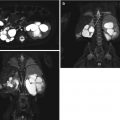
Fig. 10.1
Optimal evaluation by ultrasound requires identification of the calyceal system, renal pelvis (a), the ureteropelvic junction, and as much of the upper ureter as possible (b). Transverse images of the bladder with the relationship of the distal ureter (c) and the ureterovesical junction (d) are shown. U ureter, B bladder, UVJ ureterovesical junction, UPJ ureteropelvic junction
Prenatal Ultrasound
Perinatal urology has changed significantly since the advances of prenatal ultrasound. Prenatal ultrasound is a noninvasive tool that can determine gestational age, fetal number, viability, placental location, and some fetal anomalies. Although prenatal ultrasound screening may detect congenital abnormalities, the benefit of this knowledge in measured postnatal outcomes is unproven. The a priori deduction that early diagnosis can prevent future complications is controversial, as in many cases, early diagnosis may lead to unnecessary interventions. Additionally, it can lead to significant parental anxiety.
Although the use of prenatal ultrasound in diagnosing genitourinary abnormalities has not been proven to change postnatal outcome relative to renal function, more women are undergoing routine prenatal counseling and screening ultrasound [1].
Diuretic Renogram
In addition to anatomic detail, it is important to determine the degree of obstruction and the relative function of the kidneys. The purpose of a diuretic renogram is to distinguish between nonobstructive hydronephrosis and mechanically obstructed hydronephrosis (Figs. 10.2 and 10.3). Historically, the intravenous urogram was the study of choice, often requiring delayed images with dilute contrast in an attempt to identify the level of abnormality. The IVU has been replaced primarily by nuclear medicine as the functional modality of choice. Technetium-99m is the ideal agent because of availability, short half-life, low-energy photopeak (140 keV), and ability to bind to agents such as MAG3 (mertiatide). Appropriate gamma camera and collimator are necessary. Because radioactive isotope is excreted into the bladder, care should be taken that the bladder is emptied by continuous bladder drainage or as soon as possible after the exam, especially in females where the ovaries are adjacent. This is aided by appropriate hydration. Because time is required to acquire images, swaddling of infants or sedation if the child cannot cooperate may be necessary.
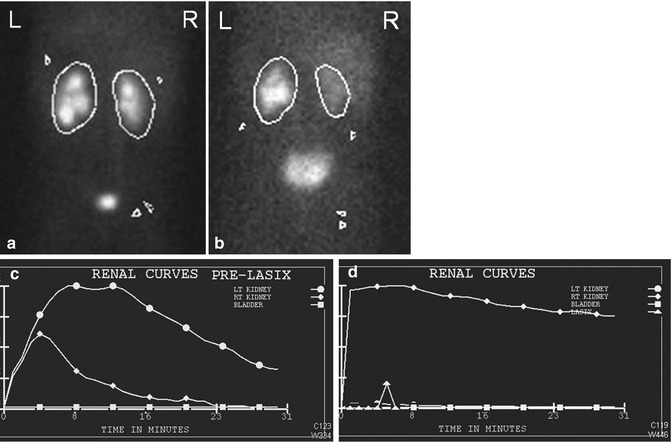
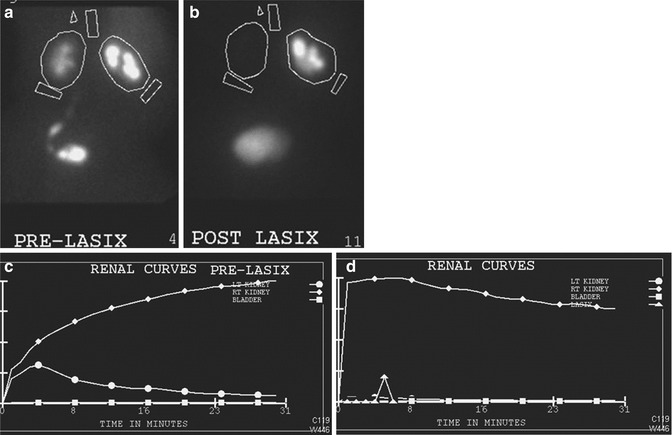

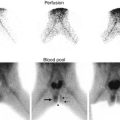
Fig. 10.2
This Tc-99m Mag3 diuretic renogram demonstrates delayed emptying of the left renal pelvis on the pre diuretic urogram (a) and flow curve (b) but with improved excretion post diuretic (c and d)

Fig. 10.3
If the degree of obstruction at the UPJ is significant, there will not be appreciable excretion of the isotope before or after diuretic administration. Here, the post diuretic images (c) and flow chart (d) show little change from pre diuretic (a and b)
Magnetic Resonance Urography
Magnetic resonance urography (MRU) is a more recently developed modality for evaluation of the genitourinary tract, particularly with obstruction. Magnetic resonance imaging was first introduced in 1986 [2]. MRU later came along and has been used to not only assess differential function and drainage as with a diuretic renogram but also to provide anatomic detail of the kidneys and collecting system. MRU also avoids exposure to radiation and iodine contrast. Volumetric differential renal function (DRF) is calculated by comparing the volume of enhancing renal parenchyma of each kidney prior to contrast excretion in the kidneys. Gadolinium-diethylenetriaminepentaacetate dimeglumine (Gd-DTPA) is the contrast used. Sequential imaging allows for evaluation of contrast parenchymal concentration, excretion, and drainage [3]. The anatomic detail of MRU is considered its main advantage. With a variety of technical sequences, exquisite anatomic detail is possible. With administration of MR contrast agents, such as gadolinium, functional information is possible as well. With the addition of diuretics, dynamic information is obtained as with renal scintigraphy but with the additional benefit of superior contrast, spatial, and temporal resolution. The major disadvantages are cost, access, and time required for imaging acquisition, necessitating sedation in younger or certain disabled patients.
Computed Tomography
Computed tomography is a modality that provides excellent anatomic detail. With intravenous contrast, it also performs the additional role of a functional modality. Non-contrast CT has become the modality of choice for the detection of calculi in adults. Contrast CT can demonstrate a delayed nephrogram, as was seen with intravenous urography, or, with delayed images, can illustrate the detail of the etiology of the obstruction such as mass, stricture, or congenital abnormality. Radiation exposure to children from CT scans can be quite high, and evaluation by alternative imaging procedures when possible is the recommended approach to evaluation.
Fluoroscopy/Voiding Cystourethrogram
The voiding cystourethrogram remains the best imaging modality to diagnose bladder outlet obstruction because it defines the anatomy of the bladder, bladder neck, and urethra. Imaging of the urethra is best obtained during the voiding phase of the VCUG.
It can, however, result in significant radiation exposure. Advances in equipment development now allow varying degrees of pulsed fluoroscopy in which the X-ray beam is functioning only intermittently rather than continuously. In addition, “fluoro store” is a function where the image on the monitor screen can be stored without requiring additional spot film exposures.
Use of Imaging Modalities in Specific Pathologic Entities
Prenatal Hydronephrosis
Prenatal hydronephrosis, or dilation of renal collecting system, is the most common abnormality detected on prenatal ultrasound. It is estimated that antenatal hydronephrosis is detected in 1–5 % of all pregnancies [4]. There are many etiologies of prenatal hydronephrosis, including transient and non-transient hydronephrosis, upper tract and lower tract obstruction, and vesicoureteral reflux (VUR) (Table 10.1).
Table 10.1
The etiology of ANH
Etiology | Incidence (%) |
|---|---|
Transient hydronephrosis | 41–88 |
UPJ obstruction | 10–30 |
VUR | 10–20 |
UVJ obstruction/megaureters | 5–10 |
Multicystic dysplastic kidney | 4–6 |
PUV/urethral atresia | 1–2 |
Ureterocele/ectopic ureter/duplex system | 5–7 |
Others: prune belly syndrome, cystic kidney disease, congenital ureteric strictures, and megalourethra | Uncommon |
The Society for Fetal Urology (SFU) categorizes antenatal hydronephrosis in the second and third trimester based on the pelvic anterior-posterior diameter (APD), which is obtained in the axial plane (Table 10.2). Factors that influence APD are maternal hydration, gestational age, and degree of bladder distension [5–8]. The degree of hydronephrosis has been shown to correlate with an increasing risk of certain postnatal pathology, such as ureteropelvic junction obstruction and posterior urethral valves (Table 10.3).
Table 10.2
Definition of ANH by APD
Degree of ANH | Second trimester (mm) | Third trimester (mm) |
|---|---|---|
Mild | 4 to <7 | 4 to <9 |
Moderate | 7 to ≤10 | 9 to ≤15 |
Severe | >10 | >15 |
Table 10.3
Risk of specific postnatal pathologic conditions by the degree of ANH
% ANH [95 % CI] | |||
|---|---|---|---|
Mild | Moderate | Severe | |
UPJ | 4.9 [2.0–11.9] | 17.0 [7.6–33.9] | 54.3 [21.7–83.6] |
VUR | 4.4 [1.5–12.1] | 14.0 [7.1–25.9] | 8.5 [4.7–15.0] |
PUV | 0.2[0.0–1.4] | 0.9[0.2–2.9] | 5.3[1.2–21.0] |
Ureteral obstruction | 1.2 [0.2–8.0] | 9.8 [6.3–14.9] | 5.3 [1.4–18.2] |
Other | 1.2 [0.3–4.0] | 3.4 [0.5–19.4] | 14.9 [3.6–44.9] |
In assessing the genitourinary system of a fetus on prenatal ultrasound, it is important to not only identify the presence of hydronephrosis but also to further characterize the kidneys, ureters, bladder, and urethra (Fig. 10.4). In examining the kidney, one should note their size, single or duplex collecting system, echogenicity of the renal parenchyma, presence of hydronephrosis, and presence of cystic structures. All of this information can suggest specific diagnoses.
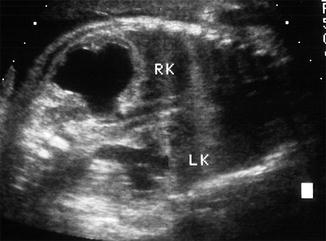

Fig. 10.4
A coronal image of a fetus demonstrates significant hydronephrosis of the right with cortical thinning (RK) and moderate hydronephrosis on the left with more substantial cortex (LK)
The echogenicity of a fetal kidney should be less than that of the liver and spleen. The medullary pyramids should be lucent. Increased echogenicity can suggest dysplasia or high-grade obstruction and is commonly seen in multicystic dysplastic kidney (MCDK) and in autosomal recessive polycystic kidney disease (ARPKD). Hydronephrosis can be due to transient or non-transient physiologic hydronephrosis, obstruction, or vesicoureteral reflux (VUR). Cystic structures in the renal parenchyma may be confused for hydronephrosis. Cysts, however, do not communicate with one another and can be a sign of MCDK or autosomal dominant polycystic kidney disease (ADPKD).
Normal ureters are not seen on prenatal ultrasound. The presence of a dilated structure between the kidneys and the bladder or the presence of cystic structures posterior to the bladder on transverse imaging suggests dilated ureters. Dilated ureters can be seen with VUR, various types of megaureters, duplication anomalies including ureterocele, and bladder outlet obstruction.
It is also important to evaluate the bladder on prenatal ultrasound. Increase in size or the presence of a thick bladder wall can indicate bladder outlet obstruction. In the case of posterior urethral valves, the posterior urethra as well as the bladder is distended giving a “keyhole” configuration.
Postnatally, the voiding cystourethrogram is the best imaging modality to diagnose bladder outlet obstruction because it defines the anatomy of the bladder, bladder neck, and urethra during its voiding phase. In bladder outlet obstruction, the bladder wall is usually thickened, and a VCUG can demonstrate a trabeculated appearance to the bladder wall or bladder diverticula. Fluoroscopy can also help determine whether hydroureteronephrosis is secondary to obstruction or due to vesicoureteral reflux. A dilated, thick-walled bladder or severely enlarged bladder is highly suggestive of bladder outlet obstruction, including posterior urethral valves, urethral atresia, congenital urethral stricture, or urethral polyp. It can also be the result of extrinsic compression of the bladder outlet as with hindgut duplication anomalies or pelvic masses. The presence of a cystic structure within the bladder can be a sign of a ureterocele, which is most commonly associated with duplication and can cause obstructive uropathy and hydronephrosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree