Fig. 1
The therapeutic index. (a) Therapeutic index for treatment with radiation alone. Radiation with dose A implies specific probabilities for tumor cure and normal tissue toxicity (e.g., 50 % cure vs <10 % toxicity). (b) If radiation is combined with radioprotectors, the radiation dose can be increased (dose B) because normal tissue toxicity is reduced (curve for normal tissue toxicity shifted to the right). (c) If radiation is combined with radiosensitizers, the probability for tumor cure increases (curve for tumor cure shifted to the left). Radioprotectors and radiosensitizers both increase the therapeutic index
Many studies on reirradiation of different cancer types report on combinations of radiation and various drugs (Haraf et al. 1996; Arcicasa et al. 1999; Schaefer et al. 2000; Mohiuddin et al. 2002; Wurm et al. 2006; Biagioli et al. 2007; Combs et al. 2008; Spencer et al. 2008; VanderSpek et al. 2008; Würschmidt et al. 2008; Dornoff et al. 2015; Minniti et al. 2015). Nevertheless, there is limited evidence from systematic, prospective clinical trials addressing reirradiation with or without response-modifying agents. This results in considerable uncertainty with regard to current clinical practice. In a Canadian survey, 28 % of respondents would use concurrent chemotherapy along with reirradiation in cases of chemosensitive tumors, 43 % would not recommend concurrent chemotherapy, and 30 % were uncertain (Joseph et al. 2008). This chapter provides an overview on the principles of combined modality treatment and reirradiation with classical chemotherapy or other response modifiers.
2 Clinical Relevance of Combined Modality Approaches
The introduction of combined modality approaches was a highly significant step in the evolution of curative radiation treatment. Parallel to analysis of altered fractionation schedules, combined treatment has actively been investigated in recent decades in both preclinical and clinical studies around the world. When judged at this time, the most pronounced increase in therapeutic gain was probably seen by combining radiation with chemotherapy and few other response modifiers.
Meanwhile, a huge body of evidence supports the use of combined modality approaches based on the combination of ionizing radiation with cytostatic drugs. In this regard, several randomized phase III trials for many relevant cancer sites provide a sound basis for level one evidence-based decision. This holds true especially for glioblastoma multiforme (Stupp et al. 2005), head and neck cancers including nasopharyngeal cancer and laryngeal cancer (Brizel et al. 1998; Forastiere et al. 2003; Budach et al. 2005), esophageal cancer (Minsky et al. 2002), colorectal and anal cancer (Sauer et al. 2004; Bartelink et al. 1997), cervical cancer (Green et al. 2001), as well as lung cancer (Schaake-Koning et al. 1992).
The most important aim of curative cancer treatment is to eradicate all clonogenic tumor cells or stem cells, which are able to give rise to a recurrence. With regard to the amount of quantitative cell kill, it has to be emphasized that important differences exist between ionizing radiation and chemotherapy (Fig. 2). In principle, radiation treatment can be designed to cover the whole tumor with a homogeneously distributed full radiation dose, maybe including a biology-driven boost, capable of inactivation of all tumor cells (Belka 2006). In contrast, pharmacotherapy is limited by the fact that the dose of the active, cell killing form of the compound is variable within the tumor and its cells. This results from problems in the delivery of drugs (perfusion, interstitial fluid pressure, tissue pH, etc.), cellular uptake, efflux, inactivation, and resistance. In many instances, the agent does not reach the relevant therapeutic targets in the required concentration and for a sufficient time period (Tannock et al. 2002; Primeau et al. 2005; Minchinton and Tannock 2006). In fact, the pharmacokinetic profile of anticancer drugs is characterized by substantial inter-patient variability where two- to threefold variation is not uncommon (Brunsvig et al. 2007). These issues gain complexity with simultaneous administration of two or more drugs and in the context of reirradiation because of the heterogeneity of reirradiated tumors (Fig. 3). As illustrated in Fig. 2, the quantitative cell kill of ionizing radiation is significantly larger than that of chemotherapy (Tannock 1992, 1998). The magnitude of this effect might vary with cell type, culture conditions, drug, exposure time, etc. Experimental evidence suggests, however, that single radiation doses result in 1 % or less cell survival compared with 10–50 % with cytotoxic drugs (Epstein 1990; Kim et al. 1992; Simoens et al. 2003; Eliaz et al. 2004). Although clinically impressive remissions of solid tumors might occur after chemotherapy, the underlying cell kill is often not larger than 1–2 log and pathological examination of tissue specimens reveals residual viable tumor cells.



Fig. 2
Differences in quantitative cell kill and time course. Influence of different therapeutic modalities on number of tumor cells during a course of treatment, based on the models by Tannock (1989, 1992) and Minchinton and Tannock (2006). The dashed line represents the border between microscopic and macroscopic tumors, defined as a size of approximately 5 mm. Compared with surgical resection and fractionated radiotherapy, multiple courses of chemotherapy (in this case six, indicated by arrows) are less efficient in cell kill. While microscopic disease might be eradicated (lower chemotherapy curve), clinical evidence suggests that most macroscopic solid tumors (exception: more sensitive testicular cancers) will shrink temporarily but eventually regrow from surviving residues (upper chemotherapy curve). As shown in the inset, the strength of chemotherapy in combination with radiation treatment (besides of spatial cooperation) is the modification of the slope of the curve

Fig. 3
In clinical reirradiation studies, the situation is complicated by very complex and heterogeneous tumor biology and changes in physiological and microenvironmental parameters resulting from the first course of radiotherapy, for example, fibrosis and impaired tissue perfusion. It has been suggested that human tumor cells derived from radiotherapy failures (head and neck carcinomas) are relatively radioresistant (Weichselbaum et al. 1988). Among radioresistant cell lines, those from previously irradiated patients were significantly more resistant than those from non-irradiated patients (Grenman et al. 1991). Importantly, some radiosensitive tumors from previously irradiated patients were also found in the latter study (three of seven examined cases)
In most clinical situations, chemotherapy augments the radiation-induced cell kill within the irradiated volume and may improve distant control. To maximize augmentation of cell kill, optimization of parameters of drug exposure is necessary. It has been shown, for example, that continuous infusion is better than bolus administration of 5-FU. The following example illustrates the efficacy of chemotherapy as a radiation enhancer. In the large randomized FFCD 9203 trial in rectal cancer, preoperative radiotherapy (45 Gy in 25 fractions) resulted in pathological complete remission (pCR) in 4 %, whereas the addition of 5-FU and folinic acid improved this figure to 12 % (Gerard et al. 2005). While radiation alone can be considered as a curative treatment in a variety of early-stage solid tumors (especially T1-2 N0 M0, e.g., skin, anal, cervix, larynx, lung, and prostate cancers), long-term control with chemotherapy alone is rarely observed. Current concepts of cancer biology suggest that most traditional chemotherapy approaches fail to eradicate cancer stem cells, which are slow-cycling cells that often express multidrug resistance (MDR) proteins (Miller et al. 2005; Prince and Ailles 2008; Moitra 2015; Yoshida and Saya 2015).
3 Basic Considerations
3.1 Therapeutic Gain
Therapeutic gain is defined by an increase of tumor control and finally survival without a parallel increase in the severity of specific side effects (Fig. 1). A very nice preclinical example is the comprehensive studies with cisplatin and 5-FU in different tumors transplanted into mice, which were reported by Kallman et al. (1992). Independently of the term “therapeutic gain,” the interaction of radiation with chemotherapy follows a precise nomenclature based on some groundbreaking theoretical considerations published in the late 1970s (Steel 1979; Steel and Peckham 1979). In every case of a scientific description and quantification of the effects of combined modality therapy in appropriate models, it is highly recommended to adhere to the proposed nomenclature. The complexity of effects increases with each step of investigation, that is, from cell culture to tumor-bearing animal to cancer patient. A thorough examination of all possible treatment combinations and administration schedules for a given drug plus radiation is very challenging, as can be seen in the publication by Kallman et al. (1992), who studied in depth the radiosensitizing effects of cisplatin. Although extensive discussion of radiobiological principles is beyond the scope of this chapter, a few definitions shall be mentioned. Since the introduction of mammalian cell survival curves, the parameters D0 and N have been used as quantitative measures of inherent radiation sensitivity, as was the shoulder width Dq (Thames and Suit 1986). The ratio alpha/beta is a measure of fractionation sensitivity.
3.2 Additivity, Synergism, and Subadditivity
When combining two treatment modalities the resulting net effect on cell killing is mainly described by the terms “additivity, synergism, and subadditivity,” which are derived from experimental work. They are not applicable to the clinical situation and do not reflect the results of clinical trials, where changes from radiation as a monotherapy to multimodal treatment usually do not result in extraordinarily favorable cure rates (or supraadditivity), although they have led to important gradual improvement. It appears prudent to refer to the term “enhancement of radiation effect” within a clinical context.
3.2.1 Synergism (Supraadditivity)
The term “synergism” describes a situation where the combination of both drugs induces more cell kill than the addition of either treatment alone. The term “radiosensitization” is also used in this regard; however, it should only be employed when the drug used is devoid of any intrinsic potential. Regardless of nomenclature, the resulting effect is a shift of the tumor control curve to the left.
3.2.2 Additivity
The term “additivity” is used to describe situations where both triggers act completely independent of each other resulting in a net kill not larger than expected from the calculated additive combinatory effect.
3.2.3 Infra (Sub)-additivity (Protection)
This term describes situations where the drug interferes negatively with the efficacy of ionizing radiation, or vice versa.
4 Interaction of Radiation and Chemotherapy
4.1 Spatial Interaction
On a large scale, chemotherapy and radiation may be effective on several levels. The concept of spatial interaction was devised to mean that chemotherapy and radiation act on spatially distinct compartments of the body, resulting in a net gain in tumor control. The concept of spatial interaction does not take into account any drug-radiation interaction on the level of the tumor itself, but rather assumes that radiation or chemotherapy would be active in different compartments, respectively. In a narrow sense, this concept describes the fact that chemotherapy would be employed for the sterilization of distant microscopic tumor seeding, whereas radiation would achieve local control (Fig. 4). Obviously, this is a theoretical consideration only, since chemotherapy also increases local control and radiotherapy reduces distant metastasis via increased local control rates; thus, when integrating the concept of spatial interaction into a more complete view on combined modality, spatial cooperation is still of major importance. Next to spatial effects, several other important mechanisms may increase the efficacy of a combined treatment approach. In this regard, inhibition of repopulation and effective killing of hypoxic radioresistant cells may contribute to the efficacy of a combined treatment.


Fig. 4
Spatial interaction. In a classical interpretation (left panel), the term “spatial interaction” refers to the fact that chemotherapy is effective on tumor compartments where radiation has no efficacy, and vice versa, resulting in a generally increased control rate. In a more complex view, spatial interaction is relevant on multiple interacting levels: increased local control by radiation reduces the risk of a secondary seeding. Furthermore, the interaction of radiation with chemotherapy increases local control; thus, in addition to the classical spatial interaction, several levels of interacting feedback loops exist, which increase efficacy of spatial interactions
4.2 Role of Repopulation
The fractionated treatment of tumors with ionizing radiation is associated with the phenomenon of repopulation (Kim and Tannock 2005). Speaking simply, a certain amount of tumor cells repair the induced damage in between two fractions and proliferate. Repopulation may neutralize around 0.5 Gy/day; however, the range of repopulation is considerably large and may reach levels exceeding 4 Gy (Trott 1990; Baumann et al. 1994; Budach et al. 1997). Based on these findings, radiation biologists advocated the use of accelerated radiation schedules. The phenomenon of repopulation must also be taken into account when trying to design combined modality regimens. In theoretical models, cell loss from neoadjuvant chemotherapy preceding fractionated radiation treatment might trigger accelerated repopulation. Then, a certain percentage of the daily radiation dose is wasted to counteract increased tumor cell proliferation. Under such conditions, despite of a response to chemotherapy, cell survival after radiotherapy is not better than after the same course of radiotherapy alone (yet toxicity results from both modalities). Whether such effects are more important than reduced interstitial fluid pressure (IFP) and improved oxygenation might depend on tumor type.
The clinical observation that the combination of 5-FU, mitomycin C, or cisplatin with radiation is of value in rapidly proliferating squamous cell cancers has led to the suspicion that the addition of drugs may influence the potential of cancer cells to repopulate. At least for mitomycin C, this effect was documented precisely using a xenograft model (Budach et al. 2002). In this model, transplanted tumors were treated with 11 fractions of 4.5 Gy under ambient conditions with or without mitomycin C followed by a graded top-up dose on days 16, 23, 30, or 37 given under hypoxic conditions. Repopulation in the interval between the fractionated treatment and the top-up dose accounted for 1.33 Gy top-up dose per day in animals not receiving mitomycin C, but only 0.68 Gy in animals receiving the drug. Thus, at least mitomycin C may increase the efficacy of radiation by the inhibition of repopulation.
4.3 Role of Hypoxia
As known for years, radiation-induced cell kill is strongly dependent on the presence of adequate oxygen tensions. With increasing tumors, for example, in head and neck cancer, areas of hypoxia and even anoxia are present leading to an increased radiation resistance of clonogenic tumor cells within such areas (Molls and Vaupel 1998; Nordsmark et al. 2005; Molls et al. 2009). It has been speculated that chemotherapeutic agents especially those which kill even hypoxic cells (mitomycin C) may overcome global radiation resistance simply by killing radioresistant hypoxic cells, thereby being of special value in highly hypoxic tumors (Teicher et al. 1981; Rockwell 1982).
Comparing the effects of several cytostatic drugs in combination with radiation on the growth of a C3H mammary carcinoma, it turned out that cyclophosphamide, adriamycin, and mitomycin C had the most significant effect on the proportional cell kill of hypoxic cells. In contrast, bleomycin and cisplatin did not exert strong effects on hypoxic cells (Grau and Overgaard 1988). In addition, it has clearly been shown that tumor blood flow in xenografts is increased after mitomycin C treatment (Durand and LePard 1994). Using two different squamous cell carcinomas, the latter authors tested the drug’s influence on outcome of radiation treatment with or without hypoxia (Durand and LePard 2000). The authors did not report an increased killing of hypoxic cell by mitomycin C, nor a consistent increase in tumor blood flow rates; however, mitomycin C in combination with radiation was associated with a slight increase in cell killing of hypoxic subpopulations of the xenograft system. Based on this observation it was concluded that the efficacy of a combined treatment with mitomycin C and radiation cannot be rationalized on either a complementary cytotoxicity or on drug-induced improvement in tumor oxygenation secondary to an increased blood flow.
In the case of paclitaxel, it has been tested whether the enhanced killing by the combination of paclitaxel and radiation is connected to the presence of oxygen. Using a MCA-4 xenograft system, the authors could show that in the absence of oxygen the paclitaxel-mediated change of the TCD50 value is strikingly less prominent (Milas et al. 1994, 1995); thus, it can be concluded that at least in part the influence of paclitaxel on the radiation response is mediated via an optimized oxygenation. In conclusion, several sets of data indicate that the efficacy of chemotherapy in combination with radiation may be related to an increased oxygenation of hypoxic tumors; however, it still remains speculative whether or to what amount the efficacy of a combined treatment is strictly related to specific influences on the hypoxic cell compartment.
5 Molecular Interactions
5.1 DNA Damage
One of the underlying molecular aspects of the efficacy of the combination of radiation and chemotherapy, which has been understood in some more detail, is influence on DNA repair. The induction of DNA damage is probably one of the most crucial events after irradiation of cells. In this regard, ionizing radiation triggers a wide array of lesions including base damage, single-strand breaks, and notably, double-strand breaks (DSBs). After irradiation, different molecular systems are involved in recognition and repair of the damage. Whereas most of the induced damage is quickly repaired, DSB repair is slow and unrepaired DSBs are considerably important for the final induction of cell death.
Many chemotherapeutic agents, especially those known to be of value in combination with radiation, also induce considerable DNA damage or interfere with effective DNA repair; therefore, two general patterns of interactions may be separated: (a) the combination of the drug with radiation directly leads to more damage; (b) the drug may interact with DNA repair pathway thus increasing the level of DNA damage more indirectly; however, none of the potential mechanisms acts without the other in real settings. Cisplatin, for example, acts by complex formation with guanosine residues and subsequent adduct formation ultimately resulting in intra- and interstrand cross-links. This type of damage is mostly removed by base excision repair and mismatch repair. Several sets of data suggest that single-strand damage induced by radiation in close vicinity to DNA damage triggered by cisplatin results in a mutual inhibition of the damage-specific repair system; thus, the amount of resulting damage leads to an increased net cell kill (Begg 1990; Yang et al. 1995). Similarly, etoposide, which is a strong topoisomerase-IIa-directed toxin, exerts DSB mostly during the S-phase of the cell cycle (Berrios et al. 1985; Earnshaw and Heck 1985). Again, several lines of evidence show that the combination of both agents results in a strongly increased level of damage (Giocanti et al. 1993; Yu et al. 2000).
The biochemical pathways implicated in DNA repair and DNA synthesis overlap in several regards; thus, drugs acting on the synthesis of DNA putatively also interfere with the repair of DNA damage induced by ionizing radiation. Several prototypical radiation sensitizers, including 5-FU, fludarabine, and gemcitabine, may act via these mechanisms. Besides cisplatin, 5-FU is probably the most commonly employed drug in clinical combined modality settings. Basically, 5-FU inhibits thymidilate synthase thereby reducing the intracellular pool of nucleoside triphosphates (Pinedo and Peters 1988; Miller and Kinsella 1992). In addition, the drug is integrated into DNA via fluorodeoxyuridine, also contributing to its anti-neoplastic effects. Several lines of evidence suggest that the amount of 5-FU integrated into DNA directly correlates with the radiosensitizing effect. In addition, the complementation of the cell culture medium with higher levels of thymidine reverses the effects of 5-FU on the radiation sensitivity (McGinn et al. 1996; Lawrence et al. 1994).
5.2 Radiation Sensitization via Cell Cycle Synchronization
The fact that striking differences in the radiation sensitivity occur as cells move through the different phases of the cell cycle has stimulated the speculation that the efficacy of a combined treatment may also be related to possible effects on the reassortment of cells in more vulnerable cell cycle phases.
Several experimental settings provide evidence that cell cycle effects are involved in the modulation of the efficacy of combined modality approaches. In this regard, the use of a temperature-sensitive p53 mutant allows the analysis of cell cycle effects very nicely. The underlying hypothesis was that fluoropyrimidine-mediated radiosensitization occurs only in tumor cells that inappropriately enter S-phase in the presence of drug resulting in a subsequent repair defect of the radiation-induced damage. The use of the mutated p53 allowed p21-mediated arrest prior to S-phase entry when cells are grown under 32 °C, in contrast to no arrest in cells grown at the nonpermissive temperatures of 38 °C. The radiation-sensitizing effect of fluoropyrimidine was directly connected to the lacking G1 arrest when cells were grown under nonpermissive temperatures; thus, the fluoropyrimidine-mediated radiosensitization clearly requires progression into S-phase (Naida et al. 1998).
In an extension of these findings, Naida et al. (1998) analyzed the effects of fluorodeoxyuridine on the radiation sensitivity in HT29 and SW620 human colon cancer cells under nearly complete inhibition of thymidylate synthase (both cell lines harbor a similar p53 mutation). Interestingly, only the HT29 cells were sensitized. As an underlying feature, the authors found that only the HT29 cells progressed into S-phase and demonstrated increased cyclin E-dependent kinase activity. In contrast, SW620 cells were found to be arrested just past the G1-S boundary and an increase in kinase activity was not detectable; thus, the findings underline the requirement of an S-phase transition for the efficacy of halogenated fluoropyrimidines in combination with radiation. These findings also highlight the role of molecules involved in cell cycle regulation as key players for the modulation of a combined modality approach (McGinn et al. 1994; Lawrence et al. 1996a, b, c). In addition to the fact that the S-phase transition is required for the radiosensitization effect, it has also been shown that fluoropyrimidines under defined dosage conditions facilitate the accumulation of cells in S-phase (Miller and Kinsella 1992).
In addition to the findings on halogenated fluoropyrimidines, several other sets of data obtained with paclitaxel suggest that an increased radiation sensitivity occurred at the time of a taxane-induced G2-M block; however, the situation for taxane combinations is highly complex in so far as other data provide evidence that the mitotic arrest is not sufficient for the effects of paclitaxel (Geard and Jones 1994; Hennequin et al. 1996). The picture becomes even more complicated when taking into account that radiation was shown to decrease the net killing of taxanes (Sauer et al. 2004). In this regard, it has been shown that the combination of paclitaxel and gamma radiation did not produce a synergistic or additive effect in a breast cancer and epidermoid cancer cell model. Instead, the overall cytotoxicity of the combination was lower than that of the drug treatment alone. Especially apoptosis induction was found to be strikingly reduced. A detailed analysis revealed that radiation resulted in cell cycle arrest at G2 phase preventing the G1-M transition-dependent cytotoxic effects of paclitaxel. Furthermore, radiation inhibited paclitaxel-induced IkappaBalpha degradation and bcl-2 phosphorylation and increased the protein levels of cyclin B1 and inhibitory phosphorylation of p34 (cdc2).
Taken together, the impact of chemotherapy-induced cell cycle alterations as major mechanism for the efficacy of the combined action is still questionable. In clinical settings, the importance of an adequate cell cycle progression for the efficacy of radiochemotherapy approaches has been impressively documented. In the case of a neoadjuvant 5-FU-based radiochemotherapy for rectal cancer, it has been shown that a decrease of the cell cycle inhibitory protein p21 during neoadjuvant treatment is strongly associated with an improved disease-specific survival. This finding has been corroborated by the observation that a parallel increase of the expression level of the proliferation marker Ki-67 is similarly associated with an improved outcome (Rau et al. 2003); thus, preclinical findings on the action of 5-FU in combination with radiation are clearly reflected by clinical observations.
6 Radiation and Platinum-Based Drugs: Example of Clinically Established Combined Modality Treatments
Contemporary clinical concepts include combined administration of radiation and three different platinum compounds (cisplatin, carboplatin, and oxaliplatin) in a variety of common solid tumors. Examples are sites such as head and neck, esophagus, lung, cervix uteri, rectum, and bladder. All these platinum drugs have demonstrated efficacy against a variety of cell lines, tumor xenografts, and human tumors. Yet, their effects vary with several molecular features of the cells, for example, p53 status and expression of drug resistance proteins. Resistance also results from increased expression of the ERCC1 gene (excision repair cross-complementing 1), which is involved in nucleotide excision repair and the removal of DNA interstrand cross-links, and other repair genes (ALTAHA et al. 2004). Both intrinsic and acquired drug resistance have been described. The simultaneous administration of platinum agents can be used to enhance the effects of radiation treatment, aiming either at additive cell kill or true radiosensitization (“radiopotentiation”) within the target volume, or to treat distant, out-of-field tumor sites based on the principle of spatial cooperation. Thereby, it is hoped to achieve a therapeutic gain.
6.1 Cisplatin
Discovered 50 years ago and initially recognized for its bacteriostatic effects (Rosenberg et al. 1965), cis-dichlorodiammine-platinum (II) or cisplatin was found in 1969 to cause antitumor effects. In 1971, the drug was, for the first time, combined with radiation in mice (Zak and Drobnik 1971) and subsequently was the first platinum-based drug entering the clinical practice of radiation oncology. Positive randomized trials were published for cervical cancer and non-small cell lung cancer (Choo et al. 1986, Schaake-Koning et al. 1990). Today, a large variety of administration schedules are in clinical use, including daily dosing with 6 mg/m2, 20 mg/m2/day on day 1–5 and 29–33 of fractionated radiotherapy, 40 mg/m2/day on day 1, 8, 15, 22, 29, and 36, 100 mg/m2/day on day 1, 22, and 43, etc. Heterogeneity of tissue concentration has been examined in various tumor models, for example, in mouse B16 melanoma, human non-small cell lung cancer xenografts (Zamboni et al. 2002), and the human prostate cancer cell line PC-3 M grown in nude mice, where the tumor concentrations ranged from 478 to 937 ppb (Coughlin et al. 1994). A prerequisite for drug efficacy is cellular uptake and avoidance of either efflux or inactivation, for example, by glutathione or other sulfur-containing molecules (Ekshyyan et al. 2009).
After transport into the cell, which appears to be linked to the copper metabolic pathway, but can also take place by passive diffusion, the chloride ligands are replaced by hydroxyl groups. This aquated the reactive form of the drug reacts with several proteins and DNA binding sites, as reviewed by Dewit (1987), and causes DNA-protein linkage and DNA interstrand and intrastrand cross-links interfering with DNA replication and repair, including repair of double-strand breaks (Taylor et al. 1976; Richmond and Powers 1976; Begg 1990; Amorino et al. 1999). The cellular responses include replication arrest, transcription inhibition, cell cycle arrest, and DNA repair via several signal transduction pathways (AKT, p53, MAPK/JNK/ERK, etc.). Cisplatin adducts might be removed by nucleotide excision repair mechanisms, following first-order kinetics. In cell culture, knockout of the nonhomologous endjoining (NHEJ) repair pathway did not change the response to cisplatin, whereas mutation of the homologous recombination repair pathway through XRCC3 resulted in increased radiation and cisplatin sensitivity (Raaphorst et al. 2005). Other data also demonstrate that yeast mutants in double-strand break repair by NHEJ and mutants in base excision repair showed no sensitivity to cis- or oxaliplatin (Wu et al. 2004). Other authors suggest that the cellular responses to cisplatin depend on DNA-activated protein kinase and DNA polymerase eta (Turchi et al. 1997; Albertella et al. 2005). It has been postulated that the loss of DNA mismatch repair is linked to the failure in detecting the DNA damage caused by cisplatin and to the lack of signal triggering the cell death mechanisms (Fink et al. 1996). Putative defective repair of oxidative damage also resulted in sensitivity to cis- and oxaliplatin in yeast (Wu et al. 2004). Cell killing after higher drug doses appears apoptosis-related, whereas after lower drug doses failure to overcome a G2 block is more important (Ormerod et al. 1994). In p53-mutated 9 L rat gliosarcoma, intraperitoneal cisplatin (1 mg/kg) led to an increase in micronuclei formation, most likely indicating induction of mitotic catastrophe, but produced little or no apotosis (Driessens et al. 2003). The drug is not cell cycle specific.
Concomitant application with radiation might reduce the likelihood of acquired resistance compared to induction chemotherapy and reduces the overall time from initiation of any treatment to completion of local radiotherapy. In early experiments, cisplatin reduced the repair of sublethal radiation-induced damage, as defined by split-dose recovery, in exponentially growing rat hepatoma cells. In plateau phase, radiation sensitization was found (Carde and Laval 1981). Later on, Dolling et al. reported inhibition of DNA double-strand break repair when cisplatin was administered prior to radiation (Dolling et al. 1998). When cis- or carboplatin were present at the time of irradiation, higher enhancement ratios were observed compared to administration 24 h prior to or 3 h after irradiation (Schwachöfer et al. 1991). Overgaard and Khan examined mouse mammary tumors exposed to radiotherapy with or without 6 mg/kg cisplatin administered intraperitoneally 30 min before irradiation (Overgaard and Khan 1981). The dose modification factor in these TCD50 experiments was 1.8, compared to 1.3 if the drug was given 30 min or 4 h after irradiation. Kallman reported a large set of animal experiments where fractionated radiotherapy was combined with cisplatin (Kallman et al. 1992). Tumor growth inhibition (RIF-1 and SCCVII tumors) and three normal tissue endpoints (duodenal crypt cell survival, lung toxicity after 5 and 10 months, respectively) were assessed. With few exceptions, the greatest therapeutic gain was achieved with multiple doses of cisplatin administered simultaneously with 5 daily fractions of radiotherapy.
6.2 Carboplatin
In chronological order, cis-diammine (1,1-cyclobutanedicarboxylate) platinum (II) (carboplatin) was the second compound that became part of clinical treatment protocols. In 20 cervical cancer cell lines, 30 % of the tumors resistant to cisplatin were also resistant to carboplatin (Monk et al. 1998). However, the toxicity profile is advantageous. Carboplatin has greater chemical stability than cisplatin and longer half-lives of ultrafilterable platinum. The terminal half-lives are comparable (5–6 days). It forms a similar spectrum of DNA adducts as cisplatin with slightly different sequence preferences (Blommaert et al. 1995). In order to obtain equivalent DNA platination levels, higher concentrations of carboplatin are needed. The sensitivity of squamous cell carcinoma cell lines to carboplatin differs at least by a factor of 4 (pekkola-Heino et al. 1992). In these experiments, no cross-resistance was observed between inherent radiosensitivity and chemosensitivity. When administered 1 h before irradiation to carboplatin-sensitive cell lines, additive effects were observed.
Carboplatin enhances the production and persistence of radiation-induced DNA single-strand breaks (Yang et al. 1995) and reduces cell survival after radiation treatment measured by clonogenic assays (supraadditive effect) (Scalliet et al. 1999). In two cell lines proficient in both excision repair and DNA double-strand break repair, and in a cell line deficient in nucleotide excision repair, carboplatin before and during irradiation enhanced radiation-induced cell killing (Yang et al. 1995). In air and under hypoxic conditions, the enhancement was characterized as both a reduction in the shoulder region of the survival curves (reduced Dq) and a reduction in D0 in the terminal region of the survival curves. Only the latter effect was observed in a cell line deficient in DNA double-strand break repair. Enhancement ratios ranged from 1.3 to 1.7, irrespective of oxygenation. Drug levels sufficient to produce cytotoxicity by themselves were required for the effect of radiation enhancement. In the extreme case, only 1/30 of the drug concentration required for other cell lines produced enhanced cell killing, as seen in the intrinsically sensitive UV41 cells. In a mouse model of Ehrlich ascites tumors, combined treatment was compared to a single dose of carboplatin alone and a single dose of radiation alone. Tumor growth delay was better with simultaneous combined treatment than each modality alone (Aratani et al. 1997). In mouse lip mucosa there was no influence of carboplatin on the response to single-dose irradiation, the capacity to repair sublethal radiation damage, and the ability to repopulate (Landuyt et al. 1987).
6.3 Oxaliplatin
Oxaliplatin (1,2-diaminocyclohexaneoxalato platinum (II)) is a third-generation lipophil platinum drug. The drug has less hematological toxicity and lacks nephrotoxicity. The dose-limiting side effect is peripheral neurotoxicity. The parent compound undergoes hydrolysis to form the effective reactive species. Cisplatin-resistant cell lines tend not to be resistant to oxaliplatin. Furthermore, oxaliplatin was more effective in several animal tumor models. Despite the fact that oxaliplatin forms covalent adducts with DNA that have similar sequence and region specificity to those formed by cisplatin, they are more cytotoxic (Pendyala and Creaven 1993; Woynarowski et al. 1998). It has been shown that cellular proteins, for example, mismatch repair proteins, recognize oxaliplatin adducts differentially and that the effects of oxaliplatin when compared to cisplatin are less dependent on intact mismatch repair (Raymond et al. 2002). Further differences exist regarding postreplicative bypass mechanisms. DNA polymerase beta and eta catalyze translesion synthesis past certain oxaliplatin adducts with greater efficiency than past cisplatin adducts. Further data provide a link between oxaliplatin sensitivity and DNA repair involving DNA polymerase beta, the major DNA polymerase involved in base excision repair (Yang et al. 2010). Like cisplatin, interstrand cross-links appear to be important toxic lesions caused by oxaliplatin (Wu et al. 2004).
Oxaliplatin showed synergistic effects with 5-FU as well as radiosensitization in human colon cancer cells (Kjellstrom et al. 2005; Folkvord et al. 2008). Additional data suggest that these effects might vary with p53 status of the colon cancer cell line (Magne et al. 2003). In p53 wild type SW403 cells, additive-synergistic effects were observed when the best sequence was administered, that is, irradiation 2 h before or at mid-drug application. Oxaliplatin was given over 2 h followed by 5-FU/folinic acid over 24 h. In p53-mutated WiDr cells, additive-antagonistic effects were seen, irrespective of sequence. Radiosensitization was also found in head and neck cancer cell lines (Espinosa et al. 2005). Oxaliplatin sensitized human HeLa and SiHa cells to ionizing radiation (Yang et al. 2009). In this model, drug pretreatment enhanced the cell cycle arrest in the G2/M phase and the radiation-induced mitotic catastrophe. Oxaliplatin modulated radiation-induced DNA double-strand breaks, as indicated by delayed abrogation of gamma-H2AX, attenuation of radiation-induced phosphorylation of ataxia telangiectasia-mutated kinase, and checkpoint kinase 2. In vivo data generated with transplanted mammary adenocarcinoma, but with the disputable endpoint of tumor growth delay rather than cure, suggest that sequence and time interval of radiation treatment and oxaliplatin do not influence the results for single-dose treatments (10 Gy and 6 or 10 mg/kg) (Cividalli et al. 2002). With 10 daily fractions of 2 Gy, however, the drug increased the efficacy of radiation treatment better when administered only twice during the treatment course as compared to daily.
7 Combined Modality Reirradiation
Two major examples where reirradiation often is combined with concomitant chemotherapy are head and neck tumors and rectal cancer. As already discussed, a therapeutic gain is expected on the basis of different models and extrapolation of results from first-line treatment approaches. With regard to the latter, there have been several meta-analyses of randomized trials performed suggesting a survival benefit with use of concurrent chemotherapy with external beam radiotherapy in head and neck cancer (Munro 1995; El-Sayed and Nelson 1996; Pignon et al. 2000, 2009). Among these meta-analyses, one was based on the collection of updated individual patient data. This Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC) previously confirmed a survival benefit for concurrent radiochemotherapy and was later updated to include 24 new trials. This update reconfirmed the benefit of concurrent treatment for patients with locoregionally advanced disease, yielding an HR of 0.81 (p < 0.001), with an absolute survival benefit of 8 % at 5 years. The 5-year absolute benefits associated with concomitant chemotherapy were 8.9 %, 8.1 %, 5.4 %, and 4 % for oral cavity, oropharynx, larynx, and hypopharynx tumors, respectively (Blanchard et al. 2011). The investigators found no significant difference between mono- and poly-chemotherapy regimens used concurrently. The benefit was found to be more pronounced for platinum-based combinations with or without 5-FU. In addition, the benefit of adding chemotherapy was more significant in younger patients and progressively decreased to become non-detectable beyond the age of 70. Together, these data support the use of concurrent radiochemotherapy as a standard of care first-line treatment option for stage III and IV head and neck cancer patients who can tolerate systemic chemotherapy.
In the reirradiation setting, such high-quality randomized trials or even meta-analyses of randomized trials comparing reirradiation with reirradiation and chemotherapy are lacking. Therefore, the question arises whether or not the theoretical advantages of combined modality treatment are detectable in the published literature. Table 1 summarizes the recently published head and neck cancer reirradiation trials that might shed some light on this question. The trials are basically retrospective in nature, of limited size and thus underpowered, included heterogeneous patient groups with tremendous variation in tumor biology and size, and applied quite individual treatment. Often, second primary tumors and locoregional relapses are lumped together. Between 20 and 92 % of the patients received chemotherapy along with reirradiation, the others reirradiation alone. Unexpectedly, no major impact of the combined approach is obvious, regardless of endpoint. However, none of the trials was really designed to prove the superiority of radiochemotherapy in this setting. The lack of efficacy might just reflect selection bias. More favorable patient groups might have received reirradiation alone, the others combined treatment. Alternatively, chemotherapy might not have improved the outcome, for example, because many patients were reexposed to platinum-based regimens, which they already had received during previous treatment, or because the drugs did not gain sufficient access to the tumor cells (altered perfusion and microenvironment after previous therapy, increased fibrosis, see Fig. 5). A small study in squamous cell head and neck cancer with 29 patients (no second primaries included) who received reirradiation with platinum-based chemotherapy was published by Nagar et al. (2004). The median dose was only 34 Gy. This study suggested better efficacy in patients previously, that is, in first line, treated with radiation alone as compared to radiochemotherapy and that rechallenge with platinum-based combination treatment might not work very well. Theoretically, agents with different modes of action might be preferable (Table 2 ). The highest level of evidence results from a small phase II randomized trial in patients with recurrent nasopharyngeal cancer (Guan et al. 2016). These relatively young patients were required to have KPS ≥70 and minimum time interval of 6 months. All 69 patients received IMRT (27 fractions of 2.2 Gy, total dose 60 Gy, inclusion between 2002 and 2008). The investigational arm also received weekly cisplatin 30 mg/m2. Despite the small sample size, overall survival was the primary endpoint. Median follow-up was 35 months. Most patients had rT3-4 N0 tumors and median volume was 28 and 29 cm3, respectively. Initial treatment was mostly 2-D radiotherapy alone, but 29 % had received radiochemotherapy before (regimens not reported in detail). In the investigational arm, more patients developed mucositis and grade 3–4 hematological toxicity. Late toxicity was not significantly increased. Survival was significantly better in the combined modality arm. In multivariate analysis, survival was associated with age, rT stage, clinical stage, and treatment arm. These analyses did not include comparisons of initial radiotherapy alone vs. radiochemotherapy.

Table 1
Agents that have been used in published reirradiation studies
Cisplatin, carboplatin |
Docetaxel, paclitaxel |
Lomustine, fotemustine |
Temozolomide |
Gemcitabine |
Hydroxyurea |
5-Fluorouracil, capecitabine |
Topotecan |

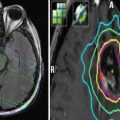
Fig. 5
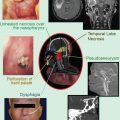
Computed tomography scan of a patient with recurrent squamous cell carcinoma of the oropharynx. Note the large lymph node metastasis which contains necrotic areas (white arrow). The inserted cartoon is an example of histological and molecular heterogeneity within a recurrent tumor. 1 tumor subvolume with insufficient blood perfusion resulting, for example, in hypoxia or poor access of systemically administered drugs. 2 cells that are primarily resistant to the mechanism of a given drug. 3 cells with acquired resistance. 4 cells that can be killed by drug treatment. 5 cells that can be killed by radiation. 6 cells that can be killed by combined radiation and drug
Table 2
Overview of clinical reirradiation studies addressing the added value of concomitant chemotherapy (Ctx), some studies included cases with additional induction and/or adjuvant Ctx
Reference
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|