thus increasing diagnostic accuracy.
Role of PET in Pancreatic Cancer
Most of the literature about the use of PET in pancreatic cancer has been described with the use of the tracer [18F]FDG. Because normal pancreas has low-glucose utilization and, on the other side, GLUT-1 transporters in pancreatic cancer are overexpressed compared with benign tissue, the criterion of a high, tumor/background [18F]FDG uptake ratio is satisfied [49].
Characterization and Detection of Lesions
The most important step in the initial approach to a patient suspected of pancreatic carcinoma is to decide whether the lesion is benign or malignant. The major limitation of morphological imaging techniques is their inability to confidently characterize small as well as cystic lesions (Fig. 17.2). PET/CT may be helpful, [50] with high sensitivity (85–100%) and moderate specificity (67–90%). Several studies have shown that [18F]FDG-PET is more accurate than conventional imaging techniques (average sensitivity and specificity is 82 and 75% for CT, respectively) [51].
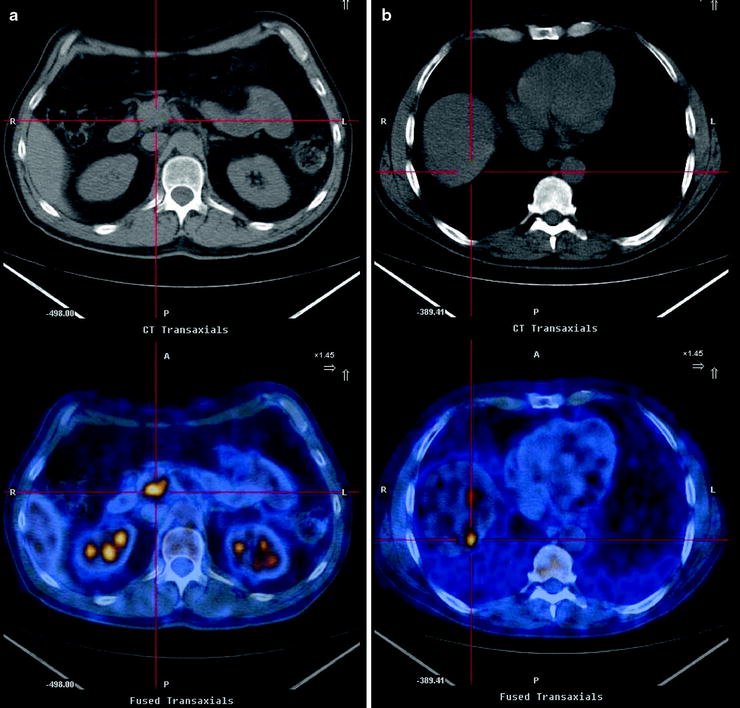

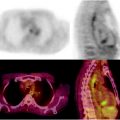
Fig. 17.2
Pancreatic mass, indeterminate at conventional imaging (suspicious at CT but negative at US). [18F]FDG-PET/CT confirms the pathologic glucose metabolism within the pancreatic head mass (a) and shows one hypermetabolic area at VIII segment of liver (b) in keeping with metastasis
Regarding the lesion characterization, the principal cause of false-positive findings with PET is inflammatory status due to chronic pancreatitis, and so it is recommended, as a first step, to always check the C-reactive protein levels (for elevated values, the specificity of [18F]FDG-PET decreases to 50%) [52].
Usually the distribution of [18F]FDG-avid areas within the parenchyma can guide diagnosis. Diffuse high uptake in the whole pancreas can be more often due to an inflammatory status (Fig. 17.3), while focal [18F]FDG uptake is mostly related to cancer [53]. Unfortunately, morphological analysis is not specific. Pancreatic cancer is sometimes accompanied by pancreatitis, and it has been demonstrated that about 24% of [18F]FDG uptake in tumor tissue may be related to inflammatory cells, meaning that it is impossible to separate clearly the two different components [54].
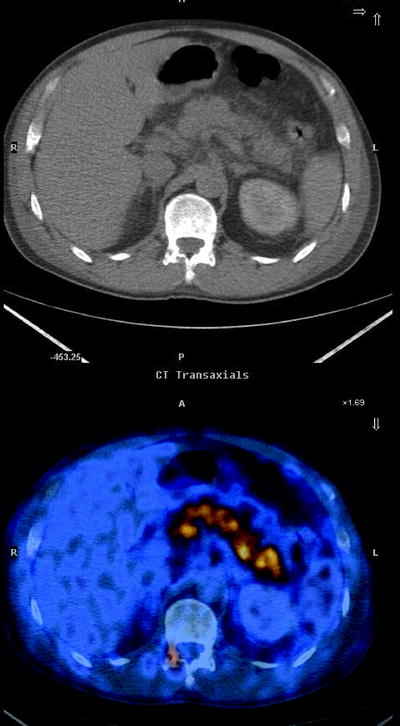

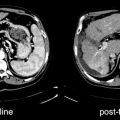
Fig. 17.3
Fused transaxial image shows diffuse high [18F]FDG uptake within the entire pancreatic parenchyma, consistent with pancreatitis
Furthermore, cancer cells can infiltrate diffusely into total pancreatic tissue, so a case with pancreatic cancer can show diffuse high [18F]FDG uptake in the whole pancreas. On the other hand, benign lesions such as autoimmune pancreatitis can assume a focal [18F]FDG uptake pattern. Unfortunately, there is a wide SUV overlap between inflammation and malignant pancreatic disease, and that is why some authors indicate that only visual qualitative interpretation can provide accurate differential diagnosis [55].
False-positive findings can also occur due to recent surgery or endoscopy, tissue inflammation after irradiation, abscess, autoimmune pancreatitis (sometimes it can assume a focal [18F]FDG uptake pattern, but high uptake at salivary glands can guide to diagnosis [56]), massive lymphocyte infiltration, retroperitoneal fibrosis, hemorrhage in pancreatic pseudocysts, inflammatory pseudotumors, pancreatic tuberculosis, and focal high-grade dysplasia.
Most false-negative results can occur for tumors of small sizes and elevated serum glucose levels (remember that many of these patients suffer from pancreatic insufficiency and diabetes; high serum glucose levels compete with [18F]FDG for glucose transporters sites, reducing the sensitivity for detecting malignant lesions) [52, 57, 58].
Tumor cell cellularity was also reported as an important cause of false-negative findings: scirrhous type, cystic type, or desmoplastic reactions are not rare features in pancreatic malignancy, and they are well known to be poor in cellularity even when tumor size is fairly large [59, 60]. Some histological types of pancreatic tumor do not have a high glucidic metabolism, such as mucinous or NETs, and therefore could be cause of false-negative reports (Fig. 17.4).
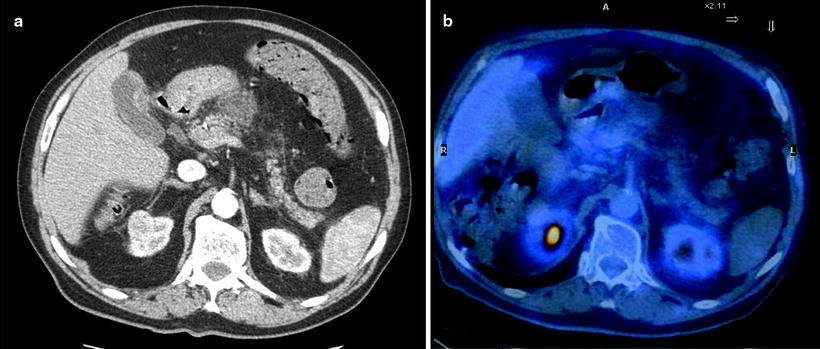

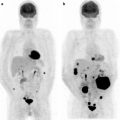
Fig. 17.4
An example of PET false-negative finding. Contrast-enhanced CT shows an exophitic lesion anterior to the pancreatic body in keeping with neoplasm and multiple peripancreatic lymphadenopathies (a); staging [18F]FDG-PET scan does not show any area of pathological [18F]FDG uptake (b). Histology will reveal a preponderant mucinous content within the malignant lesion
Regarding detection accuracy of [18F]FDG-PET/CT, a recent meta-analysis suggests that, although the addition of [18F]FDG-PET to the diagnostic work-up may enhance the diagnosis of pancreatic malignancy, its usefulness will vary depending on the pretest probability of the patient, the results of CT, and the provider’s testing thresholds. In fact, average sensitivity and specificity shift from 92 to 68% after a positive CT report to 73 and 86% after a negative CT report and 100 and 68% after an indeterminate CT report [61]. Recent advances in CT and MRCP have improved their ability to evaluate pancreatic lesions, decreasing the number of indeterminate reports and, as a consequence, also the number of [18F]FDG-PET examinations for differentiation of “equivocal” pancreatic lesions [52]. In conclusion, the greatest benefit of PET in differentiation of benign from malignant lesions is attributed to the possibility of excluding cancer without the need for biopsy or surgery, which may increase morbidity [62].
Staging
About 40% of patients with pancreatic cancers that are resectable by preoperative imaging modalities turned out to be unresectable at the time of operation [63]. Therefore, correct staging is the principal aim of imaging in pancreatic malignancies to determine the appropriate management and to define the prognosis of the disease.
Regarding T staging, the poor spatial resolution of [18F]FDG-PET limits its utilization: Anatomical imaging modalities such as multidetector CT, EUS, or MRCP are better suited to demonstrating the relationship between the tumor and the adjacent organs or vascular structures. At present no data support the usefulness of hybrid-modality PET/CT in local T staging [49].
Lymph node (LN) metastasis is one of the most important aspects of clinical management as an independent prognostic indicator for pancreatic cancer patients [64]. Unfortunately, both conventional imaging and PET/CT have low sensitivity (between 30 and 40%) for LN detection. It has been suggested that this could be caused both by the strong radioactive scatter from the main tumor on peripancreatic small LNs and by the small number of cancer cells in small metastatic LNs [65]. The principal cause of false-positive LN results at [18F]FDG-PET is the presence of reactive locoregional lymphadenopathies after biliary instrumentation (e.g., endoscopic retrograde cholangiopancreatodudenography or stent insertion).
After establishing the diagnosis of pancreatic cancer and confirming local resectability of the tumor, the main objective of staging of pancreatic cancer is the identification of patients with distant metastasis because they are currently excluded from surgical treatment. In this regard, the capability of whole-body scanning with a single examination is evidently an advantage of [18F]FDG-PET compared with the other imaging modalities. Whole-body [18F]FDG-PET detection of distant metastasis or unexpected lesions changes the management of the patient with cost-saving and improved quality of life by avoiding unnecessary surgery [52]. Several studies revealed better diagnostic accuracy of whole-body [18F]FDG-PET in the detection of distant metastasis compared with other modalities, such as CT or US. Sensitivity of PET is between 81 and 88% compared with CT (38–56%). Positive predictive value (PPV) is high for both modalities.
Regarding metastatic disease, liver is the most common organ to be affected, followed by the lungs and bone marrow. Direct spread into the peritoneum is also not uncommon and often missed on conventional anatomical imaging. The accuracy of PET in detecting liver metastasis is almost the same as for conventional imaging (94 and 90%, respectively). False-positive findings could occur for intrahepatic colestasis or in some inflammatory cases, such as abscess or intrahepatic bile duct infection mainly due to percutaneous transhepatic cholangiodrainage. False-negative PET findings could be observed in lesions of small size but also due to hepatic parenchyma heterogeneity of [18F]FDG uptake and respiratory motion artifacts [66, 67].
From the point of view of cost-effectiveness, routine use of PET has not been accepted by many centers and health care systems as a standard staging examination, and its use is recommend only in the evaluation of diagnostically challenging cases, especially in patients with biliary narrows without evidence of malignancy at conventional imaging.
Despite the encouraging results in cost-saving by excluding patients from resection because of detection of previously unknown distant metastases [50], the new 2009 edition of NCCN Clinical Practice Guidelines in Oncology™—Pancreatic Adenocarcinoma advocates the use of PET only in case of equivocal findings (recommendation category 2A) [68].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree