Tissue
Muscle
Smooth muscle
Skeletal muscle
Connective tissue
Fat tissue
Peripheral nerves
Blood vessels
Table 23.2
Common soft tissue sarcoma subtypes
Subtype |
|---|
Leiomyosarcoma |
Rhabdomyosarcoma |
Subtypes |
Alveolar (pediatric) |
Embryonal (pediatric) |
Pleomorphic (adult) |
Fibrosarcoma |
Malignant fibrous histiocytoma (MFH) |
Liposarcoma |
Subtypes |
Well differentiated |
Myxoid |
Round cell |
Pleomorphic |
De-differentiated |
Malignant peripheral nerve sheath tumor |
Malignant schwannoma |
Angiosarcoma |
Hemangiopericytoma |
Lymphangiosarcoma |
Pleomorphic sarcoma |
Synovial sarcoma |
Alveolar soft part sarcoma |
Gastrointestinal stromal tumor |
Table 23.3
Common pediatric sarcomas
Tumor histologic subtype |
|---|
Osteosarcoma |
Conventional types |
Chondroblastic |
Fibroblastic |
Osteoblastic |
Parosteal |
Telangiectatic (vascular) |
Small cell |
Periosteal |
High-grade surface types |
Secondary |
Chondrosarcomas |
Central, primary, secondary |
Peripheral |
Mesenchymal |
Clear cell |
Ewing sarcoma |
Extraosseous Ewing sarcoma |
Askin tumor (chest wall primary tumor) |
Peripheral primitive neuroectodermal tumor/peripheral neuroepithelioma (PNET) |
Giant cell tumor |
Malignant giant cell tumors |
Notochordal tumors (i.e., chordoma) |
Adults with sarcomas have an approximately 50% 5-year overall survival rate [1]. Patients with poor outcomes succumb to metastatic disease, local recurrence, or both. Local recurrence after resection occurs in 25% of patients with soft tissue sarcomas, an event that portends eventual metastatic spread [1]. Typically, pulmonary metastases are the predominant site followed, by bone and other soft tissue locations. Disease prognostic factors include patient age, tumor grade (a risk assessment based on histopathological characteristics), tumor depth (superficial or deep), size, histologic subtype, surgical margin status at resection, and disease status (primary or recurrent disease) [1].
Adverse prognostic factors affecting patient survival with soft tissue sarcomas are deep tumor location, a tumor greatest dimension of 5 cm or larger, locally recurrent disease, proximal lower extremity site, and microscopic or grossly involved tumor margins at surgical resection [1]. Sixty percent of adult soft tissue sarcomas occur in the extremities. The proximal lower extremity is a common location for soft tissue sarcomas in adults, which increases risk for poor outcome because of its association with other poor risk factors. Poor risk factors include depth beneath the fascial plane and large tumor growth before diagnosis and treatment. In most patients, once metastases occur, survival time diminishes rapidly, despite salvage combination therapy regimens [4]. Additionally, there are a number of treatment protocols for adults with sarcomas. Patients with low-grade, small sarcomas are likely to proceed to tumor resection with the possibility of adjuvant radiation. Those with high-grade large tumors are often treated with doxorubicin-based neoadjuvant chemotherapy with or without radiation followed by resection and adjuvant therapy. Surgical resection ranges from simple excision with wide margins to complex limb salvage procedures.
Adult bone tumors are most often cartilaginous histologic types. Usually, only the high-grade tumors are metastatic. The intermediate-grade and low-grade cartilage tumors have high morbidity rates due to repeated local recurrence and poor responses to neoadjuvant chemotherapy. The bone tumors are less frequent in older adults, and osteosarcomas and Ewing sarcomas are typically seen in younger adults. They are rarely observed in adults over age 50. The bone tumors are metaphyseal tumors that primarily spread to the lungs. Risk factors for osteosarcoma include prior radiation therapy and germ line mutations [5]. There are a few soft tissue sarcoma histologic types that can also occur in bone, such as synovial sarcoma, a cancer that is more biologically consistent with its soft tissue tumor counterparts. As in the soft tissue sarcomas, prognostic factors include the presence of metastases at presentation and chemotherapy resistance. Treatment for the bone sarcomas in adults is similar to the soft tissue sarcomas. Neoadjuvant doxorubicin-based chemotherapy is given and is followed by surgical resection and additional chemotherapy. Five-year survival depends largely on the level of chemotherapy resistance, which is approximately 60%. The lower-grade osteosarcomas are treated with wide local excision and limb salvage procedures and typically recur only in about 5% of the cases; however, these recurrences decrease 5-year survival significantly if the tissue recurrence degenerates into a higher grade or undifferentiated tumor type.
The pediatric sarcomas are dominated by the osteosarcomas and Ewing sarcomas. The osteosarcomas have a peak incidence in patients in their second decade of life, and the age range begins at approximately age 10. They predominately present in the extremities, and 15–20% of patients have pulmonary metastases at the time of diagnosis. In addition to prior radiation, the list of possible predisposing factors includes germ line p53 mutations, growth abnormalities, trauma, fetal x-ray exposure, and parental x-ray exposure. Metastases present at the time of diagnosis are prognostic of poor outcome. Treatment with intensive neoadjuvant chemotherapy results in 60–80% 5-year survival [5]. The treatment response rate and overall survival rate for pediatric sarcomas are significantly higher than that for adults with osteosarcomas.
The Ewing sarcomas are also a bone tumor of adolescence with a peak incidence around the age of 15 [5]. They most commonly present as a painful mass in the extremities and more often are metastatic at presentation. Bone metastases (15–30% of patients), lung metastases, and soft tissue involvement adjacent to the primary tumor mass are common. Presumably, these tumors arise from the postsynaptic neural crest precursors and are synonymous with the Peripheral Neuroectodermal Tumors (PNET), which occur more predominantly in soft tissues [5]. The presence of the EWS-FL1 fusion protein in the tumor confers poor prognosis. There are no known risk factors for causing these tumors, and they are rarely associated with other syndromes.
Patients with sarcoma have highly variable characteristics and clinical outcomes. Sarcomas are often multidrug resistant [6, 7]. The multidrug resistance systems are tissue transporters that remove toxins from tissues. Normally occurring in tissue epithelial linings in the intestines, kidneys, and the blood brain and placental barriers, their activity is upregulated in sarcomas [8–10]. This is a major cause of increased treatment failure and disease progression. The pathobiological basis of multidrug resistance activity and other sarcoma characteristics is their complexity, that is likely a result of a substantial variety of genetic abnormalities. These consist of broad categories of tumor-specific translocations that contribute to tumor diagnostic criteria and to sarcoma subtypes that have severe abnormalities in genetic and chromosomal instability [11]. Sarcomas in almost all body locations present unique challenges for diagnosis and management. These challenges have presented opportunities for evaluation and validation of new imaging techniques.
Approaches to Staging
Because sarcomas are difficult to risk-stratify for outcome, well-described staging systems have been devised to improve patient treatment and outcome. The American Joint Committee on Cancer (AJCC) criteria is often used clinically for staging soft tissue sarcoma patients (Tables 23.4 and 23.5). This system undergoes periodic analyses and revisions. The current AJCC soft tissue sarcoma system stratifies patient tumor by size (T1 ≤ 5 cm, T2 ≥ 5 cm). Additionally, the tumor is subsequently characterized by histologic grade (I–III), the presence or absence of nodal and distant metastasis, and a special description of location with respect to fascial planes, which is denoted as either “a” or “b.” The designation “a” denotes superficial location and “b” refers to deep tumor locations. Revisions that have been suggested include categories for very large tumors (T3 ≥ 15 cm), specific primary tumor sites, tumor margin statuses, histologic subtypes, the presence or absence of tumor local recurrences, and specific markers for biologic aggressiveness.
Table 23.4
AJCC definition of TNM and stage grouping for soft tissue sarcoma
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
T1 | Tumor 5 cm or less in greatest dimensiona |
T1a | Superficial tumor |
T1b | Deep tumor |
T2 | Tumor more than 5 cm in greatest dimensiona |
T2a | Superficial tumor |
T2b | Deep tumor |
aSuperficial tumor is located exclusively above the superficial fascia without invasion of the fascia; deep tumor is located either exclusively beneath the superficial fascia, superficial to the fascia with invasion of or through the fascia, or both superficial yet beneath the fascia | |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1b | Regional lymph node metastasis |
bPresence of positive nodes (N1) in M0 tumors is considered Stage III | |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Table 23.5
AJCC anatomic stage and prognostic groups for soft tissue sarcoma
Group | T category | N category | M category | |
|---|---|---|---|---|
Stage IA | T1a | N0 | M0 | G1, GX |
T1b | N0 | M0 | G1, GX | |
Stage IB | T2a | N0 | M0 | G1, GX |
T2b | N0 | M0 | G1, GX | |
Stage IIA | T1a | N0 | M0 | G2, G3 |
T1b | N0 | M0 | G2, G3 | |
Stage IIB | T2a | N0 | M0 | G2 |
T2b | N0 | M0 | G2 | |
Stage III | T2a, T2b | N0 | M0 | G3 |
Any T | N1 | M0 | Any G | |
Stage IV | Any T | Any N | M1 | Any G |
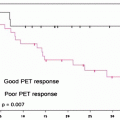
Sarcoma groups are incorporating these parameters in clinical practice more routinely each year. The pathologic tumor grade is an important component of any tumor staging system; however, there are pitfalls that exist in its application to individual patient tumors. The most widely used sarcoma grading system is the FNCLCC designed by the French Fédération Nationale des Centres de Lutte Contre le Cancer [12]. Even with well-defined grading systems, there are several challenges with grading soft tissue sarcomas. Tumors for which it is difficult to assign histologic grade because of their rare characteristics include tumors where the grade applied does not provide additional prognostic information beyond what is conferred from accurately determining the tumor histologic subtype. These include differentiated liposarcomas, the tumors that are considered “ungradable”; the epithelioid, clear cell, and angiosarcomas; and those where the histologic grade does not predict outcome well, such as the malignant peripheral nerve sheath tumors [13]. These findings reflect the heterogeneity of the biologic behavior in the sarcomas, and they provide the stimulus for incorporation of newer systems that integrate tumor characteristics from a number of different sources that identify patient risk for malignant behavior more accurately.
A comparative analysis of the AJCC, Memorial Sloan-Kettering Cancer Center system, and the Surgical Staging System of the Musculoskeletal Tumor Society soft tissue sarcoma staging systems found that schemes that include tumor depth, grade, and size are most predictive of tumor relapse in patients with extremity tumors. Wunder et al. suggest that these systems can be used to identify patients that are most likely to benefit from participation in adjuvant therapy trials [14].
Bone tumor staging follows the schemes utilized for other tumors, with a few notable exceptions. The primary tumor is assessed by whether it is confined to cortex (T1) or if it extends beyond this bone structure (T2) [15]. The tumor histologic type and grade predominate the staging criteria for prognosis. The presence or absence of distant metastases is also significant. As with soft tissue sarcoma staging, bone sarcoma staging includes a careful examination of the lungs to discover metastases, which are often present at the time of initial diagnosis. Inclusion of normograms for improved prognosis has also been developed, and their use may help to improve selection of multimodality treatment for bone sarcoma patients [16].
Imaging plays an increasingly important role in sarcoma staging. All types of examinations—including plain films, chest x-ray, CT with and without contrast, MRI, and [18F]FDG PET—are now routinely used in combination to stage sarcoma patients and to restage patients when treatment decisions are re-contemplated throughout different stages of diagnosis and during initial treatment. The bone scan is still routinely used because of its sensitivity in the detection of bone metastases, although most sarcomas predominantly metastasize to the lungs. Several types commonly have associated bone metastases, such as the osteosarcomas, Ewing sarcomas, and highly undifferentiated tumors. Imaging methods and their uses in sarcoma management are discussed more thoroughly in the following sections.
Radiopharmaceuticals
Disease staging in sarcoma involves all aspects of imaging the body with radiopharmaceuticals. Since these tumors vary considerably in their presentation, local recurrence rates, and patterns of metastatic spread, different imaging studies that provide complementary information for disease staging are used in clinical practice. These are for characterizing extent of tumor, presence of bony, lymph node, and soft tissue metastases. Some tumor types involve multifocal primary disease presentation, synchronous tumors, and skip metastases. In most sarcoma clinics, a combination of nonspecific, highly sensitive, and tissue-specific imaging agents are used in diagnosis, staging, response, termination, and restaging aspects of patient management.
99mTc-MDP
The standard bone scan is widely used in oncology for staging the skeleton for the presence of bony metastases. Sarcomas generally show osteoblastic behavior and are less likely to show lytic metastases. Often, as in the case of bone tumors, they show either a blastic or lytic appearance. For soft tissue masses, the bone scan is a standard part of disease staging. Patients with bony metastases show increased uptake in typical metastatic decrease patterns in the axial skeleton and extremities. The presence or absence of uptake is often diagnostic in separating more benign or locally aggressive tumors from malignant types. Bone scans also have special utility in primary bone tumors. These tumors form malignant osteoid matrices that are undergoing disordered calcification; therefore, they are 99mTc-MDP avid (Fig. 23.1). Both the osteosarcomas and some chondrosarcomas with osteoblastic differentiation contain malignant osteoid. Ewing sarcomas cause bone destruction and reactions that result in bone scan positivity. Primary bone tumors have frequent bone metastases and can present with skip lesions in the affected bone. The osteosarcomas frequently have bone metastases, but bone scan positive appearance in multiple sites is common in Ewing sarcoma. In this latter group of tumors, metastases are often present at the time of diagnosis in both bone and soft tissue PNET primary tumors.
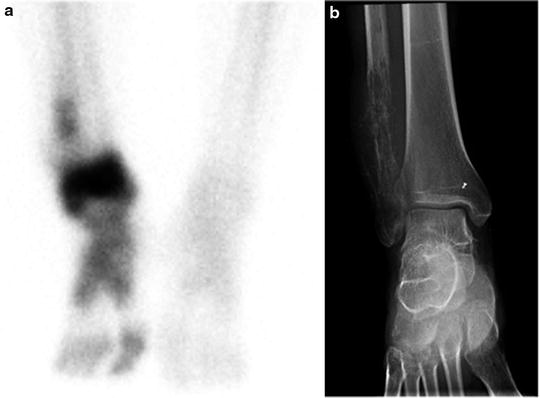

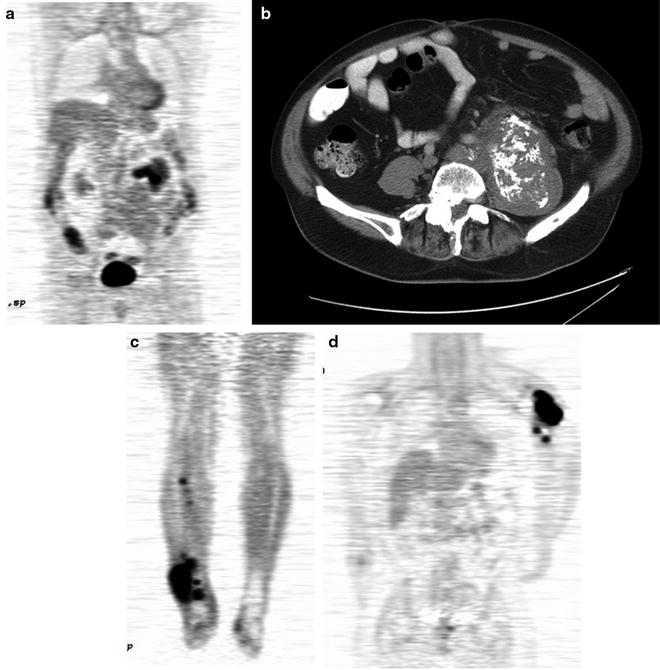
Fig. 23.1
Bone scans in sarcomas. (a and b) A right ankle Ewing’s sarcoma, with marked uptake in the proximal foot. The involved area shows fibula involvement with soft tissue extension
The bone scan is also a sensitive method for indentifying osteosarcoma pulmonary metastases (Fig. 23.2). These are often highly 99mTc-MDP avid. Some clinicians use the relative decrease in bone scan uptake in pulmonary metastases as an indicator of treatment response and suitability for wedge resection metastasectomy. In osteosarcoma patients with limited pulmonary metastasis, this procedure has been shown to increase disease-free survival [17]. The 99mTc-MDP scan has been reported to contribute to the diagnosis of bone forming malignant tumors by revealing unusual sites and presentations. As such, in these individuals, similar to primary bone tumor patients, the 99mTc-MDP bone scan is likely a means of monitoring treatment response. In these patients, a decrease in malignant osteoid matrix calcification would indicate a response to treatment in this cellular portion of the tumor. The bone scan appearance in metastatic disease, as with other tumors, can also underestimate the extent and severity of skeletal metastases. In these cases, the metastases involving the bone may only be apparent when a cortical reaction occurs. Subsequent other whole body surveys such as CT or [18F]FDG PET may provide a complimentary picture of the extent of diseased skeleton [18].
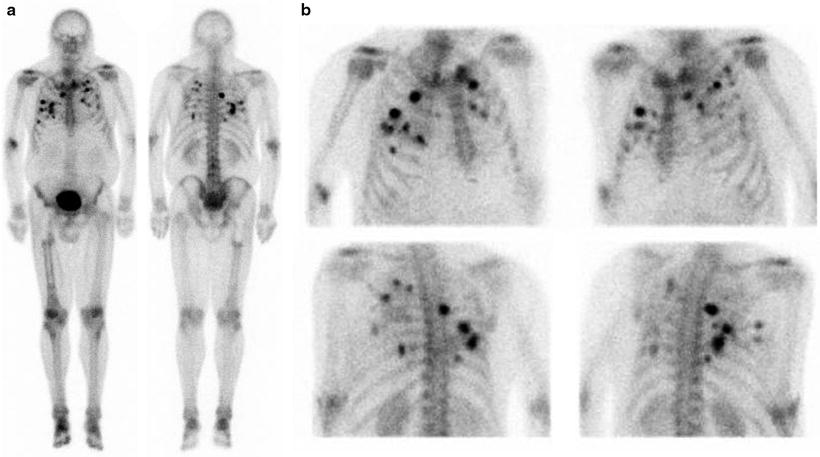

Fig. 23.2
Bone scan in a patient with active osteosarcoma pulmonary metastases
201TI-Chloride
201TI-Chloride as a nonspecific tumor imaging agent has been described for use in many tumors, including sarcoma. Most often, higher uptake is noted in malignant tumors, while lower uptake more frequently signifies benign neoplasms. Soft tissue, bone, and cartilaginous tumors have all shown positivity [19]. Typical high-grade tumor features, such as central necrosis, can also be noted in 201Tl-Chloride imaging; this finding is predictive of decreased survival [20].
18F-Fluoride
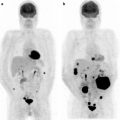
18F-Fluoride is increasingly being used as a sensitive bone scanning agent for metastatic surveys for many cancers. This is also the case for sarcomas; however, the use of 18F-fluoride may extend beyond the detection of metastases in bone sarcomas. Since the hallmark of these tumors is new bone formation, uptake and distribution levels of 18F-Fluoride will likely change in response to therapy. A particular use may be in evaluating activity in lung metastases since it is common practice to resect lung metastases when they are quiescent after successful chemotherapy. Figure 23.3 shows a whole body 18F-fluoride bone scan.
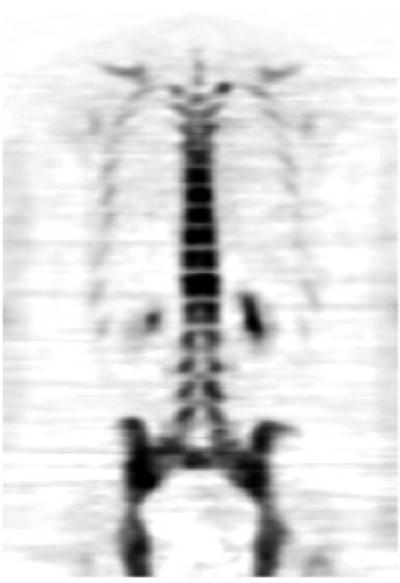

Fig. 23.3
Whole body 18F-fluoride PET bone scan
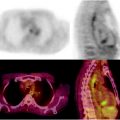
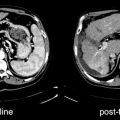
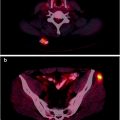
[18F]FDG and Sarcoma Diagnosis
Positron emission tomography (PET) with [18F]fluorodeoxyglucose ([18F]FDG) has been evaluated for use in sarcoma imaging. Recently, this imaging procedure has been investigated in treatment response evaluation. Diagnosis through imaging in primary cancers has several critical aspects for patient treatment planning. It is used to determine the biologic behavior of the tumor, often determining if the tumor is benign or malignant; however, sarcomas include numerous tumors that do not fit well with either category because they are locally aggressive, yet rarely metastatic. The tumor histologic type determined from biopsy and imaging data is also helpful in determining tumor grade, which is the propensity of the tumor to behave aggressively. Several prospective and retrospective studies have described the utility of [18F]FDG PET in sarcoma diagnosis. In soft tissue sarcoma, this imaging can reliably distinguish low-grade from high-grade tumors [21–23]. There is less ability to discriminate benign from low-grade tumors or intermediate-grade types from either high-grade or low-grade processes. Low-grade tumors are often difficult to distinguish through histologic criteria as well. Additionally, special features and [18F]FDG uptake are related to specific histologic types. For example, the atypical lipomas and the low-grade liposarcomas may have very similar tissue features and biological behavior. They often result in repeated local recurrence; nevertheless, in a large mass, the [18F]FDG appearance can be significant for identifying small areas of high-grade differentiation, which can lead to much worse treatment outcome than the low-grade majority of the tumor mass composition. Figure 23.4 shows several [18F]FDG PET examples of primary sarcoma tumors.