Fig. 1
Aneurysm clip. The CT image shows the spring (arrow) and blade (arrowhead) components of a titanium aneurysm clip
The advent of dual-energy CT (DECT) imaging in conjunction with Metal Artifact Reduction Software (MARS) has been shown to improve image quality of aneurysm, parent vessel, and adjacent parenchyma when compared to single-energy conventional CT examinations and offers a potential future application for CT in surveillance of treated lesions [8]. Additionally, DECT offers the capability of constructing virtual unenhanced images, offering the capability of monitoring relative contributions of hemorrhage versus contrast to hyperattenuation in the subarachnoid space related to pre- or post-procedure aneurysm rupture, extravasated contrast from the procedure itself, or both [9]. Furthermore, DECT can be useful for bone subtraction without an additional scan. Extracranial-intracranial bypass performed in conjunction with cerebral aneurysm clipping is most commonly performed between the superficial temporal artery and MCA or between the occipital artery and posterior cerebral artery (PCA). Patency of the anastomoses between bypass vessels can be assessed on CTA and MRA (Fig. 2). The STA often increases in caliber after successful revascularization.
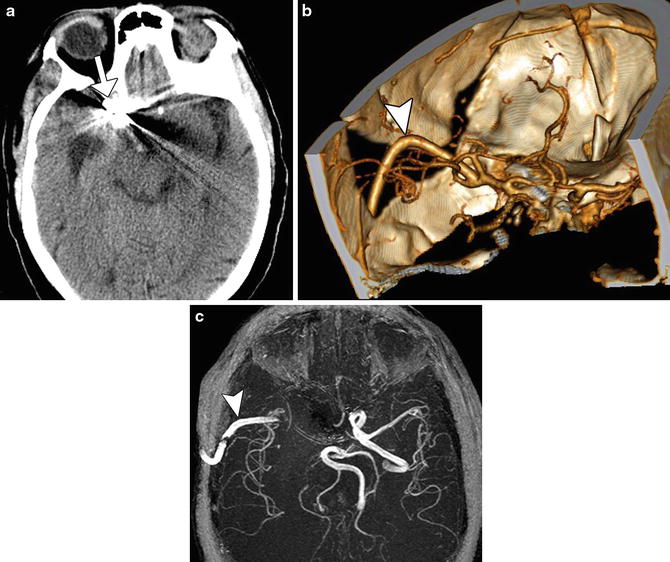

Fig. 2
Aneurysm clipping with extracranial-intracranial bypass. Axial CT image (a) shows a right paraclinoid aneurysm clip (arrow). 3D CTA image (b) and corresponding time-of-flight MTA (c) show a patent right extracranial bypass graft and anastomosis with the right MCA (arrowhead)
Following endovascular coil embolization, metallic streak artifact related to platinum alloy coils can considerably obscure the aneurysm and the parent vessel as well as the adjacent parenchyma, limiting the usefulness of CTA. On the other hand, several studies have demonstrated the sensitivity of MRA for residual aneurysm after coil embolization or stent-assisted coil embolization to be between 90 % and 100 % [9], while others have determined that MRA may be at least as sensitive as DSA if performed carefully (Fig. 3) [10]. TOF-MRA and contrast-enhanced MRA have similar accuracy for the evaluation of coiled aneurysms. Nevertheless, some institution protocols perform both techniques sequentially, employing CE-MRA data to supplement assessment by TOF-MRA. However, with regard to post-stent-assisted coiling, the excess susceptibility artifacts related to the stent material on time-of-flight MRA can obscure the aneurysm neck and parent vessel. Thus, in such cases, follow-up with contrast-enhanced MRA may be more beneficial (Fig. 4). In the case of embolization devices, the used CTA for follow-up is often preferable due to even greater artifact of MRA. Indeed, the lumen of the embolization devices and status post the treated aneurysms can be defined in detail on CTA (Fig. 5).
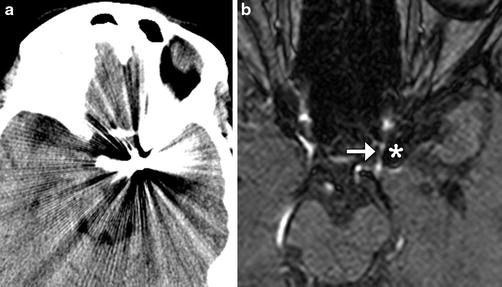
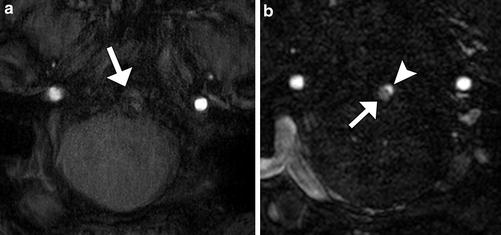
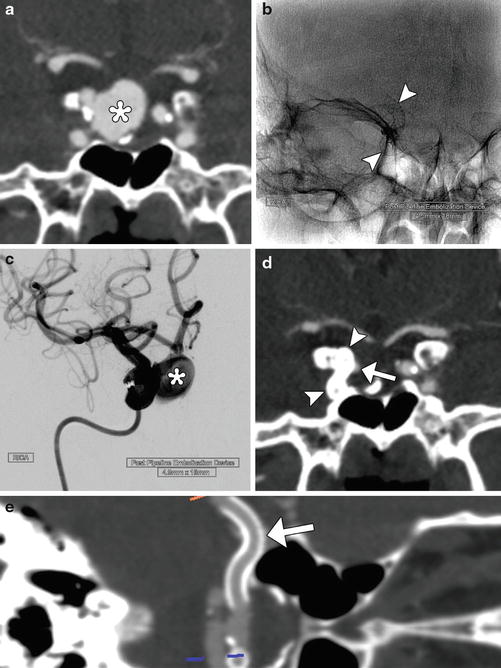

Fig. 3
CT versus MRA. Non-contrast CT (a) after coiling demonstrates extensive metallic streak artifact that obscures adjacent structures. The corresponding time-of-flight 3-T MRA (b) demonstrates minimal susceptibility artifact (*) allowing visualization of the patent parent vessel (arrow) adjacent to the coil mass

Fig. 4
The utility of contrast MRA. (a) 3D TOF-MRA post-stenting of basilar fusiform aneurysm at 3-T basilar artery obscured by susceptibility artifact (white arrow). Contrast-enhanced MRA at the same level (b) demonstrates both opacification of the vessel lumen (arrow) and a small focus of residual flow in the aneurysm (arrowhead)

Fig. 5
Pipeline device. Pretreatment coronal CTA (a) shows a large right superior hypophyseal aneurysm (*). Fluoroscopic image (b) obtained shows the Pipeline device (arrowheads) in position within the right ICA. Digital subtraction angiography (c) at the completion of Pipeline Embolization device insertion shows persistent filling of the aneurysm sac (*). Follow-up CTA obtained 9 months later shows near-complete exclusion of the aneurysm due to thrombosis of the sac with minimal residual filling of the neck (arrow) and Pipeline device in position (arrowheads). Curved planar reformatted CTA image (e) shows the full length of the patent flow-diverting stent (arrow)
There are several variables which may be considered when optimizing imaging parameters. For example, increasing receiver bandwidth can minimize susceptibility artifact related to metal implants [11]. By minimizing the amount of time during which echo sampling occurs (TE), susceptibility-related distortion of the image can be decreased, although this occurs to the detriment of signal-to-noise ratio. While protocols may vary, as a general principle, doubling the readout bandwidth yields good results. Shortening TE time may also decrease susceptibility artifact related to the coil mass and/or associated hardware [12]. Gonner et al. found that decreasing TE from 6 to 2.4 ms significantly reduced the volume of susceptibility artifact associated with the coil mass and additionally allowed improved visualization of the adjacent artery in 36 % of cases [13].
Although imaging at 3-T provides greater signal-to-noise ratio than at lower field strengths, the degree of susceptibility artifact associated with the coil mass is also increased. An in vitro study by Walker et al. showed a significant increase in the amount of susceptibility artifact and coil mass volume overestimation at 3-T versus 1.5-T [12]. They also showed that the volume of artifact depended on packing density of the aneurysm, with a more densely packed aneurysm resulting in significant differences in volume overestimation. They found that the use of a short TE protocol improved visualization, especially on 3D TOF images, concluding that with all variables being equal, 1.5-T was superior to 3-T when 3D TOF images were used. However, a prospective clinical study by Kaufmann et al. showed no significant difference in sensitivity for detecting an aneurysm remnant between 1.5-T and 3-T [14]. Ultimately, an accurate interpretation of the posttreatment MRA requires a familiarity with the pretreatment and immediate post-embolization conventional angiograms. A review of the images from the initial treatment provides the reader with a 2D projection of the 3D anatomy, demonstrating the orientation of the coil mass and aneurysm neck with respect to the parent vessel.
Digital subtraction angiography (DSA) is the modality of choice for treated aneurysm follow-up in cases when examination with MRA is contraindicated or noninvasive diagnostic imaging is inconclusive. DSA allows for exceptional spatial resolution, multiplanar projection, and the added dimension of dynamic temporal assessment of the contrast bolus, eliminating issues such as venous contamination encountered in cross-sectional techniques. The procedure is invasive, however, carrying an associated risk of neurologic complication in 2.6 %, stroke with permanent disability in 0.14 %, and death in 0.06 % [7, 15]. 2.6 % is a commonly quoted value, although it is notable that this large series showed a decreasing rate of complications in examinations performed more recently in the series. Meta-analyses also demonstrate that the risk of angiographic complication is significantly lower in patients with SAH, cerebral aneurysm, or AVM than in patients with TIA/stroke [16]. In general, CTA tended to overestimate aneurysm volume and MRA consistently underestimated volume, while DSA can both over- and underestimate aneurysm volume, providing a more accurate value on average than either of the other modalities [17].
Vasospasm and Posttreatment Complications
Aneurysmal subarachnoid hemorrhage is associated with a high mortality rate and is attributable to the initial hemorrhage event or aneurysm re-hemorrhage prior to surgical or endovascular obliteration. In survivors, the days that follow aneurysm obliteration are a critical period where patients are monitored intensely for the development of cerebral vasospasm. It is this secondary insult of vasospasm that impairs cerebral blood flow and is the major cause of delayed ischemia and stroke [18]. Although the pathophysiology of vasospasm is incompletely understood, it is generally accepted that the rapid institution of therapies, including systemic hypertension and intra-arterial vasodilator therapy, can be used in symptomatic patients to prevent permanent deficits [19]. Therefore, the period following aneurysmal subarachnoid, whether the aneurysm is treated with clipping or coiling, represents a critical period where clinical care is heavily influenced by the information provided by neuroimaging. The definition of cerebral vasospasm is new onset of intracranial arterial narrowing and it typically described qualitatively (mild, moderate, or severe) as subjectively interpreted on catheter or CT angiography [20]. Elevated transcranial Doppler velocities of >200 cm/s not thought to be a result of hyperemia are also classified as cases of vasospasm. Symptomatic vasospasm is a subgroup of vasospasm where clinical neurological symptoms are referable to a region of radiographic or sonographic vasospasm in the absence of alternative explanations.
The purpose of any imaging surveillance for vasospasm is to identify changes in vessel caliber and/or blood flow prior to neurological deterioration. Not all patients with subarachnoid hemorrhage from ruptured cerebral aneurysms go on to develop cerebral vasospasm. Radiographic grading scales, such as the Fisher scale, have been developed to describe a relationship between quantity or pattern of blood and the risk of future vasospasm development. However, patients with similar hemorrhage patterns and quantitative amount of subarachnoid blood may have a drastically different clinical course (with some patients experiencing no vasospasm at all). This emphasizes that in addition to the subarachnoid hemorrhage itself, there are patient-specific factors that predispose an individual to cerebral vasospasm. Development of radiographic biomarkers and studies assessing the efficacy of surveillance imaging will be important to improve management in these critical patients. However, there is no evidence from randomized controlled trials that guide the detection or management of vasospasm following aneurysmal subarachnoid hemorrhage. Practice patterns vary widely from institution to institution, where various means of radiographic and sonographic surveillance aid to augment serial clinical examinations. In patients with Glasgow Coma Scale scores less than 9 (comatose), these radiographic surveillance methods are the main method of vasospasm detection, as it is challenging to detect subtle differences in neurological exam in a poor neurological state.
Doppler ultrasound is a noninvasive modality that can be used to measure cerebral blood flow [21]. The general concept of the modality is that blood velocity increases as vessel diameter decreases, as is seen in the setting of vasospasm. However, hyperemia can also result in elevated velocity recordings, confounding interpretation. A variety of measurement techniques have been used to help differentiate between these two conditions (vasospasm and hyperemia), such as the calculation of the Lindegaard ratio, which compares the blood flow in the more proximal carotid artery with the MCA [22]. Identification of these differences in blood flow is potentially useful in determining focal areas of vasospasm as compared to global changes in blood flow that are seen with hyperemic states or diffuse vasospasm. Drawbacks of transcranial Doppler ultrasound to detect vasospasm are inherent in its limited sensitivity and specificity. Some reports caution that there is a lack of evidence and lack of accuracy of transcranial Doppler, criticizing its use in routine clinical practice [23]. Nevertheless, because of its noninvasive nature, lack of radiation exposure, and ease of daily bedside acquisition, it is routinely used at several high volume neurovascular institutions.
CTA is also useful for the detection of cerebral vasospasm following subarachnoid hemorrhage post-clipping, particularly when there is severe spasm located in proximal artery locations, such as the A1 segment of the anterior cerebral artery (ACA) or the M1 segment of the MCA (Fig. 6). In addition, CT perfusion can be performed concurrently to provide insight into the extent of cerebral ischemia resulting from vasospasm. However, CTA may be less accurate for detecting mild and moderate spasm in distal vessel locations [24]. The use of CTA in patients with renal impairment is limited secondary to the necessity of iodinated contrast. CTA is also associated with a radiation dose that can be significant in critical patients undergoing serial imaging in the vasospasm “window.” Future study in the form of randomized trials is needed to define the efficacy of either symptom-prompted CTA or routine surveillance CTA following aneurysmal subarachnoid hemorrhage and its effect on outcome.
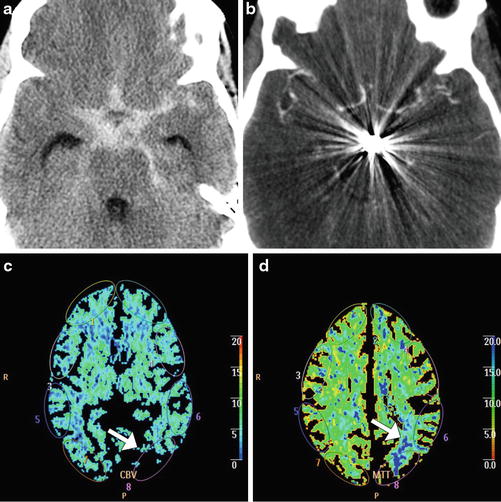

Fig. 6
Cerebral vasospasm depicted on CTA and CT perfusion. Axial non-contrast CT (a) shows extensive subarachnoid hemorrhage related to a ruptured basilar artery aneurysm. Axial CTA MIP image (b) obtained several days after aneurysm coiling shows severe diffuse narrowing of the proximal circle of Willis branches, consistent with vasospasm. The corresponding CBV (c) and MTT (d) maps show perfusion deficits, particularly severe in the left parietal lobe (arrows)
Ultimately, catheter-based angiography has been considered to be the historical gold standard to diagnose vasospasm [25]. Advantages of catheter-based angiography include its high specificity and sensitivity. It also allows for the immediate administration of intra-arterial therapy should severe vasospasm be detected (Fig. 7). The main risks of the diagnostic procedure include stroke (estimated at 1 % or less), local access site complications, the need for iodinated contrast, and radiation exposure [16].
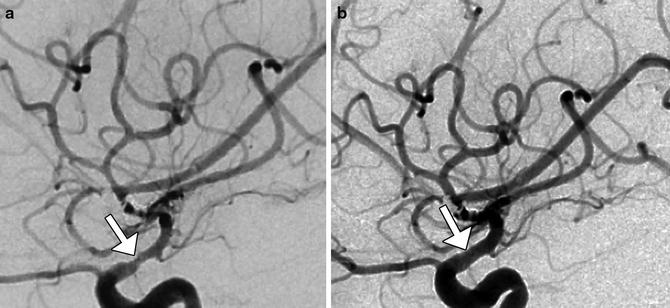

Fig. 7
Digital subtraction angiography of cerebral vasospasm. Five days postoperatively, the patient was suspected to have developed left MCA vasospasm based on elevated transcranial Doppler velocities in that distribution. Initial lateral projection DSA image (a) shows severe stenosis of the MCA (arrow). The patient was treated with intra-arterial administration of milrinone and nicardipine, with nearly resolved spasm of the MCA (arrow), as demonstrated on follow-up lateral DSA projection (b)
In addition to direct imaging of the cerebral vasculature, there are several imaging modalities that may be able to detect aberrations in cerebral perfusion irrespective of changes in large vessel caliber. This is an important consideration because vasospasm of the microcirculation may not routinely be detected on either CT angiography or digital subtraction angiography. The most commonly used dynamic imaging technique for this purpose is CT perfusion, likely as a result of its widespread availability and ease of acquisition. Other dynamic imaging techniques, such as positron emission tomography, have also been used to identify regions of impaired regional blood flow or regional cerebral metabolic rate of oxygen. Emphasis on imaging of brain perfusion may be able to better evaluate for vasospasm, particularly since “large vessel” constriction may only occur in a portion of affected cases.
Periprocedural aneurysm rupture is a documented complication of coil embolization occurring in 4.6 % of all cases and 20.4 % of balloon-assisted cases in one large series (Fig. 8) [26]. While a distressing complication, the majority of patients demonstrated an absence of clinical sequelae (74 %) related to the event. Early rebleeding is also a well-documented complication following coil embolization [27]. The ISAT study found rates of rebleeding among ruptured aneurysms treated with coiling of 2.5 % in the first year and 0.2 % in subsequent years. The CARAT trial demonstrated rebleeding risk in the first 3 years to be 2.2 %, 0.2 %, and 0 %, respectively [28]. Hemorrhage at any point following surgical clipping is considered to be a rare event with accumulative estimated risk of between 1 % and 2 % [3]. Unruptured aneurysms treated with coil embolization demonstrate an exceptionally low rate of subsequent bleeding, estimated at 0.2 % annually in one meta-analysis [29].
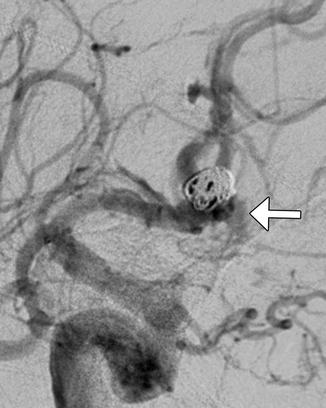

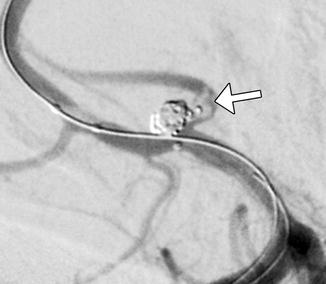
Fig. 8
Intraprocedural aneurysm rupture: DSA demonstrates extravasation of contrast from a partially coiled ACoA aneurysm (arrow)
Thromboembolic complications are a well-described complication of coil embolization and range from silent incidental findings on subsequent imaging to devastating findings. Silent thromboembolic events are common, presenting as multifocal diffusion restriction on DWI sequence images in the postoperative period. Lesions are typically small, often multifocal, and typically localize to the vascular territory of the vessel being treated (Fig. 9). One of the earlier descriptions by Rordorf et al. showed multifocal restricted diffusion in 61 % of patients who were imaged within 48 h of uncomplicated coiling [30]. A recent larger series of 342 patients by Kang et al. showed an incidence of DWI-positive lesions of 54.5 %, while symptoms were only detected in 4.1 % of patients, resolving in approximately half. The presence of ≥6 lesions was, however, correlated with the presence of symptoms – 78.6 % of symptomatic patients had ≥6 lesions implying the burden of DWI could potentially be employed as a surrogate marker for symptomatic ischemia [31]. Occasionally, larger territorial infarcts result when a branch associated with an aneurysm is sacrificed or as a result of parent vessel is purposely embolized (Fig. 10). Stent-assisted coiling is also associated with silent thromboembolism, although limited literature at present precludes assessment of whether the risk is significantly elevated in this population. Altay et al. found DWI lesions in 30 % of 46 patients with unruptured aneurysms treated with stent-assisted coiling [32]. Hahnemann et al. recently reported on a series of 75 patients treated with stent assistance in which 48 (64 %) were found to develop ischemic lesions [33]. Stent-assisted coiling techniques are associated with an increased risk of thrombosis compared to coil embolization alone. In addition to early thrombosis, stent-assisted coiling results in the risk of both delayed in-stent stenosis and delayed thrombosis. A recent meta-analysis determined a rate of delayed stenosis of 5.3 % (range of 0–20.6 %), which correlates with a prospective database assessing the Enterprise stent that found rates of delayed stenosis and thrombosis of 3 % each [34]. Rates of in-stent stenosis and thrombosis compare favorably with rates documented following stenting of intracranial vessels for atherosclerosis during the SAMMPRIS trial of 28.3 % and 3.9 %, respectively [35].
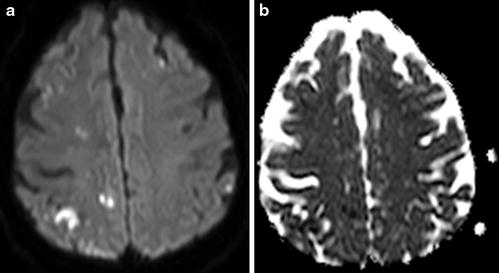
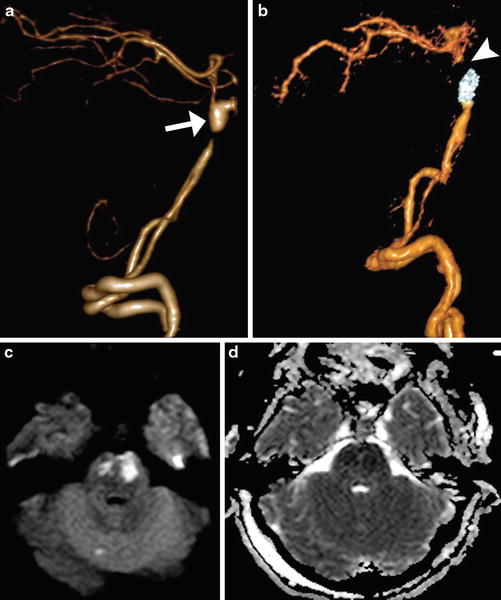

Fig. 9
Silent thromboembolic events. At 6 days post-coiling of a ruptured 6 mm ACoA aneurysm, there is multifocal diffusion restriction depicted on DWI (a) and ADC (b)

Fig. 10
Infarct due to parent vessel sacrifice with coil embolization. Pretreatment 3D surface-rendered CTA image (a) shows a dissecting aneurysm of the basilar artery with proximal stenosis. Post-embolization 3D surface-rendered CTA image (b) shows coils within the lesion and absent opacification of the distal basilar artery (arrowhead). Axial DWI (c) and ADC map (d) shows associated restricted diffusion in the pons, which correspond to acute infarcts in the perforator artery territory
Clinically significant thromboembolism is a much less common complication, although this is the most common cause of periprocedural morbidity associated with coil embolization of intracranial aneurysms, reported to occur in 1–17 % of cases, including 4.7 % of cases in one large retrospective series [36, 37]. During as many as 11 % of procedures, distal emboli or intraluminal thrombus related to the coil mass/aneurysm may be demonstrated (Fig. 11) [38]. If encountered during the coiling procedure, therapy including infusion of a glycoprotein IIB-IIIA inhibitor such as abciximab (ReoPro™) may be employed to acutely lyse thrombus, although thromboembolic complications occurring after the completion of the procedure pose a more difficult therapeutic conundrum, especially if the treated aneurysm was ruptured at the time of presentation. Risk factors for symptomatic thromboembolism in one series of 159 procedures included mass effect by the treated lesion, residual flow within the coil mass at completion of the procedure, prolapsed coils, and mean aneurysm diameter larger than 12 mm [37]. Remodeling of the aneurysm neck is also a risk factor for thromboembolic complication. Use of a stent construct to remodel the aneurysm neck resulted in acute or subacute in-stent thrombosis in 4.5 % of cases in a series of 161 patients, and 2.3 % experienced delayed stenosis or occlusion [39]. These results were in keeping with a recent meta-analysis which found a 4.6 % risk of intraprocedural and 4.3 % post-procedural risk of stent-related thromboembolism [40]. Balloon remodeling of the neck was also associated with an increased risk of thromboembolic complications. The increased risk of thromboembolism with neck remodeling is at least partially attributable to selection bias wherein larger, more complex aneurysms requiring longer procedure times may impart an inherently higher risk of thromboembolism.